Abstract
The unique endoplasmic reticulum (ER) subdomain termed the mitochondria-associated ER membrane (MAM) engages the physical connection between the ER and the mitochondrial outer membrane and plays a role in regulating IP3 receptor-mediated Ca2+ influx and the phospholipid transport between the two organelles. The MAM contains certain signaling and membrane-tethering proteins but also lipids including cholesterol. The biophysical role of lipids at the MAM, specifically in the physical interaction between the MAM of the ER and mitochondria, remains not totally clarified. Here we employed the in vitro membrane association assay to investigate the role of cholesterol in the association between MAMs and mitochondria. The purified MAMs and mitochondria were mixed in vitro in a test tube and then the physical association of the two subcellular organelles was quantified indirectly by measuring the presence of the MAM-specific protein sigma-1 receptors in the mitochondria fraction. Purified MAMs contained free cholesterol approximately 7 times higher than that in microsomes. We found that depletion of cholesterol in MAMs with methyl-β-cyclodextrin (MβC) significantly increased the association between MAMs and mitochondria, whereas MβC saturated with cholesterol did not change the association. 14C-Serine pulse-labeling demonstrated that the treatment of living cells with MβC decreases the level of de novo synthesized 14C-phosphatidylsrine (PtSer) and concomitantly increases greatly the synthesis of 14C-phosphatidylethanolamine (PtEt). Apparently, cholesterol depletion increased the PtSer transport from MAMs to mitochondria. Our findings suggest that cholesterol is an important substrate in regulating the association between MAMs of the ER and mitochondria.
Keywords: MAM, mitochondria-associated membrane, phospholipid transport, cholesterol, sigma-1 receptor, lipid raft
Introduction
The coordination of complex intracellular transports allows organelles to develop physical membrane-to-membrane interactions between organelles. As shown by a number of studies, the endoplasmic reticulum (ER) membranes associate with mitochondria, Golgi intermediate compartments, peroxisomes, and plasma membranes to facilitate transport proteins, lipids, and Ca2+ [1,2,3,4,5,6,7,8]. The association between ER and mitochondria membranes recently receives growing attentions mainly because of the vast importance of the association in regulation of Ca2+ signaling, mitochondrial bioenergetics, apoptosis, and lipid metabolism [5,6,7,9]. Ca2+ transferred directly from ER to mitochondria via the membrane contact is known to activate the tricarboxylic acid cycle, whereas overloading of mitochondrial Ca2+ causes apoptosis [9,10]. Phosphatidylserine (PtSer) synthesized at the ER directly moves to mitochondria via the physical membrane contact for its carboxylation to form phosphatidylethanolamine (PtEt) [5,6]. Particularly in hepatocytes, PtEt synthesized in mitochondria is transported back to the ER via the membrane contacts for its subsequent methylation to form phosphatidylcholine (PtChol) [5,6]. Thus, the interface between ER and mitochondria serves as a center place for the phospholipid biosynthesis.
The unique subdomain of the ER that associates with mitochondria is termed ‘the mitochondria-associated ER membrane (MAM) [6].’ The MAM accommodates specific proteins, such as Ca2+ signaling proteins (e.g., IP3 receptor), molecular chaperones (e.g., sigma-1 receptor chaperone, BiP, calreticulin), Bcl-2 family proteins (e.g., Bcl-2), ubiquitin ligases (e.g., AMFR/gp78), membrane tethering/vesicular transport proteins (e.g., mitofusion-2, PACS-2), and lipid synthases (e.g., PtSer synthase, acetyl-CoA: cholesterol acyltransferase) [11,12,13]. However, the mechanism regulating the protein recruitment to the MAM remains unknown. The association of MAMs with mitochondria is highly dynamic. Elevation of cytoplasmic Ca2+ is shown to cause the reversible rapid dissociation of MAMs from mitochondria [14]. The molecular mechanism regulating the dynamics of the MAM-mitochondrion association is also largely unknown.
We previously found that, in contrast to the bulk of ER membranes, the MAM is highly enriched with cholesterol and ceramides, thus containing lipid raft-like microdomains [15]. We also found that depletion of cholesterol or ceramide causes the relocation of the MAM-enriched proteins sigma-1 receptors and IP3 receptors from MAMs to the bulk of ER membrane [15]. Those results suggest that lipids may be important in promoting formation of specialized protein assemblies at the MAM and might relate to the association of MAMs with mitochondria. To support the hypothesis, we decided to focus on cholesterol and examined specifically whether depletion of cholesterol in purified MAMs may affect their association with mitochondria. To the best of our knowledge, this is the first report showing that cholesterol affects the physical association between isolated MAMs and mitochondrial membranes.
Materials and Methods
Reagents
Reagents for cell culture were purchased from Invitrogen (Carlsbad, CA). Antibodies were from the following sources: anti-ATP synthase inhibitor and anti-cytochrome c oxidase subunit I were from Invitrogen. Anti-IP3 receptor type-3 and anti-BiP were from BD Biosciences (San Jose, CA). Anti-sigma-1 receptor antibodies were developed as described previously [16]. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
Chinese hamster ovary (CHO) cells (American Type Culture Collection, Manassas, VA) were maintained in Minimal essential medium-α Glutamax containing 10% (v/v) heat-inactivated fetal bovine serum at 37 °C with 5% CO2. CHO cells were treated with methyl-β-cyclodextrin (MβC) or cholesterol-conjugated MβC (MβC-Chol) at 5 mM in culture medium without serum. MβC-Chol was prepared by rotating 1 ml of MβC solution (100 mM) with a cholesterol film (4 mg, dried under N2) overnight at room temperature.
MAM Preparation
The MAM fraction was prepared as described previously [17] with minor modifications. Briefly, CHO cells in two 15-cm dishes (100% confluency) were homogenized by a glass Dounce homogenizer with homogenization buffer (0.25 M sucrose, 10 mM HEPES/KOH, pH 7.4). The homogenate was centrifuged at 600g. The pellet (P1) contains nuclei and unbroken cells. The supernatant was centrifuged at 10,300g for 20 min to pellet the crude mitochondrial fraction. The supernatant was centrifuged at 100,000g for 1 h to yield P3 microsomal and cytosolic fractions. The crude mitochondrial fraction in 0.5 ml of isolation medium (250 mM mannitol, 5 mM HEPES/KOH at pH 7.4, and 0.5 mM EGTA/KOH) was layered on a Percoll solution [225 mM mannitol, 25 mM HEPES/KOH at pH 7.4, 1 mM EGTA/KOH, and 30% (v/v) Percoll (GE Healthcare, Buckinghamshire, UK)] followed by a centrifugation at 95,000g for 30 min in an SW 55Ti rotor. Purified mitochondrial and MAM fractions were washed three times successively with the isolation medium, 50 mM phosphate buffer (pH 7.2) containing 0.25M sucrose and 5 mM β-mercaptoethanol, and saline. Protein concentrations in each fraction were measured by a Micro BCA Assay (Thermo Fisher Scientific, Rockford, IL).
MAM-mitochondria association assay and Western Blotting
The MAM-mitochondria association assay reported by de Brito and Scorrano (2009) was modified. MAMs were centrifuged at 6,300g for 10 min, and the supernatant was immediately used in the assay. Twenty microgram of MAMs and mitochondria were mixed in reaction buffer (RB) [10 mM Tris (pH7.4), 150 mM KCl, 1 mM KH2PO4, 5 mM MgCl2, 10 mM sodium succinate], and incubated at 37 °C for 1–30 min. In some experiments, MAMs were incubated with 5 mM of MβC or MβC-Chol with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) at 37 °C for 30 min. The membranes treated were washed to remove MβC or MβC-Chol prior to the assay. After the incubation of the mixture of two membranes, mitochondria and MAMs associating with mitochondria were pelleted by a centrifugation at 6,300g for 10 min. The supernatant was collected as a source of unbound MAMs (note that MAMs not associated with mitochondria can sediment only at 100,000g or higher). The level of MAM-specific protein sigma-1 receptors in the pellet and the supernatant was measured by Western blotting according to the protocol described before [16]. Protein bands were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and Kodak Image Station 440CF (Carestream, Rochester, NY).
Free cholesterol measurement
Cholesterol in 50 µg of mitochondria, MAMs, and microsome membranes were extracted by a Bligh & Dyer lipid extract method. Total lipid extracts were dissolved in 100 µl of isopropanol by incubating at 37 °C for 10 min with vortex. Free cholesterol was measured by a Free Cholesterol E kit (Wako Chemicals USA, Richmond VA).
High performance thin-layer chromatography (HTLC)
CHO cells were cultured in 6-cm dishes, and treated with MβC or MβC-Chol for 2 hrs in culture medium without serum. Medium was replaced with 1 ml of fresh culture medium containing 0.3 µCi/ml of 14C-serine with MβC or MβC-Chol. After 1 hr of pulse-labeling, cells were harvested and total lipids were extracted as described above. Lipids were resolved by a HTLC with the CHCl3-methanol-H2O mixture (13:5:0.8). Lipids were visualized by direct autoradiography.
Results and Discussion
Three previous studies have examined the effect of MβC that extracts cholesterol from membranes on the function of the MAM. One study found that the MβC treatment normalizes overloading of mitochondrial Ca2+ that is caused by the pathological accumulation of GM1 ganglioside in fibroblasts of lysosomal β-galactosidase knockout mice [18]. The MβC treatment was also shown to alleviate targeting of sigma-1 receptors or human cytomegalovirus protein UL37exon 1 to lipid microdomains at the MAM [15,19]. Further a recent study using confocal microscopy showed that the treatment of CHO cells with MβC does not significantly change the colocalization co-efficiency between green fluorescent proteins and DsRed proteins respectively expressed in ER and mitochondria [15]. However, any of currently available data do not provide direct and sufficient evidence supporting the possibility that depletion of cholesterol changes the physical association of the two subcellular membranes. Further, microscopic observations appear to have a certain limitation in precisely determining the physical membrane association. Therefore, we here employed an in vitro assay [13] to monitor the direct physical association between MAMs and mitochondria in vitro, and examined the effect of the MβC treatment on the association.
The principal of the assay is based on the unique property of the MAM-mitochondria association that can be reconstituted rapidly and energy-independently in a test tube [5,6]. Simply mixing purified MAMs with mitochondria promotes the interaction of the two distinct membranes. MAMs associated with mitochondria can be separated by a low-speed centrifugation as free MAMs stay exclusively in the supernatant. Accordingly, we first prepared MAMs and mitochondria membranes from CHO cells, and verified respective purity (Figure 1A). Immunoblotting confirmed that mitochondrial proteins are present mostly in the prepared mitochondrial fraction (Figure 1A). Sigma-1 receptors, the ER proteins localized at MAMs [15,16], were highly enriched in the MAM fraction (Figure 1A). Sigma-1 receptors were also detected in the mitochondria fraction, but the level is significantly lower than that in the MAM fraction. When the MAM alone was centrifuged at 6300g where the MAM stays in the supernatant, sigma-1 receptors were detected solely in the supernatant (Figure 1B). In contrast, when MAMs were incubated together with purified mitochondria in the test tube, sigma-1 receptors was now detected in the pellet after the centrifugation with a concomitant decrease of its level in the supernatant (Figure 1B). Similar results were obtained when membranes were prepared from the rat liver (Figure 1B). Co-sedimentation of MAMs with mitochondria was observed as early as 1 min after the incubation of MAMs with mitochondria, and continued to increase up to 30 min (data not shown). These results clearly demonstrate the validity of the MAM-mitochondria association assay with accompanying sigma-1 receptor immunoblotting for the monitoring of the physical association of the two membranes in vitro.
Fig. 1. The in vitro MAM-mitochondria association assay.
A. MAM and mitochondrial membranes from CHO cells. P1, nuclear; Mito, mitochondrial; P3, microsomal; Cyt, cytosolic fractions. Sig-1R, sigma-1 receptor; IP3R3, IP3 receptor type-3; CRT, calreticulin. Levels of respective organelle markers were measured by immunoblotting. Note that ER chaperones involved in the ER-to-Golgi vesicle transport (i.e., BiP, CRT) are also detected in the cytoplasmic fraction. B. In vitro association of MAMs with purified mitochondria. MAMs and mitochondria membranes were prepared from CHO cells or rat livers. In the MAM(+)-Mito(+) sample, the two membranes were incubated together for 30 min. Pellets and supernatants (SNT) were obtained by a centrifugation at 6,300g. The level of MAM-enriched protein sigma-1 receptors (Sig-1R) was measured by immunoblotting.
The level of cholesterol in the MAM and its alteration after the MβC treatment were examined. The MAM contains 5–7 times higher levels of cholesterol when compared with mitochondria or microsomes (Figure 2A). This result supports a previous finding showing that MAMs are highly enriched with lipid raft-like microdomains [15], and suggests the potential significance of examining the role of cholesterol in the association between MAMs and mitochondria. When purified MAMs were incubated with MβC at 37°C, the cholesterol level was time-dependently decreased and the cholesterol level reached approximately 50% of the original in 30 min (Figure 2B). In contrast, at 4 °C, MβC was ineffective in causing a reduction of the membrane cholesterol (Figure 2B).
Fig. 2. Depletion of cholesterol in MAMs with MβC.
A. The level of free cholesterol in isolated mitochondria, MAMs, and microsomal membranes. N=3. Mean±SEM. B. Time-dependent effect of MβC in depleting membrane cholesterol. The crude mitochondrial fraction (containing both mitochondria and MAMs) were incubated with 5 mM of M®C at 37 or 4 °C for indicated periods of time.
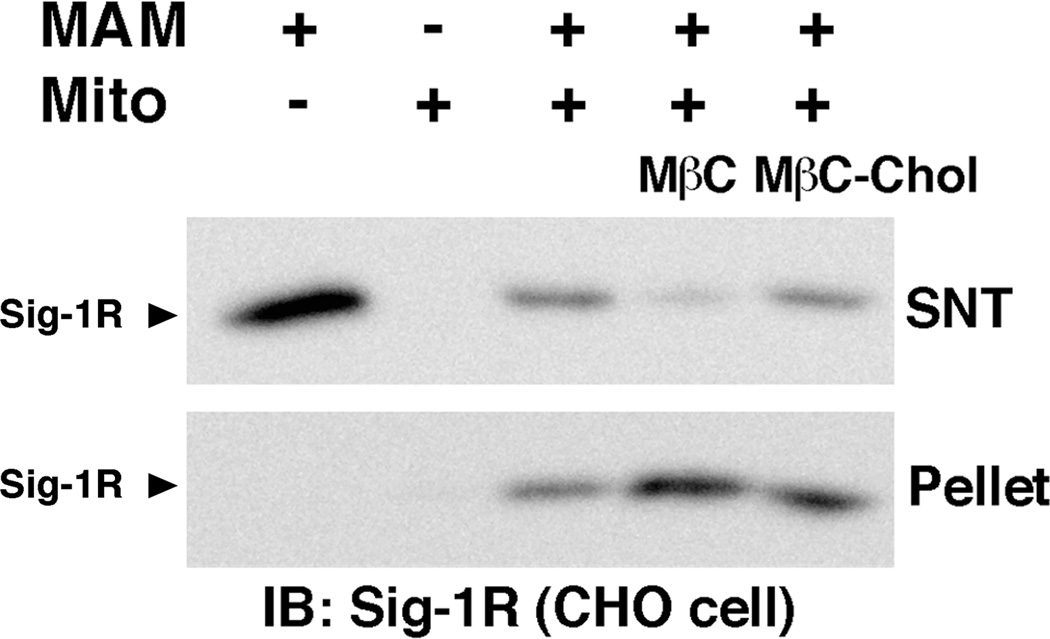
The MAM-mitochondria association assay was then conducted with MAMs pre-incubated with MβC for 30 min at 37°C. Depletion of cholesterol in MAMs significantly increased MAMs co-sedimented with mitochondria (Figure 3). The treatment with MβC did not cause sedimentation of MAMs in the absence of mitochondria (data not shown). The pre-incubation of MAMs with MβC conjugated with cholesterol no longer potentiated the co-sedimentation of MAMs with mitochondria (Figure 3). These findings suggest that the effect of MβC is specific in depleting cholesterol from the membrane and that the depletion of cholesterol in MAMs facilitates the association between MAMs and mitochondria.
Fig. 3. Effect of cholesterol depletion on the association between MAMs and mitochondria.
MAMs were incubated with MβC or MβC-Chol at 37 °C for 30 min. The MAM-mitochondria association assay was then performed as described in Figure 1. Mito, mitochondria; SNT, supernatant. Sigma-1 receptors (Sig-1R) were measured by immunoblotting.
Since cholesterol at the MAM plays a role in formation of lipid microdomains [15], which leads to the formation of protein assemblies at the loci [15], we originally speculated that cholesterol may promote the association between MAMs and mitochondria, and therefore depletion of cholesterol at MAMs might reduce their association with mitochondria. However, here we found that, in contrast to our speculation, depletion of cholesterol increased the membrane association. Although details of the underlying mechanism is unknown at present, our finding may suggest the existence of two distinct systems at the MAM in regulating its association with mitochondria: one that is to promote the membrane association via specific membrane-tethering proteins such as mitofusin-2 [13] and the other that is used to restrict the association wherein cholesterol may play a role. Probably, the overwhelmed membrane association between ER and mitochondria is unfavorable for the proper cellular functioning. Thus the latter mechanism serves as a brake in a coordinated manner with the membrane-tethering mechanism to fine-tune the ER-mitochondria association. In fact, a manipulation causing a tighter association between MAM and mitochondrial membranes has been shown to promote apoptosis due to overloading of mitochondrial Ca2+ [20]. Further, in the most types of cells, the ER associates with only a limited portion of mitochondria (mostly 10–20% of the entire mitochondria surface) [20,21]. Therefore, the two systems at the MAM may create a yin-yang effect to regulate number and/or size of the contact sites. Our data suggest that certain lipids such as cholesterol might have a novel biophysical action limiting coalescence of two distinct intracellular membranes.
How cholesterol alters the association of MAM with mitochondrial membranes is unknown at present. Depletion of cholesterol in the MAM might change the membrane integration or the structure of proteins involved in the membrane tethering and/or dissociation. Alternatively, since the MAM-mitochondria association can be partially achieved (approximately 50%) with co-incubation of proteinase K-treated MAMs and mitochondria [22], a protein-independent mechanism regulating the membrane association may exist. Membrane lipids per se may thus be active substrates in regulating the MAM-mitochondria association.
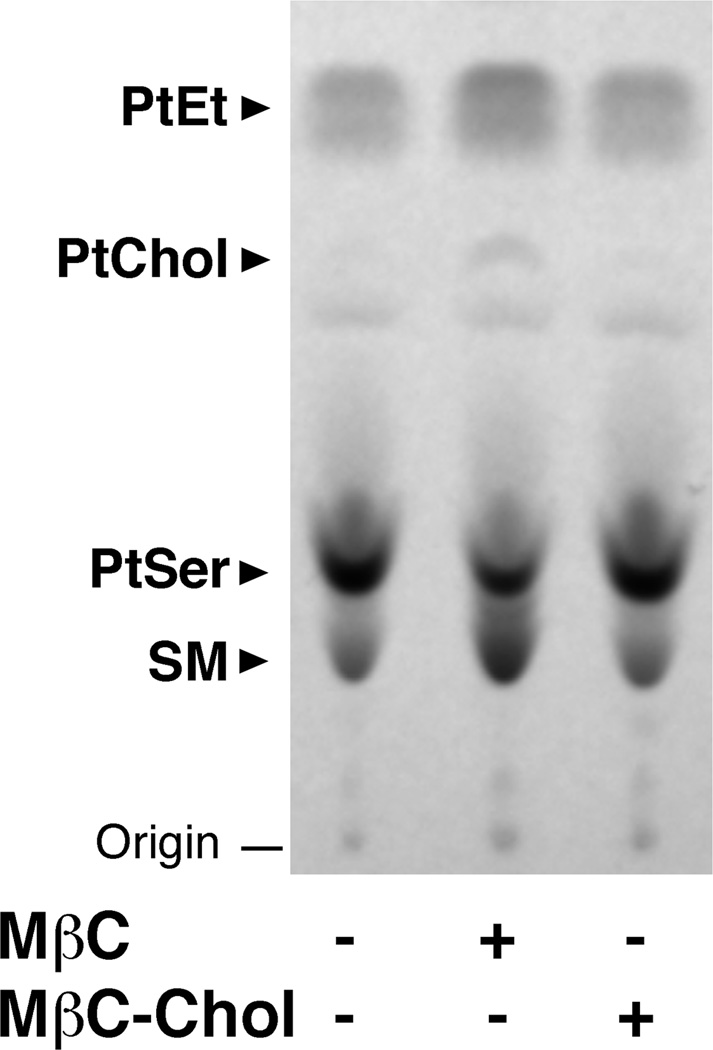
Since above experiments were all performed with isolated membrane preparations, the result may not necessarily reflect the in vivo process. To provide the physiological insight of our finding, we examined whether the MβC treatment alters the MAM-to-mitochondria phospholipid transport in living cells. When transported from MAMs to mitochondria, PtSer is rapidly converted to PtEt, where the PtEt synthesis predominantly depends on activity of the intermembrane transport of PtSer via the physical membrane contacts [5,6,22]. Therefore, monitoring the synthesis of PtSer and PtEt helps indirectly assess the degree of the MAM-mitochondria association [22,23]. Accordingly, CHO cells were pulse-labeled with 14C-serine for 1 hr, permitting the near maximal velocity in the PtSer transport as well as the mitochondrial PtEt synthesis in CHO cells [23]. The level of synthesized 14C-PtSer and 14C-PtEt were then measured. Although the MβC treatment of living CHO cells is shown to initially deplete cholesterol on the cell surface, an extended treatment (e.g., 2 hrs) can decrease cholesterol in subcellular membranes including MAMs [15]. Therefore, cells were pretreated with MβC or MβC-chol for 2hr prior to being pulse-labeled with 14C-serine. The treatment with MβC slightly decreased the level of 14C-PtSer synthesized during the 1-hr incubation with 14C-serine, but significantly increased the synthesis of 14C-PtEt (Figure 4). This result indicates that the MβC treatment does not significantly compromise the PtSer synthesis at the ER, but promoted the availability of PtSer for the biosynthesis of PtEt in mitochondria.
Fig. 4. Effect of cholesterol depletion on the de novo biosynthesis of PtSer and PtEt in CHO cells.
CHO cells were treated with 5 mM of MβC or MβC-Chol for 2 h prior to 14C-serine pulse-labeling for 1 h. Total lipids were extracted and visualized by HPTLC followed by autoradiography. SM, sphingomyelin.
Albeit MβC appears to slightly increase the 14C-PtChol synthesis (Figure 4), the data may not be conclusive because of the inherited low level of PtChol derived from serine in CHO cells. The result is likely due to low expression of the PtEt N-methyltransferase activity in CHO cells [23]. In addition, in an agreement with a previous report showing that cholesterol depletion increases the de novo synthesis of the sphingosine backbone [24], we found that the MβC treatment increased the synthesis of sphingomyelin (Figure 4). Details of this mechanism however remain unknown.
When MβC was pre-conjugated or saturated with cholesterol, the MβC effect on the de novo synthesis of phospholipids disappeared. This result suggests that the effect of MβC on the phospholipid synthesis is mainly due to its cholesterol-depleting action. Because the physical association of the two organelles is important in the PtSer transport and the subsequent PtEt biosynthesis [5,6,22], this finding with MβC-saturated cholesterol further supports our notion that cholesterol negatively regulates the membrane association between MAMs and mitochondria.
Although cholesterol and sphingolipids are synthesized at the ER, they are actively transported by the vesicular or non-vesicular transport to Golgi and other organelles [25]. Thus, the bulk of ER membranes are composed mostly of phospholipids [25]. In contrast, the MAM of the ER contains the exceptionally high level of cholesterol [15]. Cholesterol at the MAM therefore leads to formation of lipid microdomains that recruit and compartmentalize signaling proteins at the ER-mitochondria interface [15]. Extending from the facts that at the plasma membrane lipid rafts control signal transductions at connections between the plasma membrane and ER membrane [26,27], we suggest that intracellular lipid microdomains, including those at the ER, might be universally utilized by cells to regulate docking of distinct organelle membranes or substrates. In summary, our results showing the potentiation of biological membrane associations induced by cholesterol depletion may provide a new insight into the role of lipids in the dynamics of membrane association and dissociation in the biological system.
Highlights.
The endoplasmic reticulum subdomain termed MAM associates with mitochondria.
The biophysical role of lipids in the MAM-mitochondria association is unknown.
The in vitro membrane association assay was used to examine the role of lipids.
Cholesterol was found to negatively regulate the association.
Acknowledgements
This study was supported by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. M. Fujimoto is also supported by Japan Society for the Promotion of Sciences (JSPS) Fellowship for Japanese Biochemical and Behavioral Researchers at NIH. We thank Dr. Mary Pfeiffer for carefully editing the manuscript.
Abbreviations
- CHO
Chinese hamster ovary
- ER
endoplasmic reticulum
- HTLC
high performance thin-layer chromatography
- MAM
mitochondria-associated ER membrane
- MβC
methyl-β-cyclodextrin
- MβC-Chol
MβC conjugated with cholesterol
- PtChol
phosphatidylcholine
- PtEt
phosphatidylethanolamine
- PtSer
phosphatidylserine
- SM
sphingomyelin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflict of interest.
References
- 1.Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- 4.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voelker DR. Bridging gaps in phospholipid transport. Trends Biochem Sci. 2005;30:396–404. doi: 10.1016/j.tibs.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 7.Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- 8.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajnoczky G, Csordas G, Madesh M, Pacher P. Control of apoptosis by IP(3) and ryanodine receptor driven calcium signals. Cell Calcium. 2000;28:349–363. doi: 10.1054/ceca.2000.0169. [DOI] [PubMed] [Google Scholar]
- 10.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Brito OM, Scorrano L. Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: the role of Ras. Mitochondrion. 2009;9:222–226. doi: 10.1016/j.mito.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Dai J, Kuo KH, Leo JM, van Breemen C, Lee CH. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium. 2005;37:333–340. doi: 10.1016/j.ceca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010;77:517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 18.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson CD, Zhang A, Colberg-Poley AM. The Human Cytomegalovirus Protein UL37 Exon 1 Associates with Internal Lipid Rafts. J Virol. 2011;85:2100–2111. doi: 10.1128/JVI.01830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 22.Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem. 1999;264:545–553. doi: 10.1046/j.1432-1327.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 23.Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270:11190–11198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- 24.Leppimaki P, Kronqvist R, Slotte JP. The rate of sphingomyelin synthesis de novo is influenced by the level of cholesterol in cultured human skin fibroblasts. Biochem J. 1998;335(Pt 2):285–291. doi: 10.1042/bj3350285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 27.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]