Abstract
Brain metastases are a serious obstacle in the treatment of patients with solid tumors and contribute to the morbidity and mortality of these cancers. It is speculated that the frequency of brain metastasis is increasing for several reasons, including improved systemic therapy and survival, and detection of metastases in asymptomatic patients. The lack of preclinical models that recapitulate the clinical setting and the exclusion of patients with brain metastases from most clinical trials have slowed progress. Molecular factors contributing to brain metastases are being elucidated, such as genes involved in cell adhesion, extravasation, metabolism, and cellular signaling. Furthermore, the role of the unique brain microenvironment is beginning to be explored. Although the presence and function of the blood–brain barrier in metastatic tumors is still poorly understood, it is likely that some tumor cells are protected from therapeutics by the blood–tumor barrier, creating a sanctuary site. This Review discusses what is known about the biology of brain metastases, what preclinical models are available to study the disease, and which novel therapeutic strategies are being studied in patients.
Introduction
Metastatic brain tumors are the most frequently occurring intracranial neoplasms in adults with the annual incidence in the USA estimated to be 200,000 cases.1 Furthermore, an estimated 8–10% of adults with cancer develop symptomatic brain metastases.2,3 The majority of brain metastases originate from primary cancers in the lung (40–50%) or breast (15–25%), or from melanoma (5–20%).2,3 The frequency of diagnosis of metastatic brain tumors seems to be increasing as a result of improved imaging modalities and earlier detection as well as longer survival after primary cancer diagnosis because of more-effective treatment of systemic disease.
The distribution of brain metastases correlates with blood flow and tissue volume, with 80% detected in the cerebral hemispheres, 15% in the cerebellum, and 5% in the brainstem.4 The majority of patients exhibit multiple tumors at the time of brain-metastasis diagnosis. Clinical features include headache, neurological deficit, and seizures. Neuropsychological testing demonstrates cognitive impairment in 65% of patients with brain metastases,5,6 which might be a result of destruction or displacement of brain tissue by the expanding tumor, peritumoral edema leading to further disruption of surrounding white matter tracts, increased intracranial pressure, and/or vascular compromise.
Therapeutic approaches for brain metastases include surgery, whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), chemotherapy, growth factor inhibitors, or a combination of these therapies. Survival for patients with brain metastases treated with WBRT typically ranges from 4–6 months, but can be as long as 12–24 months for selected patients.6 Strong positive prognostic factors include good functional status, age <65 years, no sites of metastasis outside of the central nervous system (CNS), controlled primary tumor,7 the presence of a single metastasis in the brain, long interval from primary diagnosis to brain relapse, and certain cancer subtypes such as HER2-positive breast cancer and EGFR-mutant non-small-cell lung cancer (NSCLC).8–10 In up to half of patients the cause of death is attributed to CNS progression.11 Randomized clinical trials have shown that surgery or SRS combined with WBRT improves overall survival compared with WBRT alone in patients with a single metastasis in the brain. In patients with four or fewer brain metastases, SRS results in equivalent overall survival but worse intracranial disease control compared with SRS plus WBRT.11–15 The combination of radiotherapy and chemotherapy improves response rate and/or progression-free survival in some studies, but not overall survival.16–18
We review our current understanding of the biology of brain metastasis, present preclinical approaches that are available to model the disease, including emerging imaging techniques, and discuss novel therapeutic strategies to target the unique features of CNS metastases.
Brain metastasis formation
The metastatic cascade, whereby cancer cells escape from the primary tumor site, invade surrounding tissue, intravasate into the bloodstream or lymphatics, and arrest, extravasate, survive and proliferate within a secondary site (Figure 1), is an inherently inefficient process.19 Certain tumor types demonstrate an organ-specific pattern of spread; for example, prostate cancer frequently metastasizes to bone and melanoma frequently metastasizes to the lung, liver and brain.20,21 Stephen Paget was the first to hypothesize that the propensity of cancer cells to spread to specific sites was dependent upon two factors: the cancer cell (the ‘seed’) and the receiving organ environment (the ‘soil’).22 An alternative hypothesis attributed to pathologist James Ewing proposes that circulatory patterns between the primary tumor and specific secondary organs are sufficient to explain the majority of organ-specific metastatic spread.23 A newer hypothesis is that cancer cells can bring their own soil—stromal components from the primary site including activated fibroblasts—to secondary sites.24 Researchers have sought to understand how molecular and genetic features of the primary cancer cell, the brain microenvironment, and the angiogenic pathways influence spread of cancer to the brain and its growth there (Table 1).
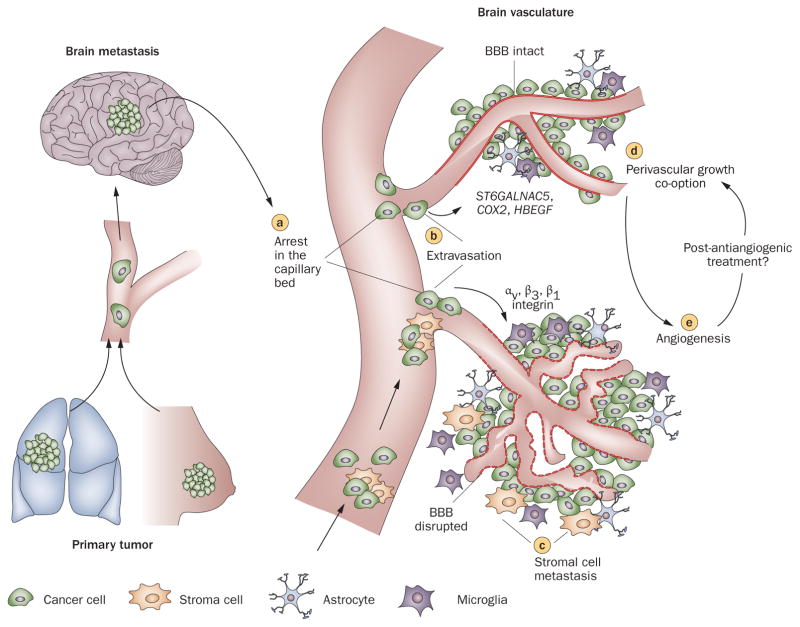
Figure 1.
Steps in the formation of hematogenous metastasis to the brain. Once tumor cells shed from primary tumors arrive in brain vasculature, a | they arrest in the capillary bed primarily owing to size restriction, and b | subsequently extravasate across the BBB and enter the brain parenchyma. Three genes mediate cancer cell trans-BBB migration: HBEGF, COX2, and ST6GALNAC5.78 Also, activation of integrins, such as αvβ354,139 and β1,27 is suggested to control tumor-cell arrest and adhesion to the vasculature. c | Metastasizing tumor cells (seeds) may bring their own host cells (soil) in metastasis.24 After extravasation, tumor cells either d | grow along pre-existing blood vessels (perivascular growth),49 or e | recruit new blood vessels (angiogenesis) to obtain sufficient nutrients to support their proliferation. Abbreviation: BBB, blood–brain barrier.
Table 1.
Genes involved in the formation of brain metastasis
| Genes | Function | Primary tumor type | Comments |
|---|---|---|---|
| COX2 | Important in prostaglandin production, possibly leading to increased permeability of BBB | Breast | Inhibition suppresses penetration of an artificial BBB, and enhances brain-metastasis-free survival78 |
|
| |||
| HBEGF | EGFR ligand—increases cell growth, motility, and invasiveness | Breast | Inhibition suppresses penetration of an artificial BBB, and enhances brain-metastasis-free survival78 |
|
| |||
| ST6GALNAC5 | Sialyltransferases catalyze the addition of sialic acid to gangliosides and glycoproteins, and cell-surface sialylation has been implicated in cell–cell interactions | Breast | Inhibition suppresses penetration of an artificial BBB, and enhances brain-metastasis-free survival78 |
|
| |||
| HK2 | Important in glucose metabolism, oxidative phosphorylation, and antiapoptosis | Breast | High HK2 expression is associated with poor patient survival after craniotomy140 |
|
| |||
| FOXC1 | Transcription factor essential for mesoderm development; involved in brain development and brain tumorigenesis | Breast | Predicts poor overall survival in basal-like breast cancer, a higher incidence of brain metastasis and a shorter brain-metastasis-free survival in lymph-node-negative patients141 |
|
| |||
| HER2 | Receptor tyrosine kinase of the EGFR family | Breast | Overexpression increased the incidence of large brain metastases (>50 μm2)73 |
|
| |||
| VEGFA | Angiogenic growth factor | Breast | Increased in brain-metastatic clones, and VEGFR inhibition decreased brain tumor burden via a reduced number of blood vessels, decreased proliferation and increased apoptosis52 |
| Melanoma | Overexpression accelerated growth, accompanied by dilation of co-opted tumor vessels with concomitant induction of vascular permeability48 | ||
| Lung and colon | Decreased expression significantly decreased the incidence of brain metastases51 | ||
|
| |||
| LEF1 | A transcriptional effector of the canonical WNT pathway | Lung | Part of a signature that predicts lung metastasis to the brain; knockdown inhibited brain metastasis, and decreased colony formation and invasion in vitro142 |
|
| |||
| HOXB9 | Belongs to the homeobox transcription factor gene family, which is critical for embryonic segmentation and limb patterning—a TCF4 target | Lung | Part of a signature that predicts lung metastasis to the brain; knockdown inhibited brain metastasis, and decreased colony formation and invasion in vitro142 |
|
| |||
| CDH2, KIFC1, and FALZ3 | N-cadherin is a calcium-dependent cell–cell adhesion molecule | Lung | Highly predictive of brain metastasis in early-stage and advanced-stage lung cancers—causal role is not clear143 |
|
| |||
| STAT3 | Important transcription factor in cellular signaling pathways | Melanoma | Reduction suppressed brain metastases—affected angiogenesis in vivo and cell invasion in vitro144 |
|
| |||
| αvβ3 | Important for sprouting endothelial cells, contributes to angiogenesis, supports invasion and metastasis | MDA-MB-453 | Activated αvβ3 enhances brain metastatic tumor growth through continuous upregulation of VEGF, leading to increased angiogenesis and decreased hypoxia54 |
|
| |||
| HDAC3, JAG2, NUMB, APH1B, HES4, and PSEN1 | Notch signaling pathway genes that determine cell fates through communication with their environment | MDA-MB-453 | Inactivation of Notch significantly inhibited migration and invasion145 |
Abbreviation: BBB, blood–brain barrier.
Brain microenvironment
Once metastatic cancer cells enter the brain circulation, they might arrest in sites of slow flow within the capillary bed at vascular branch points,25 which is then followed by early changes in the brain microenvironment.26 The arrested cancer cells encounter brain vascular endothelial cells, which seem to promote metastatic tumor cell growth and invasion.25,27 In addition, stromal cells such as fibroblasts associated with the primary tumor are involved in metastatic nodules in the brain (Figure 1).24 These co-disseminating stromal cells provide survival and proliferative advantages to the tumor cells and facilitate early colonization of metastatic foci. The brain also provides an environment that differs from most other organs, and the factors that either promote or suppress colonization and proliferation are poorly understood (Table 1). Thus, successful treatment of CNS metastases may require targeting of both tumor and host responses.
Response of host stromal cells
After infiltration into the brain tissue, cancer cells encounter a number of host cell types, including microglia and astrocytes. Microglia displaying stellate morphology with thick cellular processes, characteristic of an activated state, have been observed around extravastated cancer cells within 7 days of intracarotid injection of breast cancer cell lines into mice.26 Activated astrocytes with thick processes and upregulated expression of glial fibrillary acidic protein can be seen even earlier, when cancer cells are still in the intravascular space, and they remain closely associated with cancer cells throughout their growth into macrometastases.26 In a xenograft model, reactive astrocytes and microglia were in direct contact with tumor cells along the border of the tumors as well as infiltrating the inner tumor mass. Similar findings were reported in human specimens. In addition, in vitro co-cultures demonstrated that glia induced a fivefold increase in metastatic cell proliferation, leading the researchers to hypothesize that reactive glia creates an altered brain microenvironment that is more permissive to tumor growth and invasion.28 Consistent with these findings, expression of endothelin 1, a regulator of numerous transforming processes, was observed in peritumoral astrocytes in 85% of hematogeneous metastases of the human brain.29 Furthermore, additional studies suggest that microglia can enhance the invasion and colonization in the brain tissue by breast cancer cells similar to other tissue-specific stromal cells such as osteoblasts and osteoclasts in the bone.30,31 Most likely, during the early stages of brain metastasis formation, there is a balance between the protective or trophic, and cytotoxic functions of microglia, dependent on the release of a variety of factors and ultimately determined by signals from tumor cells.32
Protective roles of astrocytes
Astrocytes within the brain microenvironment might serve to protect brain metastases from cytotoxicity induced by chemotherapeutic drugs.33 In co-culture experiments, the presence of astrocytes but not fibroblasts dramatically reduced 5-fluorouracil-induced and cisplatin-induced apoptosis in human tumor cells.34 The protective effect was lost when cells were separated with a trans-well membrane, suggesting that direct contact of astrocytes with tumor cells, rather than secreted factors, was mediating the chemoprotective effect. Similar effects have also been demonstrated in human melanoma cell lines,35 breast cancer cells, and lung cancer cells.36
Soluble factors also have an important role in tumor cell–astrocyte interaction. Activated astrocytes surround brain metastatic lesions from lung cancer, both in an experimental mouse model and in the human brain.37 Tumor cell-derived factors (including migration inhibitory factor, interleukin [IL]-8, and plasminogen activator inhibitor 1) induced astrocytic activation and release of molecules (IL-6, TNF-α, and IL-1β) that promoted tumor-cell proliferation in vitro. Furthermore, neurotrophins and their receptors have a role in the invasion and colonization of brain-prone melanoma cells.38,39
Angiogenesis
The growth and proliferation of primary and metastatic tumors is dependent on the establishment of an adequate blood supply.40–42 A tumor can recruit blood vessels via different mechanisms: angiogenesis (sprouting from existing blood vessels), vasculogenesis (formation of a de novo vascular system from endothelial precursor cells), co-option (growth of cancer cells along existing blood vessels), intussusception (vessel remodeling and expansion by the insertion of interstitial tissue columns into the lumen of pre-existing vessels), vasculogenic mimicry (cancer cells lining blood vessels), cancer cells that transdifferentiate into endothelial cells,43 and cancer stem-like cells that form an inner lining of blood vessels in the brain.44–47
Changes in brain vasculature
Kienast et al.25 used multiphoton laser scanning microscopy and a mouse cranial window model to follow in real time brain metastasis formation from both lung cancer and melanoma cell lines. After extravasation, there was a persistent correlation between tumor cells with micro-vessels and either vessel co-option (with melanoma) or angiogenesis (with lung carcinoma). Previous studies showed a similar association between metastasizing tumor cells and blood vessels.27,28,48,49 Kusters et al.48 showed that a melanoma brain metastasis could grow up to 3 mm through co-opting pre-existing blood vessels. Similar observations in murine experimental brain metastases models from breast cancer and melanoma cells indicate that an active adhesion mechanism exists between tumor cells and the vascular basement membrane.27
Metastatic brain tumor vessels in preclinical models and human surgical specimens have significantly larger diameters and thicker basement membranes when compared with the vessels of normal brain.50 Brain metastases from murine melanoma, murine fibrosarcoma, human lung carcinoma, and human colon carcinoma have a lower microvascular density than the surrounding normal brain parenchyma, and they all contain dilated blood vessels with large lumens.50 The mechanism for this enlargement seems to involve endothelial cell proliferation.
Angiogenic factors in brain metastasis
The progressive growth of the majority of metastatic brain tumors is critically dependent on the expression of VEGF.51 In a brain metastatic variant of MDA-MB-231 cells, there was a significant increase in VEGF-A production, and inhibition of VEGFR activity significantly reduced brain tumor burden after intracarotid injection in mice.52 Moreover, when human lung cancer cells transfected with antisense VEGF165 were injected into the carotid artery of nude mice, the formation of brain metastases was substantially decreased compared with untransfected cells.51 However, in cells transfected with VEGF121 or VEGF165, no increase in metastasis was observed, indicating that VEGF expression is necessary but not sufficient for the production of brain metastases. In another study, VEGFR inhibition with cediranib did not impede tumor cell extravasation in a murine model of brain metastasis from a prostate cancer primary.53 These data suggest heterogeneity between different tumor cells and types.
Activation of integrin αvβ3 on tumor cells strongly promotes metastatic breast tumor growth in the brain by enabling tumor cells to attract blood vessels independent of hypoxia, an effect mediated by post-transcriptional control of VEGF expression.54 Other growth factors including angiopoietin 1 and 2, bFGF, PlGF, SDF1α, PDGF and IL-8 have been implicated in the angiogenesis pathway in primary tumors and gliomas, but their role remains to be established in metastatic brain tumors.43,55
Hostile microenvironment impairs drug delivery
In cancer, the balance between proangiogenic and anti-angiogenic factors tips towards angiogenesis owing to the overexpression of proangiogenic growth factors. This overexpression leads to blood vessels that are structurally and functionally abnormal, exhibiting heterogeneous and often sluggish blood flow and hyperpermeability.50,56–59 A heterogeneous and inefficient blood vessel network creates regions of hypoperfusion or no perfusion where reduced nutrients and oxygen, and impaired waste removal, creates an abnormal metabolic environment, characterized by hypoxia and acidosis. Furthermore, vascular hyperpermeability induces tumor interstitial hypertension, which hinders drug penetration owing to the lack of convective transport.60,61
Clearly, host–tumor interactions including angiogenesis have an important role in the formation and expansion of brain metastases. However, the knowledge of the molecular mechanisms required during the formation of meta-static lesions is still lacking. What is certain is that the molecular and cellular changes within metastatic brain tumor vasculature and the microenvironment should be considered when designing strategies to improve the delivery and efficacy of therapeutic agents.
Modeling and imaging brain metastases
Substantial progress has been made over the past two decades in modeling human cancer in the mouse and, more recently, model systems have emerged that recapitulate many aspects of metastatic disease to the CNS. An important barrier to the development of reliable CNS mouse models is that in most orthotopically transplanted primary tumors that give rise to metastatic foci, the animals succumb to systemic disease before the brain can be reliably studied. Therefore, current models often rely on either direct implantation of tumors into the brain or iterative selection of brain-seeking clones resulting from experimental hematogeneous dissemination to the brain to enrich metastatic disease in the CNS. These experimental metastasis models inevitably miss the initial steps of the metastatic cascade and thus may not reflect the full clinical manifestation of the disease.
Animal models of brain metastasis
Various preclinical animal models for the study of brain metastases and their advantages and limitations are summarized in Table 2. Rodent model systems for brain metastasis have been reported for multiple cancer types, including melanoma,62,63 lung carcinoma,64 and breast carcinoma.65–71 The emergence of new animal models of brain metastasis that more closely mimic human disease provides the tools necessary to understand all of the steps involved in the process. Unfortunately, the field is still limited by the few spontaneous models available and the complexity of more clinically relevant models.
Table 2.
Preclinical rodent animal models of brain metastasis
| Tumor dissemination routes | Methods | Advantages | Disadvantages |
|---|---|---|---|
| Direct implantation model | |||
| Leptomeningeal metastasis75,146,147 | Inoculation of tumor cells or fragments into the subarachnoid space, or subarachnoid catheter for delivery to the cerebrospinal fluid space | Relatively simple procedure; convenient for longitudinal imaging study with molecular or intravital microscopic imaging | Bypasses precolonization steps of metastasis; does not model cerebral brain metastases |
| Intraparenchymal implantation27,148,149 | Inoculation of tumor cells or fragments directly into the brain parenchyma by hand or stereotactic guidance | Simple way to study metastatic tumor growth inside the brain; the procedure has been extended by placement of a cranial window or burr hole (at the site of inoculation) | Bypasses precolonization steps of metastasis |
| Hematogeneous metastasis model | |||
| Intracardiac injection25,78 | Injection of single-cell suspension of tumor into the left ventricle of an animal | Relatively simple procedure and can bypass pulmonary arrest and retention | Dissemination of tumor cells to sites other than cerebral target; decreased reproducibility due to the blind nature of the inoculation compared with direct implantation |
| Intracarotid artery injection72,150,151 | Injection of metastatic cells into the internal carotid artery followed by permanent ligation | Produces predominately cerebral tumors and minimal noncerebral metastasis | Murine carotid is very small and significant microsurgical skill is required |
| Spontaneous metastasis model | |||
| Spontaneous formation63,77,152 | Spontaneous brain metastases subsequent to removal of primary tumor from either parental or metastatic variants | Recapitulates complete metastatic steps; generated from other simpler model systems; useful for confirming hypotheses | Longer duration requiring resection of primary tumors or multiple preselection processes; most complicated method to run a controlled experiment |
Melanoma
Melanoma mouse models have been established using a variety of murine and human melanoma cell lines injected via an intracardiac, intracarotid or, less successfully, intravenous route. Most models show a combination of parenchymal and leptomeningeal deposits at the time of sacrifice, with occasional dural deposits.72
Breast cancer
Yoneda et al.70 established a brain-seeking clone (MDA-MB-231BR) of the human breast cancer cell line MDA-MB-231 by serial in vivo and in vitro passages. In a mouse xenograft, when the MDA-MB-231BR cell line was transfected with HER2, the HER2-overexpressing clones demonstrated a threefold increase in the number of large brain metastases compared with untransfected MDA-MB-231BR cells; however, the number of micro-metastases was unaffected.73 This model has also been used to probe the effect of anti-HER2 therapies on the development and propagation of brain metastases.74 Alternatively, human breast cancer cells implanted near the surface of the brain provide a model to directly observe tumor and vessel growth using a cranial window and intravital microscopy (Figure 2a–c).75,76
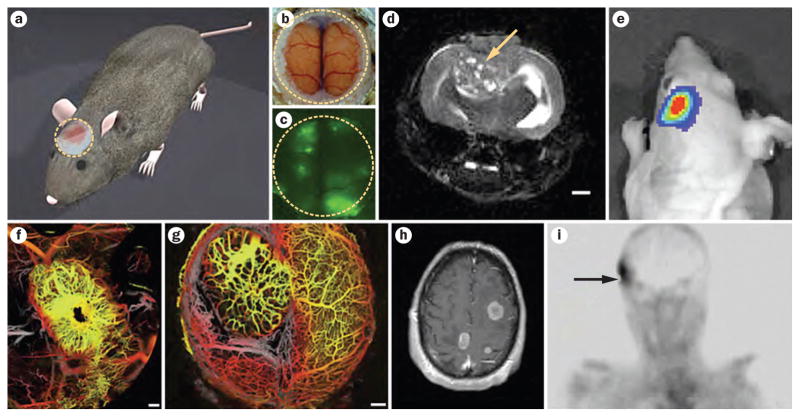
Figure 2.
Imaging brain metastases in preclinical models and patients. a | Chronic cranial window (yellow dotted line) for intravital brain imaging (courtesy of Lance Munn). Intravital imaging through cranial window showing b | normal brain (bright field image) and c | metastatic brain tumors (green fluorescent protein-expressing breast cancer cells). d | T2-weighted MRI image of a metastatic brain tumor (arrow) after direct implantation of breast cancer cells (courtesy of Christian Farrar). Scale bar: 1 mm. e | Whole-body bioluminescence imaging of brain metastasis using Gaussia luciferase (Chung, E. et al. unpublished data). Optical frequency domain imaging of murine mammary carcinoma grown in f | breast (primary) and g | brain (metastasis). Scale bars: 0.5 mm. h | T1-weighted post-contrast MRI showing multiple enhancing mass lesions consistent with brain metastases (courtesy of A. F. Eichler). i | 99mTc-folate image of human brain metastasis (arrow) from a primary breast cancer (Courtesy of Phillip S. Low and Endocyte Inc.). Permission for parts f and g obtained from Nature Publishing Group © Vakoc, B. J. et al. Nat. Med. 15, 1219–1223 (2009).
Spontaneous metastasis
Few reports describe cell lines capable of reproducibly spontaneously metastasizing to the CNS from a primary tumor. These include a variant of the murine B16 melanoma cell line (named G3.5), generated through successive rounds of selection of brain metastases following intravenous injection, which leads to spontaneous metastasis to the CNS from subcutaneous primary tumors in 80% of mice.77 Using a similar method, a brain metastatic variant of the triple-negative breast cancer cell line CN34 (CN34-BrM2) capable of metastasizing to the CNS from a primary mammary tumor in about 42% of mice was generated.78 A procedure that might more accurately replicate the selection mechanisms of clinical brain metastasis formation was established from long-term, low-dose metronomic chemotherapy treatment of mice injected subdermally with a highly metastatic variant of the WM239A human melanoma cell line.63 Among long-term surviving mice, 20% had spontaneous brain metastases, and cell lines generated from these metastases spontaneously metastasized to the brain parenchyma after orthotopic transplantation and removal of the primary tumor.63
Preclinical imaging techniques
Visualization and quantification of metastatic disease burden in the brain is an indispensible component of any metastasis model system. Several novel methods of in vivo imaging have been developed that allow for serial measurements over time before animal sacrifice, including window models (Figure 2a–c,f,g). For instance, the MDA-MB-231 human breast cancer cell line has been engineered to express a naturally secreted Gaussia luciferase (Gluc) and in an orthotopic primary and brain metastatic mouse model, quantitative serum and urine Gluc levels correlated well with overall tumor burden.76 Importantly, Gluc activity in the blood revealed early growth of metastatic brain tumors before they could be detected by bioluminescence imaging (Figure 2e).
An alternative adaptation of quantitative fluorescent and phosphorescent imaging techniques has recently been developed that interrogates blood–tumor barrier (BTB) permeability within and surrounding metastatic brain tumors in mice, using tumor cells expressing enhanced green fluorescent protein (eGFP) and Texas red dextran as a marker of passive permeability.79 This model also allows simultaneous quantification of BTB permeability using radiolabeled chemotherapeutic drugs. Combining these techniques with the MDA-MB-231BR HER2-overexpressing mouse model previously described,70 Lockman et al.79 analyzed over 2,000 brain metastases and demonstrated that BTB permeability was compromised in over 89% of lesions.79 However, 14C-paclitaxel and 14C-doxorubicin only reached cytotoxic concentrations in about 10% of metastases.79
Other powerful imaging technologies include MRI, PET and near-infrared optical imaging.80,81 In the past, the majority of MRI studies of brain tumors in mice were performed using high-field-strength MRI (≥7 Tesla) because of its high spatial resolution and signal-to-noise ratio (Figure 2d).82,83 However, several techniques enhance the sensitivity of clinical-strength MRI scanners (1.5–3 Tesla) for the detection of small brain metastases in rodents. When MDA-BR-231-eGFP cells are labeled with fluorescent μm-sized superparamagnetic iron oxide (MPIO) particles, single labeled breast cancer cells can be detected in the rat brain parenchyma as small dark or hypointense voxels on T2★-weighted MRI that then develop into metastases after 4 weeks.84 The same cells labeled with ferumoxides-protamine sulfate (FEPro) could be detected and quantified within 2 weeks of intracardiac injection using T2★-weighted 3-Tesla MRI with better sensitivity than bioluminescence imaging, providing a powerful tool for both spatial and temporal monitoring of metastatic tumor growth and progression.85,86
Novel therapeutic strategies
The role of chemotherapy in the treatment of brain metastasis has been limited owing to multiple factors: poor blood–brain barrier (BBB) penetrability of many systemically active chemotherapeutic drugs; the tendency for many patients to have had multiple rounds of chemotherapy prior the development of CNS metastatic disease; and the historical exclusion of patients with brain metastases from clinical trials testing new agents. New therapies and strategies are increasingly being explored that aim to enhance drug delivery to the brain, complement the effects of radiation therapy, substitute the role of radiation therapy, or act as preventative agents against the development of new metastases. Furthermore, with the development of new models and imaging techniques (Figure 2h,i), preclinical treatment strategies can become more focused on established brain metastases, necessary for translational potential.
Crossing the blood–brain barrier
The BBB is a selective barrier between the systemic circulation and cerebrospinal fluid that is formed by specialized endothelial cells lining the cerebral microvasculature, together with pericytes and astrocytic perivascular endfeet (Figure 3).87 Tight junctions between adjacent cells force most molecules to pass through, rather than around, endothelial cells, creating a physical barrier. Transport systems on the luminal and abluminal membranes regulate the passage of small hydrophilic molecules. Large hydrophilic molecules, including many chemotherapeutic and molecular-targeted drugs, are excluded from the CNS unless they can be actively transported by receptor-mediated transcytosis. In addition, the BBB expresses high levels of drug efflux pumps such as the P-glycoprotein (PgP)/multi-drug resistance proteins,88 which actively remove some chemotherapeutic drugs from the brain.
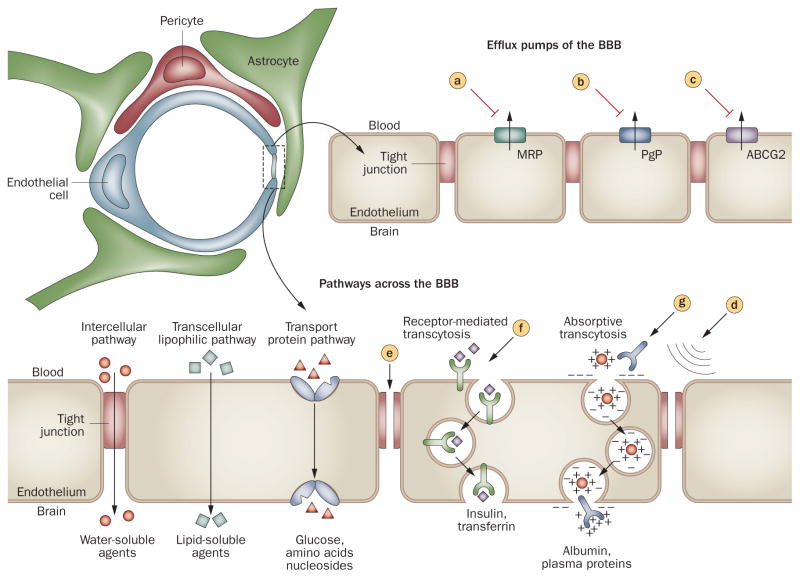
Figure 3.
Approaches to enhance drug delivery to the brain. Schematic diagram of the BBB with an enhanced illustration of the brain capillary endothelial cell. The main drug efflux transporters of brain capillary endothelial cells include MRPs, PgP, and ABCG2. All of these transport proteins have been targeted for pharmacological inhibition. a | probenecid, sulfinpyrazone and MK-571; b | verapamil, cyclosporin A, quinidine, valspodar, elacridar, biricodar, zosuquidar, and tariquidar; and c | GF120918, elacridar, and fumitremorgin C. Tight junctions normally restrict penetration of water-soluble compounds across the BBB, but they can be disrupted by mechanical and pharmacological methods, via d | ultrasound and e | bradykinin analogs, respectively. f | Receptor-mediated transcytosis of transferrin or insulin has been used to increase transport of drugs across the BBB, and g | cationization (that is, antibodies) can increase uptake of molecules by absorptive transcytosis. Abbreviations: ABCG2, breast cancer resistant protein; BBB, blood–brain barrier; MRPs, multidrug resistant proteins; PgP, P-glycoprotein.
When metastatic tumors grow beyond 1–2 mm in diameter within the brain parenchyma, the BBB becomes structurally and functionally compromised.56,58,75,89–92 In an experimental brain metastasis model of eight human tumor lines, lesions smaller than 0.2 mm2 contained an intact BBB; however, larger tumor-cell clusters resulted in leakage of sodium fluorescein indicating that the function of the BBB is directly related to the size of the lesion, and a growing tumor mass may disrupt the interaction of astrocytes and endothelial cells.93 In addition to changes in blood vessel permeability, there was a significant reduction in PgP expression to 5% and 40% of normal levels in brain metastases from melanoma and lung carcinoma, respectively.94 The disruption of the BBB might not be homogeneous, and the BBB might remain intact at least in parts of tumors. Thus, barriers to drug delivery to such lesions might still need to be overcome.
It is widely thought that macroscopic metastatic brain tumors have a disrupted BBB, as evidenced by homogeneous contrast enhancement on MRI. Brain metastases, distinct from infiltrative gliomas, have sharp borders and can usually be completely resected if their location allows. However, not all single metastases are amenable to surgical resection, and even those tumors that are assumed to be totally resected at the time of surgery have a recurrence rate of up to 50% when postoperative radiation therapy is not employed.95 In addition, there are likely micrometastatic deposits in many patients at the time of diagnosis that are not well visualized by MRI, have an intact BBB, and contribute to CNS recurrence after initial local therapy, and some macroscopic tumors have a relatively intact BBB.79 Therefore, strategies to circumvent the challenges of an intact BBB are likely to be needed in some patients for improved intracranial disease control (Table 3).
Table 3.
Strategies to circumvent the blood–brain barrier
| Strategy | Methods | Potential applications |
|---|---|---|
| Physical | ||
| Convection-enhanced delivery153 | Catheters placed around the resection cavity at the time of surgery, left in place for infusion of drug for 1–2 days | Large brain metastases that cannot be totally resected; to improve local control rates after gross total resection |
| Osmotic BBB disruption154 | Intra-arterial infusion of hyperosmotic agent before infusion of chemotherapeutic, antibody, or nanoparticle drug | Multiple brain metastases from a chemosensitive primary tumor |
| Targeted ultrasound BBB disruption153 | Intravenous injection of preformed gas bubbles before pulsed ultrasound treatment | Single or limited number of brain metastases from a chemosensitive primary; single refractory or recurrent brain metastasis |
| Pharmacological | ||
| Bradykinin analogs155–157 | Intravenous delivery to transiently increase the permeability of the BBB | In combination with chemotherapy (for example, RMP-7 increased the delivery of carboplatinum into intracranial brain tumors) |
| Exploiting RMT:158 TfR, IR, IGF-1R, LRP-1 | To achieve RMT, chemotherapy of choice linked to an antibody that targets the TfR, IR, IGF-1R, or LRP-1 | Broad applicability for single or multiple brain metastases |
| PgP inhibitors159–161 | Inhibiting the drug efflux pump (for example, HM30181A, cyclosporine A, valspodar, elacridir, zosuquidar) | Administration concurrently with chemotherapy for broad applications |
Abbreviations: BBB, blood–brain barrier; IGF-1R, insulin-like growth factor-1 receptor; IR, insulin receptor; LRP-1, low density lipoprotein receptor-related protein 1; PgP, P-glycoprotein; RMT, receptor-mediated transcytosis; TfR, transferrin receptor.
An alternative approach to physical and local disruption techniques is to design drugs that can be shuttled across the BBB using receptors that are naturally expressed on the endothelial cells of the BBB (Figure 3). Preclinical studies have demonstrated feasibility using antibodies against transferrin and insulin receptors, as well as peptides targeting the receptor lipoprotein receptor-related protein (LRP-1).96–98 LRP-1 is highly expressed at the BBB, is involved in the transport of proteins and peptides such as β-amyloid, tissue plasminogen activator, melanotransferrin and receptor-associated peptide, and is upregulated in brain tumors.96,97,99–101 GRN1005 (Angiochem, Montreal, QC, Canada), is paclitaxel linked to angiopep2 (a ligand for LRP-1) and is in phase I clinical trials in patients with metastatic brain tumors or malignant gliomas.102,103 GRN1005 has antitumor activity in subcutaneously implanted glioblastoma and lung tumors and extends the survival of mice with intracerebral tumors.104 In addition, in the MDA-MB-231BR mouse model, there was a fourfold to 54-fold increase in in vivo uptake of GRN1005 into brain and brain metastases compared with paclitaxel.105 Another mechanism to increase the concentration of anticancer agent in the CNS is to inhibit their efflux by targeting the efflux transporters that comprise the BBB (Figure 3a–c).
Sensitizing tumor cells to radiation
Although WBRT leads to stabilization or shrinkage of tumors in at least half of patients, many patients have tumor recurrence either at sites of original disease or in new sites, some of which may have been present at the time of initial treatment but below the threshold of detection. There is, therefore, continued interest in developing drugs that act to sensitize tumor cells to radiation therapy, with the goal of improving local and distant control rates over radiation alone while sparing toxicity to normal tissue. Multiple agents with preclinical radiosensitizing properties have failed to show benefit in randomized controlled trials, including lonidamine, metronidazole, misonidazole, motexafin gadolinium, bromodeoxyuridine, and efiproxiral.6,106–111 Several chemotherapeutic agents with preclinical evidence of both radiosensitizing properties and BBB penetration have been tested in trials in combination with WBRT, including temozolomide and topotecan, but none has emerged as clearly superior to WBRT alone.16,18,112 The histone deacetylase inhibitor vorinostat has shown promise as a radiosensitizer in multiple cells lines as well as in brain using the MDA-MB-231BR mouse model, where an improvement in both tumor growth delay and overall survival was seen compared with radiation alone.113 In a separate study using the MDA-MB-231BR mouse model, vorinostat as a single agent reduced the size and number of brain metastases formed compared with vehicle-treated controls, raising the possibility that this agent might be useful as a prophylactic agent in patients who are at high-risk of tumor relapse in the brain.114 The mechanism of the prevention of metastasis by vorinostat seems to be the induction of DNA double-strand breaks associated with the downregulation of the DNA repair gene RAD52, indicating that vorinostat sensitizes the tumor to DNA-targeting therapies such as radiation.114 Vorinostat in combination with WBRT is being tested in a phase I clinical trial in patients with brain metastases from multiple solid tumor types and in a phase I trial in combination with SRS in patients with brain metastases from NSCLC.115,116
Targeting angiogenesis
There are conflicting preclinical data as to whether inhibition of angiogenesis results in tumor growth delay and a reduction in metastatic potential, or promotes altered growth patterns via vessel co-option and increased metastatic potential.48,49,52 There are limited clinical data to support the phenomenon of vessel co-option and infiltrative growth of metastatic brain tumors under the influence of antiangiogenic therapy,46 but angiogenesis inhibitors have not been systematically studied in brain metastases mainly owing to concerns about the potential for intracranial hemorrhage. Such concerns have been substantially allayed with the publication of reviews of large clinical trial datasets and two prospective clinical trials. These studies show that the risk of CNS hemorrhage in patients with solid tumors that have not spread to the CNS, as well as in patients with stable brain metastases at the time of initiation of anti-angiogenic therapy, is low (0.8–3.3%) and not above rates that would be expected independent of antiangiogenic agent exposure.117–120 Therefore, clinical trials have been launched to determine the safety and efficacy of various antiangiogenic agents in combination with either radiation therapy or single-agent chemotherapy for the treatment of new or progressive brain metastases from solid tumors.121
Other targeted therapies
NSCLC
Brain metastases from NSCLC have been shown to respond to the EGFR inhibitors gefitinib and erlotinib. In patients with unselected NSCLC brain metastasis, there was a response rate of 10–38% to gefitinib (complete and partial), with a median duration of response of 9–13.5 months.122,123 Similar findings have been documented with erlotinib.124–126 As for extracranial disease, response is highly dependent on the presence of an activating EGFR mutation.122,123 Patients who were treatment naive were particularly responsive: in 23 Asian never-smokers with brain metastases from a NSCLC primary treated with first-line erlotinib or gefitinib, a 70% CNS response rate was observed,127 and all seven patients with brain metastases enrolled on a phase II study of erlotinib for chemotherapy-naive advanced-stage NSCLC achieved an objective CNS response.128 Retrospective data indicate that patients with brain metastases from EGFR-mutant NSCLC might have improved overall survival compared with EGFR wild-type cancers, particularly when they receive treatment with an EGFR inhibitor.10 As new genetic subpopulations of NSCLC are identified that can be targeted with small-molecule inhibitors, such as cancers harboring an ALK rearrangement that are responsive to crizotinib,129 it will be important to assess whether there is a differential response to targeted therapies in the CNS compared with extracranial sites of disease. The CNS responses seen with the first generation of small-molecule EGFR inhibitors in properly selected patients suggest that the use of drugs that are highly effective is at least as important as drug delivery for treating patients with brain metastases.
HER2-amplified breast cancer
Patients with HER2-positive breast tumors are at high risk of developing brain metastases, with a frequency as high as 35% in patients with advanced-stage disease.130,131 A combination of factors likely explains the increased incidence of CNS disease in these patients, including the ability of HER2 to increase brain colonization73 via its downstream molecules including heparanase—a target of microRNA-1258132—and improved control of systemic disease with the anti-HER2 monoclonal antibody, trastuzumab, which has poor BBB penetration.130 Lapatinib, a small-molecule tyrosine kinase inhibitor of EGFR and HER2, has limited activity as a single agent for patients with refractory brain metastases,133 but slightly higher activity in combination with capecitabine.134 In a phase III randomized study of capecitabine plus lapatinib versus capecitabine alone for advanced-stage, trastuzumab-refractory breast cancer, fewer patients in the combination arm had symptomatic CNS progression as part of the first progression event compared with those not receiving lapatinib.135 Furthermore, in a preclinical study using an experimental model of HER2-positive breast cancer brain metastases, lapatinib inhibited the formation of large brain metastases by 54%, suggesting preventative activity and supporting the clinical findings.74 It should be noted that trastuzumab not only directly affects HER2-expressing tumor cells but also acts as an antiangiogenic cocktail by downregulating multiple proangiogenic factors and inducing endogenous antiangiogenic factors, and normalizes blood vessels of breast cancer grown in the leptomeningeal space.75 Unfortunately, the decrease in VEGF expression in cancer cells is compensated by an increase in VEGF expression in host cells. These findings suggest that an anti-VEGF agent (for example bevacizumab) might be beneficial when combined with trastuzumab or lapatinib.75 Ongoing clinical trials of combined anti-VEGF and anti-HER2 therapies in patients with metastatic breast cancer will likely shed on whether this hypothesis is supported by the data.136
Melanoma
Melanoma accounts for 5–20% of all brain metastases, and 40–60% of melanomas carry an activating mutation in the gene encoding BRAF, a serine–threonine protein kinase.137 In a phase I study, administration of an inhibitor of mutated BRAF, PLX4032 (Plexxikon, Berkeley, CA, USA), to patients with metastatic melanoma harboring this mutation resulted in complete or partial tumor regression in the majority of patients. However, patients with active brain metastases were excluded from this study.137 In a separate study using the BRAF inhibitor GSK2118436 (GlaxoSmithKline, Brentford, UK), all seven evaluable patients with previously untreated brain metastases showed CNS tumor shrinkage, including three complete responses, and parallel extracranial responses were noted in most patients.138
Conclusions
Many genes whose products are necessary for the steps of brain metastases formation—in particular extravasation and colonization—have been identified. New developments of more clinically relevant models and advanced imaging techniques over the past couple of years have begun to facilitate understanding of the intricacies of brain metastases. It is increasingly evident that the brain microenvironment has a key role in the metastatic growth process, as well as in resistance to antitumor therapies. Delivery of drugs across the BBB is a challenge, albeit more so in smaller lesions. Although the BTB is not as tight as the BBB, concentrations of drugs in brain lesions are usually lower compared with extracranial sites. The grand challenge now is to integrate this knowledge and develop novel strategies to target the unique microenvironment of brain metastasis.
Key points.
Longer survival of patients and more-sensitive detection of metastatic disease by improved imaging modalities might contribute to the increased incidence of detected brain metastases
Novel preclinical models that more accurately represent clinical brain metastases and imaging techniques that allow study of the formation of brain metastases and their response to treatments are emerging
Brain metastases grow by co-opting existing blood vessels and/or by forming new blood vessels; the brain microenvironment promotes tumor cell survival, tumor growth and resistance to therapy
Although a lesser problem in large metastases, the blood–brain barrier (BBB) could prevent therapeutic access to micrometastases; strategies to enhance drug delivery across the BBB are under investigation
Differential expression of genes involved in intravasation and extravasation, metabolism, cell adhesion, and cellular signaling in brain-specific metastatic clones have been identified
Targeted therapies, including inhibitors of EGFR, HER2, PI3K and BRAF, have shown promise in the treatment of brain metastases, but require testing in randomized trials
Review criteria.
Information for this Review was compiled by searching the PubMed database for articles published before 1 March 2011. Search terms included “brain metastasis animal model”, “brain metastasis blood–brain barrier”, “brain metastasis genes”, “brain metastasis microenvironment”, “brain metastasis therapy”, “brain tumor angiogenesis”, “central nervous system metastasis animal model”, ”intravital microscopy”, and ”small animal imaging”. Full articles were checked for additional material when appropriate, and articles that cite key publications were also checked.
Acknowledgments
We thank J. Engelman, S. Goel, L. Xu, I. J. Fidler, R. S. Kerbel, W. Cruz-Munoz and S. Mohla for their helpful comments on the manuscript. This work was supported by the National Institutes of Health grants P01-CA080124 (R. K. Jain and D. Fukumura), R01-CA085140, R01-CA115767 and R01-CA126642 (R. K. Jain), R01-CA096915 (D. Fukumura), R21-CA135605 and U01-CA062490 (A. F. Eichler), a Federal Share Income Grant (R. K. Jain and D. Fukumura), T32-CA073479 (D. P. Kodak) and Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (R. K. Jain). E. Chung was supported by a Tosteson postdoctoral fellowship award from the Massachusetts Biomedical Research Corporation. E. Chung also acknowledges support from his current institute: Gwangju Institute of Science and Technology, Gwangju, Republic of Korea.
Footnotes
Competing interests
R. K. Jain declares associations with the following companies: Astellas, AstraZeneca, Dyax, Enlight Biosciences, Genzyme, MedImmune, Millenium, MPM Capital, Noxxon, Roche, SynDevRx. The other authors declare no competing interests.
Author contributions
A. F. Eichler and R. K. Jain contributed equally to the preparation of this manuscript. All authors contributed to researching data for the article, discussions of content, writing of the manuscript, and to reviewing and editing of the article before submission.
References
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 4.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60:277–283. doi: 10.1227/01.NEU.0000249272.64439.B1. [DOI] [PubMed] [Google Scholar]
- 6.Mehta MP, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar L, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 8.Melisko ME, Moore DH, Sneed PK, De Franco J, Rugo HS. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88:359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 9.Eichler AF, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 10.Eichler AF, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patchell RA, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 12.Vecht CJ, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 13.Andrews DW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama H, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 15.Kocher M, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonadou D, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20:3644–3650. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 17.Robinet G, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95–1. Ann Oncol. 2001;12:59–67. doi: 10.1023/a:1008338312647. [DOI] [PubMed] [Google Scholar]
- 18.Verger E, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 19.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess KR, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 21.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–10. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 22.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 23.Ewing J. Neoplastic Diseases. A Treatise on Tumors. W. B. Saunders Co; Philadelphia and London: 1928. [Google Scholar]
- 24.Duda DG, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 26.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald DP, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J Neurol Sci. 1995;134:26–32. doi: 10.1016/0022-510x(95)00227-9. [DOI] [PubMed] [Google Scholar]
- 30.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pukrop T, et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58:1477–1489. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- 32.He BP, et al. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006;12:161–170. doi: 10.2119/2006-00033.He. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–112. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Langley RR, et al. Generation of an immortalized astrocyte cell line from H-2Kb-tsA58 mice to study the role of astrocytes in brain metastasis. Int J Oncol. 2009;35:665–672. doi: 10.3892/ijo_00000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Q, et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2010;12:748–754. doi: 10.1593/neo.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SJ, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13:286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seike T, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2010;28:13–25. doi: 10.1007/s10585-010-9354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denkins Y, et al. Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol. 2004;6:154–165. doi: 10.1215/S115285170300067X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menter DG, Herrmann JL, Nicolson GL. The role of trophic factors and autocrine/paracrine growth factors in brain metastasis. Clin Exp Metastasis. 1995;13:67–88. doi: 10.1007/BF00133612. [DOI] [PubMed] [Google Scholar]
- 40.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 42.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. doi: 10.1038/nature10144. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain RK, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 44.Ricci-Vitiani L, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 46.Di Tomaso E, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soda Y, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusters B, et al. Vascular endothelial growth induces progression of melanoma factor-A165 brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002;62:341–345. [PubMed] [Google Scholar]
- 49.Leenders WP, et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res. 2004;10:6222–6230. doi: 10.1158/1078-0432.CCR-04-0823. [DOI] [PubMed] [Google Scholar]
- 50.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 51.Yano S, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60:4959–4967. [PubMed] [Google Scholar]
- 52.Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis. 2004;21:107–118. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- 53.JuanYin J, et al. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin Exp Metastasis. 2009;26:403–414. doi: 10.1007/s10585-009-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin controls angiogenesis and αvβ3 metastatic growth in the brain. Proc Natl Acad Sci USA. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiratsuka S, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci USA. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bullitt E, et al. Vessel tortuosity and brain tumor malignancy: a blinded study. Acad Radiol. 2005;12:1232–1240. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feigin I, Allen LB, Lipkin L, Gross SW. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer. 1958;11:264–277. doi: 10.1002/1097-0142(195803/04)11:2<264::aid-cncr2820110207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 58.Yuan F, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 59.Hiratsuka S, et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin upregulation. Proc Natl Acad Sci USA. 2011;108:3725–3730. doi: 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 61.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cranmer LD, Trevor KT, Bandlamuri S, Hersh EM. Rodent models of brain metastasis in melanoma. Melanoma Res. 2005;15:325–356. doi: 10.1097/00008390-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68:4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 64.Mathieu A, et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-α, GST-μ, and GST-π. Cancer. 2004;101:1908–1918. doi: 10.1002/cncr.20571. [DOI] [PubMed] [Google Scholar]
- 65.Chen EI, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–1486. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 66.Monsky WL, et al. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 67.Price JE. Metastasis from human breast cancer cell lines. Breast Cancer Res Treat. 1996;39:93–102. doi: 10.1007/BF01806081. [DOI] [PubMed] [Google Scholar]
- 68.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 69.Rye PD, et al. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int J Cancer. 1996;68:682–687. doi: 10.1002/(SICI)1097-0215(19961127)68:5<682::AID-IJC20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 70.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 71.Zhang RD, Fidler IJ, Price JE. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis. 1991;11:204–215. [PubMed] [Google Scholar]
- 72.Fidler IJ, Schackert G, Zhang RD, Radinsky R, Fujimaki T. The biology of melanoma brain metastasis. Cancer Metastasis Rev. 1999;18:387–400. doi: 10.1023/a:1006329410433. [DOI] [PubMed] [Google Scholar]
- 73.Palmieri D, et al. HER-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 74.Gril B, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 76.Chung E, et al. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS ONE. 2009;4:e8316. doi: 10.1371/journal.pone.0008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alterman AL, Stackpole CW. B16 melanoma spontaneous brain metastasis: occurrence and development within leptomeninges blood vessels. Clin Exp Metastasis. 1989;7:15–23. doi: 10.1007/BF02057178. [DOI] [PubMed] [Google Scholar]
- 78.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lockman PR, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer. 2002;2:11–18. doi: 10.1038/nrc701. [DOI] [PubMed] [Google Scholar]
- 81.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anderson SA, Frank JA. MRI of mouse models of neurological disorders. NMR Biomed. 2007;20:200–215. doi: 10.1002/nbm.1167. [DOI] [PubMed] [Google Scholar]
- 83.Frank JA, et al. Methods for magnetically labeling stem and other cells for detection by in vivo magnetic resonance imaging. Cytotherapy. 2004;6:621–625. doi: 10.1080/14653240410005267-1. [DOI] [PubMed] [Google Scholar]
- 84.Heyn C, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 85.Song HT, et al. Quantitative T2* imaging of metastatic human breast cancer to brain in the nude rat at 3 T. NMR Biomed. 2011;24:325–334. doi: 10.1002/nbm.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song HT, et al. Rat model of metastatic breast cancer monitored by MRI at 3 tesla and bioluminescence imaging with histological correlation. J Transl Med. 2009;7:88. doi: 10.1186/1479-5876-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 88.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 89.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 90.Fukumura D, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 91.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monsky WL, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 93.Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141:1115–1124. [PMC free article] [PubMed] [Google Scholar]
- 94.Regina A, et al. Multidrug resistance in brain tumors: roles of the blood-brain barrier. Cancer Metastasis Rev. 2001;20:13–25. doi: 10.1023/a:1013104423154. [DOI] [PubMed] [Google Scholar]
- 95.Patchell RA, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 96.de Boer AG, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet. 2007;46:553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 97.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19:1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 99.Bu G, Maksymovitch EA, Geuze H, Schwartz AL. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J Biol Chem. 1994;269:29874–29882. [PubMed] [Google Scholar]
- 100.Demeule M, et al. High transcytosis of melanotransferrin (P97) across the blood-brain barrier. J Neurochem. 2002;83:924–933. doi: 10.1046/j.1471-4159.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- 101.Pan W, et al. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J Cell Sci. 2004;117:5071–5078. doi: 10.1242/jcs.01381. [DOI] [PubMed] [Google Scholar]
- 102.US National Library of Medicine. Clinicaltrials.gov. 2010 [online], http://clinicaltrials.gov/ct2/show/NCT00539383.
- 103.US National Library of Medicine. Clinicaltrials.gov. 2010 [online], http://clinicaltrials.gov/ct2/show/NCT00539344.
- 104.Regina A, et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas FC, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeAngelis LM, et al. The combined use of radiation therapy and lonidamine in the treatment of brain metastases. J Neurooncol. 1989;7:241–247. doi: 10.1007/BF00172917. [DOI] [PubMed] [Google Scholar]
- 107.Eyre HJ, et al. Randomized trial of radiotherapy versus radiotherapy plus metronidazole for the treatment metastatic cancer to brain. A Southwest Oncology Group study. J Neurooncol. 1984;2:325–330. doi: 10.1007/BF00178115. [DOI] [PubMed] [Google Scholar]
- 108.Komarnicky LT, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 109.Mehta MP, et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73:1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 110.Phillips TL, Scott CB, Leibel SA, Rotman M, Weigensberg IJ. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 111.Suh JH, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 112.Neuhaus T, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer. 2009;100:291–297. doi: 10.1038/sj.bjc.6604835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baschnagel A, et al. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther. 2009;8:1589–1595. doi: 10.1158/1535-7163.MCT-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palmieri D, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.US National Library of Medicine. Clinicaltrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT00838929.
- 116.US National Library of Medicine. Clinicaltrials.gov. 2010 [online], http://clinicaltrials.gov/ct2/show/NCT00946673.
- 117.Besse B, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16:269–278. doi: 10.1158/1078-0432.CCR-09-2439. [DOI] [PubMed] [Google Scholar]
- 118.Socinski MA, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27:5255–5261. doi: 10.1200/JCO.2009.22.0616. [DOI] [PubMed] [Google Scholar]
- 119.Polikoff J, et al. Safety of bevacizumab (Bv) therapy in combination with chemotherapy in subjects with non-small cell lung cancer (NSCLC) treated on ATLAS [abstract] J Clin Oncol. 2008;26 (15 Suppl):a8079. [Google Scholar]
- 120.De Braganca, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 2010;100:443–447. doi: 10.1007/s11060-010-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eichler AF, et al. A phase I study of cediranib plus whole-brain radiation therapy in patients with brain metastases from non-small cell lung cancer [abstract] J Clin Oncol. 2010;28(15 Suppl):TPS177. [Google Scholar]
- 122.Ceresoli GL, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 123.Wu C, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer. 2007;57:359–364. doi: 10.1016/j.lungcan.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Fekrazad MH, Ravindranathan M, Jones DV., Jr Response of intracranial metastases to erlotinib therapy. J Clin Oncol. 2007;25:5024–5026. doi: 10.1200/JCO.2007.13.3751. [DOI] [PubMed] [Google Scholar]
- 125.Lai CS, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61:91. doi: 10.1136/thx.2005.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Popat S, et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer. 2007;56:135–137. doi: 10.1016/j.lungcan.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 127.Kim JE, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351–354. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 128.Paz-Ares L, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24:1428–1434. doi: 10.1200/JCO.2005.04.3299. [DOI] [PubMed] [Google Scholar]
- 129.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 131.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 132.Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71:645–654. doi: 10.1158/0008-5472.CAN-10-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin NU, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin NU, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 135.Cameron D, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 136.US National Library of Medicine. Clinicaltrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT01004172.
- 137.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Long GV, et al. Phase 1/2 study of GSK2118436, a selective inhibitor of V600 mutant (MUT) BRAF kinase; evidence of activity in melanoma brain metastases (METS) [abstract] Ann Oncol. 2010;21:viii12. [Google Scholar]
- 139.Felding-Habermann B, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palmieri D, et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7:1438–1445. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ray PS, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–3876. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 142.Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grinberg-Rashi H, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15:1755–1761. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 144.Xie TX, et al. Activation of Stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 145.Nam DH, et al. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 2008;14:4059–4066. doi: 10.1158/1078-0432.CCR-07-4039. [DOI] [PubMed] [Google Scholar]
- 146.Siegal T, et al. Alteration of blood-brain-CSF barrier in experimental meningeal carcinomatosis. A morphologic and adriamycin-penetration study. J Neurooncol. 1987;4:233–242. doi: 10.1007/BF00150615. [DOI] [PubMed] [Google Scholar]
- 147.Schabet M, Herrlinger U. Animal models of leptomeningeal metastasis. J Neurooncol. 1998;38:199–205. doi: 10.1023/a:1005936304256. [DOI] [PubMed] [Google Scholar]
- 148.Schackert G, Price JE, Bucana CD, Fidler IJ. Unique patterns of brain metastasis produced by different human carcinomas in athymic nude mice. Int J Cancer. 1989;44:892–897. doi: 10.1002/ijc.2910440524. [DOI] [PubMed] [Google Scholar]
- 149.Broder H, Anderson A, Kremen TJ, Odesa SK, Liau LM. MART-1 adenovirus-transduced dendritic cell immunization in a murine model of metastatic central nervous system tumor. J Neurooncol. 2003;64:21–30. doi: 10.1007/BF02700017. [DOI] [PubMed] [Google Scholar]
- 150.Schackert G, Fidler IJ. Development of in vivo models for studies of brain metastasis. Int J Cancer. 1988;41:589–594. doi: 10.1002/ijc.2910410419. [DOI] [PubMed] [Google Scholar]
- 151.Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988;48:3478–3484. [PubMed] [Google Scholar]
- 152.Yang M, et al. Genetically fluorescent melanoma bone and organ metastasis models. Clin Cancer Res. 1999;5:3549–3559. [PubMed] [Google Scholar]
- 153.Muldoon LL, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 154.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gregor A, et al. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J Neurooncol. 1999;44:137–145. doi: 10.1023/a:1006379332212. [DOI] [PubMed] [Google Scholar]
- 156.Matsukado K, et al. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 1996;39:125–133. doi: 10.1097/00006123-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 157.Prados MD, et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003;5:96–103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joo KM, et al. Oral paclitaxel chemotherapy for brain tumors: ideal combination treatment of paclitaxel and p-glycoprotein inhibitor. Oncol Rep. 2008;19:17–23. [PubMed] [Google Scholar]
- 160.Joo KM, et al. Response of brain specific microenvironment to p-glycoprotein inhibitor: an important factor determining therapeutic effect of p-glycoprotein inhibitor on brain metastatic tumors. Int J Oncol. 2008;33:705–712. [PubMed] [Google Scholar]
- 161.Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med. 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]