Abstract
A high-throughput screen led to the discovery of 2-amino-4-oxo-4-phenylbutanoate inhibitors of the 1,4-dihydroxy-2-naphthoyl-CoA synthase (MenB) from the menaquinone biosynthesis pathway in Mycobacterium tuberculosis. However, these compounds are unstable in solution and eliminate to form the corresponding 4-oxo-4-phenylbut-2-enoates that then react with CoA in situ to form nanomolar inhibitors of MenB. The potency of these compounds results from interaction of the CoA adduct carboxylate with the MenB oxyanion hole, a conserved structural motif in the crotonase superfamily. 4-Oxo-4-chlorophenylbutenoyl methyl ester has minimum inhibitory concentrations of 0.6 and 1.5 μg/mL against replicating and nonreplicating M. tuberculosis, respectively, and it is proposed that the methyl ester penetrates the cell where it is hydrolyzed and reacts with CoA to generate the active antibacterial. The CoA adducts thus represent an important foundation for the development of novel MenB inhibitors and suggest a general approach to the development of potent inhibitors of acyl-CoA binding enzymes.
Keywords: Menaquinone, MenB, 1, 4-dihydroxy-2-naphthoyl-CoA synthase, CoA, HTS, o-succinylbenzoic acid
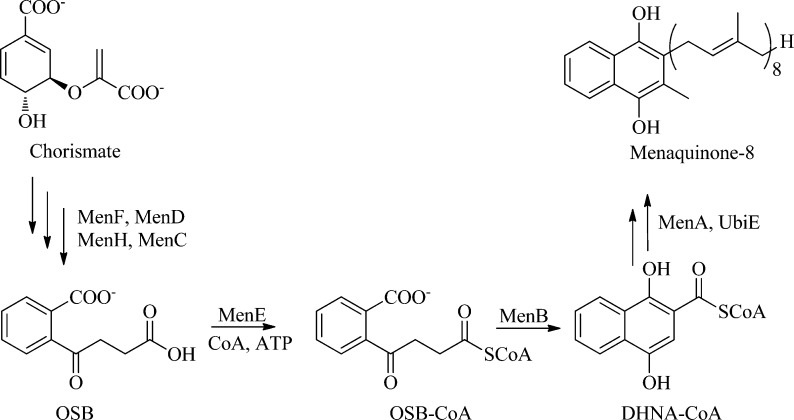
Tuberculosis is a global health threat, and efforts to combat the spread of this disease are hindered by the emergence of drug resistance, coupled with the ability of Mycobacterium tuberculosis to survive in a latent, nonreplicating state for many years.1,2 Because latent mycobacteria are thought to consume ATP to remain viable,3 compounds that interfere with bacterial respiration hold the promise of targeting both replicating as well as nonreplicating bacterial populations.4M. tuberculosis utilizes menaquinone (vitamin K2), a polyisoprenylated naphthoquinone, as the lipid-soluble redox cofactor in the electron transport chain, and efforts are underway to validate enzymes in the menaquinone biosynthesis pathway as targets for drug discovery.5,6 The menaquinone biosynthesis pathway in M. tuberculosis mirrors that found in Escherichia coli (Figure 1),7,8 and transposon site hybridization has identified menC, menD, and menE as essential for growth of M. tuberculosis.9 Recently, Sassetti and co-workers have shown that menB is also essential (Dr. Sassetti, personal communication), while inhibitors of MenA are active against both replicating and nonreplicating bacterial populations.6 In the present study, we now report the discovery of a series of 2-CoA-4-oxo-4-phenylbutanoic acids that target MenB, the 1,4-dihydroxy-2-naphthoyl-CoA (DHNA-CoA) synthase that catalyzes an intramolecular Claisen condensation (Dieckmann) reaction leading to the formation of DHNA-CoA from o-succinylbenzoate (OSB) (Figure 1).8
Figure 1.
Menaquinone biosynthesis pathway. This pathway converts chorismate to menaquinone in prokaryotes such as E. coli, S. aureus, B. subtilis, and M. tuberculosis. Shown in detail are the reactions catalyzed by the OSB-CoA synthase MenE and the 1,4,-dihydroxy-2-naphthoyl-CoA synthase MenB.
The substrate for MenB is unstable, and consequently, we used a coupled assay8 to screen 105 091 small molecules (see the Supporting Information).10 Following the primary screen, in which compounds were tested at a single concentration of 12.5 μg/mL, we obtained 455 hits that had at least 30% inhibition relative to control. Within these hits, we identified seven compounds (Figure 2) that possessed the backbone structure of OSB, the substrate for MenE. We hypothesized that these molecules could inhibit either MenE or MenB, and so, compounds were first evaluated for their ability to inhibit MenE directly.11 Using this assay, it was found that 1548L21 (Figure 2) did not inhibit MenE (100 nM) up to a concentration of 300 μM (data not shown).
Figure 2.
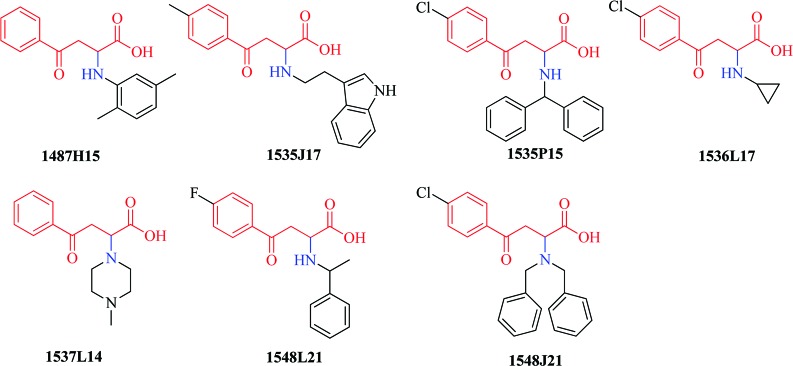
HTS hits containing the OSB core. These compounds were identified in the initial HTS screen conducted using the MenE/MenB coupled assay.
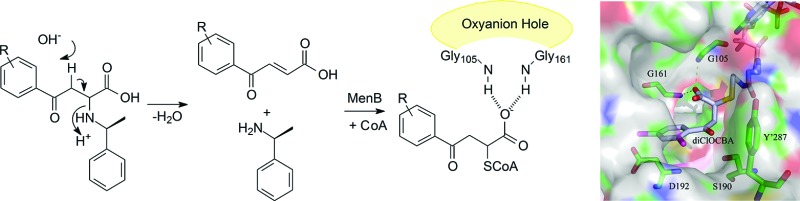
Analogues of 1548L21 were synthesized (Scheme S1 in the Supporting Information) and evaluated for their ability to inhibit MenB as well as bacterial growth (Table 1 and the Supporting Information). Although several compounds inhibited MenB with IC50 values below 10 μM (4 and 5), there was generally a poor correlation between enzyme inhibition and antibacterial activity. For example, 1 is at least 10-fold more active in the antibacterial assay than 4 [minimum inhibitory concentration (MIC) is 6.25 μg/mL as compared to 50 μg/mL] but is a significantly poorer inhibitor of MenB (IC50 = 52 μM). Subsequent studies revealed that the inhibitors undergo retro-Michael addition,12 leading to the regeneration of the (E)-benzoylacrylic acid and the amine in solution. The 2-aminobutanoates have half-lives of ∼10 min to 12 h at pH 7, which is significant since the MIC measurements take at least 24–48 h to complete. In addition, the IC50 values were determined using the same protocol as that developed for the high-throughput screening (HTS) in which compounds were preincubated in reaction buffer with all components except MenE for 1 h prior to determining enzyme activity, suggesting that compound stability was also a factor in the enzyme inhibition studies. Indeed, these compounds were found not to inhibit MenB if the reaction was initiated immediately after addition of inhibitor (Table 1).
Table 1. Activity of the 2-Amino-4-oxo-4-phenylbutanoic Acids.
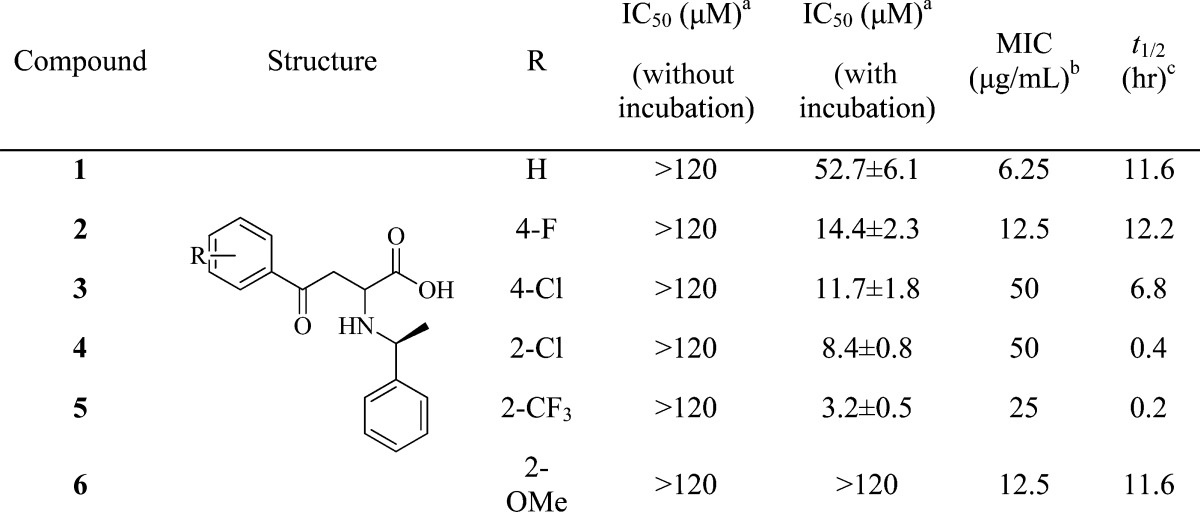
IC50 values for MenB inhibition were determined at a MenB concentration of 150 nM. Assays were initiated by the addition of MenE either immediately after adding inhibitor to the assay (without incubation) or after a 1 h incubation (with incubation).
Antibacterial activity against M. tuberculosis H37Rv.
Stability at pH 7.4 and 25 °C.
To strengthen the connection between compound stability and enzyme inhibition, we synthesized a series of compounds that were unable to undergo retro-Michael addition (Figure 1S in the Supporting Information). This included analogues 1S and 2S in which the α-protons were replaced with fluorines or methyl groups, 3S in which the ketone was replaced with a hydroxyl group, and 4S, 5S, and 6S in which the amine was replaced with an amide, heterocycle, or sulfur, respectively. Compounds 1S to 6S were stable in solution and did not inhibit MenB up to a concentration of 50 μM. In addition, we also synthesized the monomethyl and deuterium analogues 7S and 8S, which were still able to undergo elimination and, as a consequence, also retained the ability to inhibit MenB. On the basis of these results, we hypothesized that the inhibitory activity of the compounds was related to their stability in solution.
Because the (E)-benzoylacrylic acid is a Michael acceptor, we speculated that CoA in the reaction mixture might react with the acid, and it was found that the IC50 value for inhibition of MenB by compound 3a decreased as the concentration of CoA increased (data not shown). This suggested that inhibition of MenB might result from an adduct formed between the (E)-benzoylacrylic acid and CoA. Subsequently, the (E)-benzoylacrylic acid and CoA were incubated together at pH 7.0 for 2 h, and the major product was purified by high-performance liquid chromatography. Analytical data confirmed that the CoA thiol had added to the C2 carbon of the acid.
To generate structure–activity relationship data for MenB inhibition, a series of CoA adducts were synthesized and evaluated (Table 2). In general, the addition of a bulky substituent at either the meta or the para position of the phenyl group resulted in significant reduction in enzyme inhibition. Additionally, incorporation of electron-donating substituents at either the ortho or the para positions also decreased inhibitor potency. In contrast, introduction of an electron-withdrawing substituent into the aromatic ring resulted in an increase in enzyme inhibition. Perusal of the data in Table 2 indicates that the most potent compounds (10 and 16) have IC50 values of ∼100 nM, which is close to 1/2[Eo]. To gain further mechanistic insight into the functioning of these compounds, we thus used steady state kinetic methods to study the inhibition of MenB by 7, 10 and 16. These compounds were found to be noncompetitive (mixed) inhibitors of MenB. Consistent with their respective IC50 values (106 and 470 nM, respectively), the Ki and Ki′ values of the 2,4-diCl derivative 16 (49 and 290 nM, respectively) were significantly lower than the corresponding values for the 4-Cl analogue 7 (0.35 and 1.6 μM, respectively). Clearly, there is a preference for an electron-withdrawing substituent ortho to the succinyl side chain, which of course is the position normally occupied by the OSB carboxyl group. To account for noncompetitive inhibition, we are currently exploring the possibility that binding of inhibitor to one subunit in the MenB homohexamer can modulate the activity of adjacent subunits.
Table 2. Inhibition of MenB by the 2-CoA-4-oxo-4-phenylbutanoic Acids.
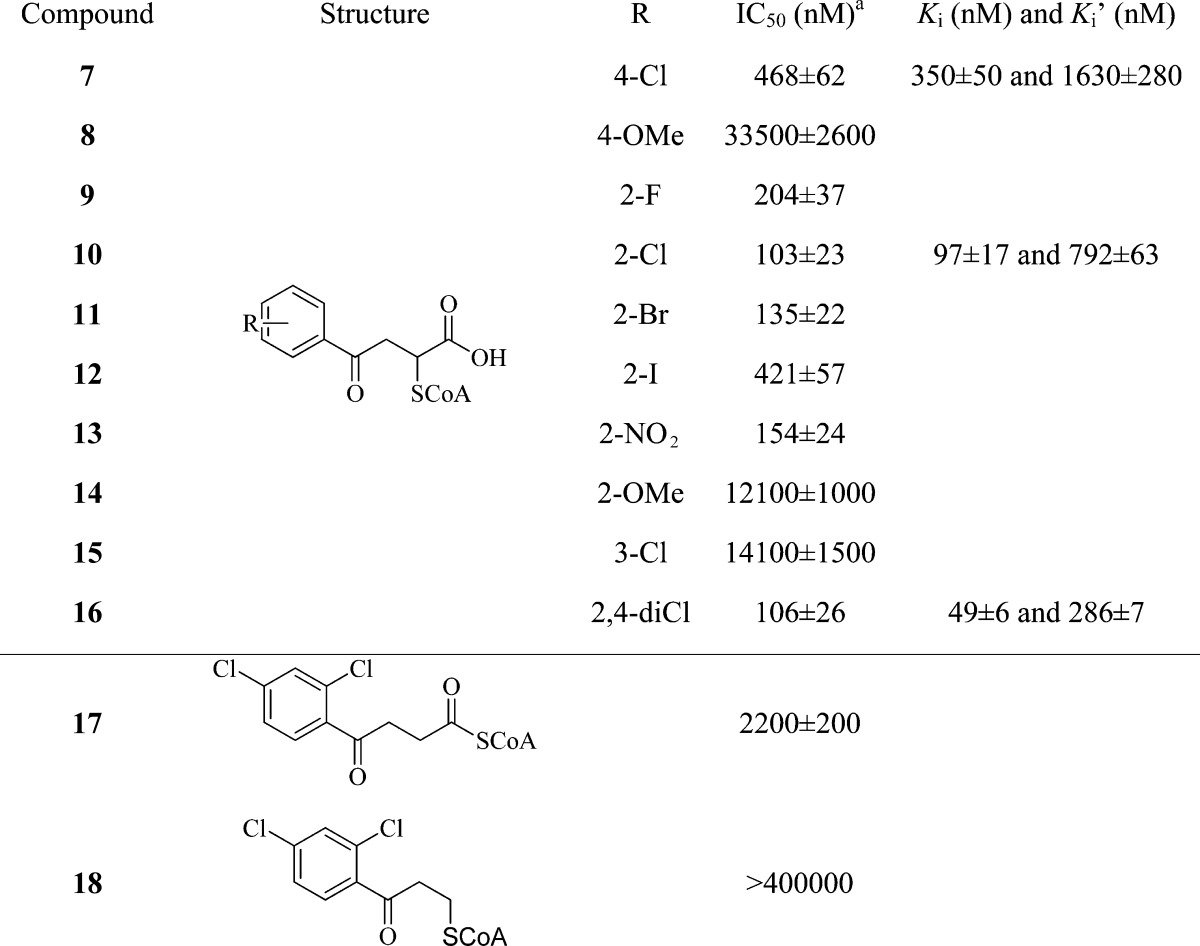
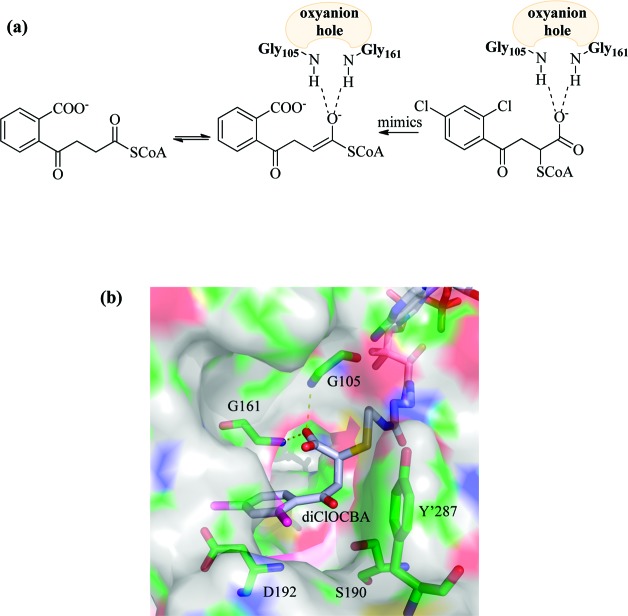
The affinity of 16 is noteworthy as, in our experience, substrate or product analogues of CoA-dependent enzymes do not generally bind with high affinity to their respective enzymes. Indeed, potent inhibitors of CoA-binding enzymes are largely unknown except in a few specific cases such as 2-octynoyl-CoA, which is a mechanism-based inhibitor of acyl-CoA dehydrogenase.13 However, analysis of the MenB reaction suggests an explanation for the potent inhibition of MenB by the CoA adducts. A mechanism for the MenB-catalyzed reaction has been proposed in which an intramolecular proton abstraction by the OSB carboxylate leads to the formation of a resonance stabilized carbanion.8 Model building suggests that the CoA adducts adopt a bound structure that resembles the intermediate required for α-proton abstraction (Figure 3), which may account for the high affinity of these compounds for MenB. Of key importance is the location of the free carboxylate of the adduct, which is bound in the oxyanion hole formed by Gly-105 and Gly-161.8,14 The oxyanion hole is a conserved structural feature within the crotonase superfamily and plays a central role in catalysis by stabilizing the carbanion/enolate formed during reactions catalyzed by this superfamily.15 Indeed, many other CoA binding enzymes outside the crotonase superfamily also function to increase either thioester electrophilicity or α-proton acidity through interactions with the acyl-CoA thioester carbonyl, and thus, our data suggest a general structural feature that may facilitate the development of inhibitors of CoA-dependent drug targets.
Figure 3.
Proposed structure of a CoA adduct bound to MenB. (a) Proposed structure of the CoA adduct bound to MenB. The CoA adduct adopts a structure that mimics the resonance-stabilized carbanion formed during the MenB-catalyzed reaction. (b) The 2,4-dichloro CoA adduct (16) modeled into the active site of MenB. This model was built using the structure of acetoacetyl-CoA bound to MenB (1Q51.pdb)8 and shows the proposed interaction between the adduct carboxylate and the MenB oxyanion hole (G105 and G161). Also shown are three conserved MenB residues (S190, D192, and Y′287). The figure was made using pymol.23
To investigate the importance of the proposed interaction between the oxyanion hole and the adduct carboxylate, we synthesized two additional compounds 17 and 18 (Scheme S1 in the Supporting Information), which have similar structures to the CoA adducts but lack a free carboxylate group. As compared with the CoA adducts, the CoA thioester (17) has a significantly lower affinity for MenB (Table 2) with an IC50 value of 2.2 μM as compared to 100 nM for the corresponding adduct (16). In addition, compound 18 displayed no significant inhibition up to a concentration of 400 μM. These two compounds thus support the importance of the free carboxylate for CoA adduct binding.
The CoA adducts were found to have limited antibacterial activity (data not shown), presumably due to poor uptake by the bacteria. We speculated that CoA addition could occur once the benzoylacrylic acid had penetrated the cell, and while the benzoylacrylic acid 3a had limited antibacterial activity, the corresponding benzoylacrylic acid methyl ester 3b, as well as the fluoro analogue 2b, displayed potent antibacterial activity (Table 3, MIC = 0.64 μg/mL). Indeed, 3b also had potent activity in a low oxygen recovery assay (LORA) against nonreplicating M. tuberculosis (NRP-MTB) (Table 3, MIC = 1.5 μg/mL), which is promising given that apart from rifampicin (MIC = 0.4 μg/mL), many current drugs are inactive against NRP-MTB.16 Interestingly, it has been reported that the menB gene is upregulated when H37Rv is grown under oxygen-limiting conditions,17 supporting the possibility that this enzyme is an intracellular target for the CoA adducts. On the basis of these results, we propose that protection of the acid aids the entry of the compounds into the cell where they then undergo reaction(s), such as a Michael addition by CoA, which results in the formation of the antibacterial species. Although we cannot rule out the possibility that the compounds react with other nucleophiles in the cell, we observed that the addition of 1,4-dihydroxy-2-naphthoic acid (DHNA; 100 μg/mL) to the media was able to rescue the growth of M. tuberculosis H37Rv that had been treated with 2b or 3b at 2× MIC. DHNA is a downstream product of the MenB reaction that has previously been used in complementation experiments to elucidate the order and identity of enzymes that comprise the menaquinone biosynthesis pathway.18 Thus, the ability of DHNA to rescue growth supports our current hypothesis that the Michael acceptors inhibit menaquinone biosynthesis in M. tuberculosis.
Table 3. Antibacterial Activity of the 4-Oxo-4-phenylbut-2-enoates against H37Rv.
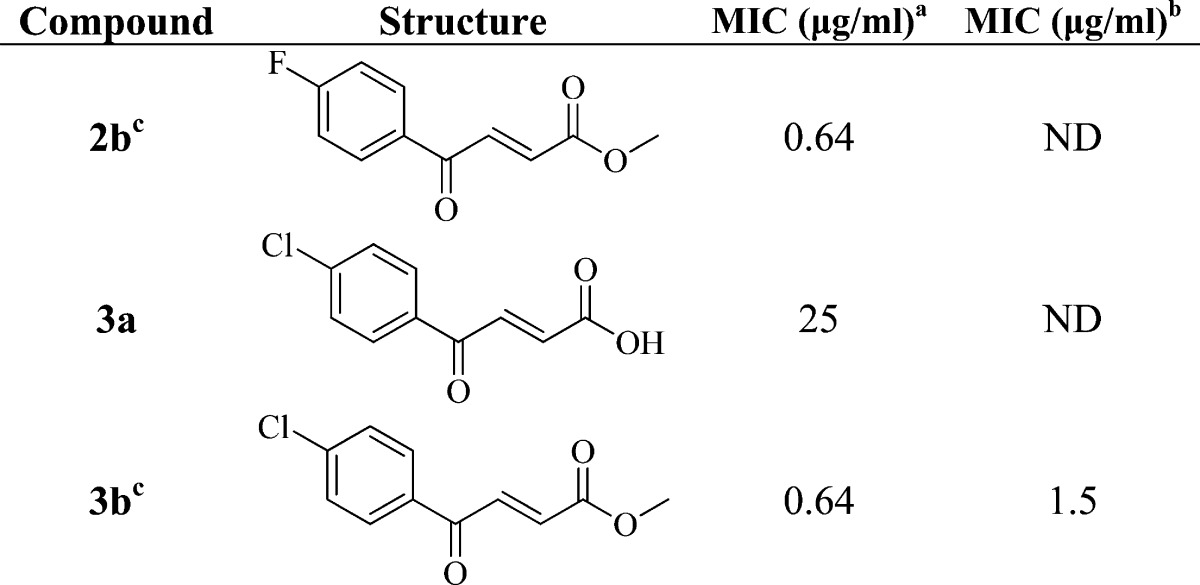
MIC determined in the microplate dilution assay under aerobic conditions.
MIC determined in the in low oxygen recovery assay against NRP-MTB. ND, not determined.
Cytoxicity assays indicated that the selectivity index of 2b and 3b is greater than 20.
Further studies are required to confirm that CoA addition and MenB inhibition occur within the cell and to fully elucidate the mechanism of action of these compounds. In addition, it will be important to further improve the affinity of the adducts for MenB and also to replace the CoA portion of the adducts by more druglike groups, and in this regard, we note the studies by Pereira et al. in which inhibitors of E. coli acetyltransferase GlmU were identified that occupy the CoA binding site in this enzyme.19 Because the benzoylacrylic acid and CoA bind to adjacent sites on MenB, we are also exploring the possibility that MenB catalyzes adduct formation in an analogous fashion to that observed, for example, in the assembly of inhibitors via in situ click chemistry.20 It is interesting to note that the IC50 values for enzyme inhibition by the parent 2-aminobutanoates (Table 1) correlate with their stability and hence reactivity of the butenoate that is formed. There is also a correlation between IC50 and Ki values for inhibition of MenB by the resulting adducts, indicating that the more electrophilic butenoates bind more tightly to the enzyme, presumably due to favorable interactions between the enzyme and the halogens at the 2- and 4-positions. A current goal of our research program is to develop butenoates with increased affinity for the enzyme but reduced electrophilicity, thus improving the selectivity of MenB inhibition by increasing the likelihood that adduct formation would occur on the enzyme. In this regard, we note the recent discussions concerning the utility of covalent enzyme inhibitors that contain appropriately tuned electrophilic groups.21
Finally, in addition to providing a promising starting point for the development of MenB inhibitors, the work described here also serves to reinforce issues that can arise with leads identified through HTS.22 Although the present leads are stable in DMSO, their reactivity under aqueous conditions reinforces the necessity to consider compound stability under the assay conditions employed, which is of particular importance for measurements that require prolonged incubation such as antibacterial assays. In the present work, the use of a coupled assay that included free CoA resulted in the discovery of CoA adducts that bind with high affinity to MenB and that represent a promising foundation for the development of novel antibacterial agents that target menaquinone biosynthesis in M. tuberculosis as well as other pathogenic bacteria that have this pathway. Importantly, our data also support the proposal that MenB may be an appropriate target in nonreplicating populations of M. tuberculosis.
Supporting Information Available
Detailed experimental procedures for the synthesis of compounds and biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
We thank NERCE/NSRB for assistance with compound screening (NIH Grant AI057159). This work was funded by the NIH Grants AI044639, AI070383, and AI058785 to P.J.T.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Bloom B. R.; Murray C. J. Tuberculosis: Commentary on a reemergent killer. Science 1992, 257, 1055–1064. [DOI] [PubMed] [Google Scholar]
- Boshoff H. I.; Barry C. E. 3rd. Tuberculosis—Metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 2005, 3, 70–80. [DOI] [PubMed] [Google Scholar]
- Gengenbacher M.; Rao S. P.; Pethe K.; Dick T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 2010, 156, 81–87. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–237. [DOI] [PubMed] [Google Scholar]
- Lu X.; Zhang H.; Tonge P. J.; Tan D. S. Mechanism-based inhibitors of MenE, an acyl-CoA synthetase involved in bacterial menaquinone biosynthesis. Bioorg. Med. Chem. Lett. 2008, 18, 5963–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman R. K.; Mahapatra S.; Slayden R. A.; Boyne M. E.; Lenaerts A.; Hinshaw J. C.; Angala S. K.; Chatterjee D.; Biswas K.; Narayanasamy P.; Kurosu M.; Crick D. C. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol. Microbiol. 2009, 72, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan R. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q): A perspective on enzymatic mechanisms. Vitam. Horm. 2001, 61, 173–218. [DOI] [PubMed] [Google Scholar]
- Truglio J. J.; Theis K.; Feng Y.; Gajda R.; Machutta C.; Tonge P. J.; Kisker C. Crystal Structure of Mycobacterium tuberculosis MenB, a Key Enzyme in Vitamin K2 Biosynthesis. J. Biol. Chem. 2003, 278, 42352–42360. [DOI] [PubMed] [Google Scholar]
- Sassetti C. M.; Boyd D. H.; Rubin E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [DOI] [PubMed] [Google Scholar]
- Li X.; Liu N.; Zhang H.; Knudson S. E.; Slayden R. A.; Tonge P. J. Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: Novel antibacterial agents against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2010, 20, 6306–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrien W. E. Continuous spectrophotometric assay for argininosuccinate synthetase based on pyrophosphate formation. Anal. Biochem. 1976, 76, 423–430. [DOI] [PubMed] [Google Scholar]
- Jenner G. Comparative study of physical and chemical activation modes. The case of the synthesis of beta-amino derivatives. Tetrahedron 1996, 52, 13557–13568. [Google Scholar]
- Powell P. J.; Thorpe C. 2-Octynoyl coenzyme A is a mechanism-based inhibitor of pig kidney medium-chain acyl coenzyme A dehydrogenase: isolation of the target peptide. Biochemistry 1988, 27, 8022–8028. [DOI] [PubMed] [Google Scholar]
- Li H. J.; Li X.; Liu N.; Zhang H.; Truglio J. J.; Mishra S.; Kisker C. F.; Garcia-Diaz M.; Tonge P. J.. Mechanism of the Intramolecular Claisen Condensation Reaction Catalyzed by MenB, a Crotonase Superfamily Member. Biochemistry 2011, DOI: 10.1021/bi200877x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Machutta C. A.; Tonge P. J.. Fatty Acid Biosynthesis and Oxidation. In Comprehensive Natural Products Chemistry II Chemistry and Biology; Mander L., Lui H.-W., Eds.; Elsevier: Amsterdam, 2010; Vol. 8, pp 231–275. [Google Scholar]
- Hurdle J. G.; Lee R. B.; Budha N. R.; Carson E. I.; Qi J.; Scherman M. S.; Cho S. H.; McNeil M. R.; Lenaerts A. J.; Franzblau S. G.; Meibohm B.; Lee R. E. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J. Antimicrob. Chemother. 2008, 62, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandra P.; Sturm A. W. Expression of the naphthoate synthase gene in Mycobacterium tuberculosis in a self-generated oxygen depleted liquid culture system. Anaerobe 2010, 16, 610–613. [DOI] [PubMed] [Google Scholar]
- Taber H. W.; Dellers E. A.; Lombardo L. R. Menaquinone biosynthesis in Bacillus subtilis—Isolation of men mutants and evidence for clustering of men genes. J. Bacteriol. 1981, 145, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. P.; Blanchard J. E.; Murphy C.; Roderick S. L.; Brown E. D. High-throughput screening identifies novel inhibitors of the acetyltransferase activity of Escherichia coli GlmU. Antimicrob. Agents Chemother. 2009, 53, 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. G.; Green L. G.; Grynszpan F.; Radic Z.; Carlier P. R.; Taylor P.; Finn M. G.; Sharpless K. B. Click chemistry in situ: Acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem., Int. Ed. Engl. 2002, 41, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Singh J.; Petter R. C.; Baillie T. A.; Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discovery 2011, 10, 307–317. [DOI] [PubMed] [Google Scholar]
- Baell J. B.; Holloway G. A. New substructure filters for removal of Pan Assay Interference Compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [DOI] [PubMed] [Google Scholar]
- Delano W. L. The PyMOL Molecular Graphics System; http://www.pymol.org, 2002.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.