Abstract
GroEL is an Escherichia coli chaperonin that is composed of two heptameric rings stacked back-to-back. GroEL assists protein folding with its cochaperonin GroES in an ATP-dependent manner in vitro and in vivo. However, it is still unclear whether GroES binds to both rings of GroEL simultaneously under physiological conditions. In this study, we monitored the GroEL-GroES interaction in the reaction cycle using fluorescence resonance energy transfer. We found that nearly equivalent amounts of symmetric GroEL-(GroES)2 (football-shaped) complex and asymmetric GroEL-GroES (bullet-shaped) complex coexist during the functional reaction cycle. We also found that D398A, an ATP hydrolysis defective mutant of GroEL, forms a football-shaped complex with ATP bound to the two rings. Furthermore, we showed that ADP prevents the association of ATP to the trans-ring of GroEL, and as a consequence, the second GroES cannot bind to GroEL. Considering the concentrations of ADP and ATP in E. coli, ADP is expected to have a small effect on the inhibition of GroES binding to the trans-ring of GroEL in vivo. These results suggest that we should reconsider the chaperonin-mediated protein-folding mechanism that involves the football-shaped complex.
Chaperonins belong to a ubiquitous class of molecular chaperones that promote protein folding in the cell, and they are found in bacteria, chloroplasts, mitochondria, archaea, and eukaryotic cytosol (1, 2). The most characterized members of the family are the Escherichia coli chaperonin GroEL and its partner GroES (3, 4). GroEL is a large cylindrical protein complex comprising two heptameric rings of identical 57-kDa subunits, and these rings are stacked back-to-back. GroES contains seven identical 10-kDa subunits assembled as a heptamer ring. GroEL interacts with GroES in a nucleotide-dependent manner and facilitates protein folding.
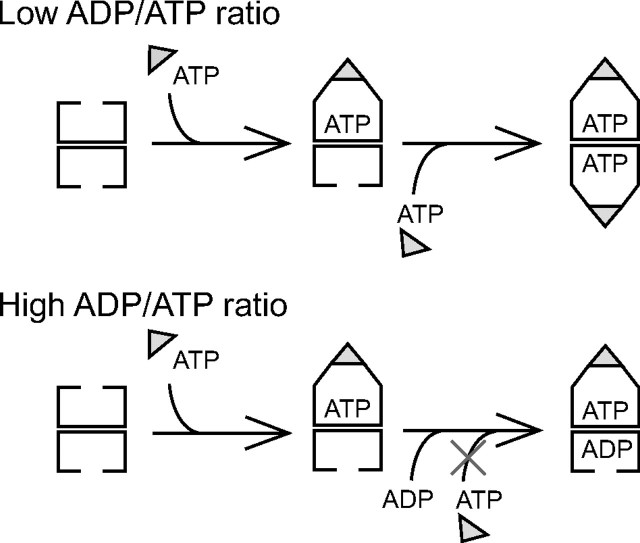
The current understanding of the GroEL-GroES reaction cycle is as follows (3, 4). First, the unfolded protein binds to one of the GroEL rings. Subsequently, ATP and GroES bind to the same ring (the cis-ring; a GroEL ring that has a bound GroES) and encapsulate the unfolded protein in the cavity of a GroEL-GroES complex. Until ATP in the cis-ring is hydrolyzed, ATP and the second GroES cannot bind to the opposite GroEL ring (the trans-ring; a GroEL ring that does not have GroES) due to the negative cooperativity between the two rings of GroEL. After ATP in the cis-ring is hydrolyzed, GroES, ADP, and the trapped protein are released from the cis-ring by the binding of ATP and an unfolded protein to the trans-ring. At the same time, the second GroES associates with the trans-ring of GroEL to form a new cis-ring. In short, GroES binds to each ring of GroEL alternatively (two-stroke model), and thus it is widely believed that an asymmetric GroEL-GroES complex (termed a bullet-shaped complex) is the only form of the GroEL-GroES complex, and a symmetric GroEL-(GroES)2 complex (termed a football-shaped complex) is not formed during the reaction cycle (5–7). On the other hand, football-shaped complexes have been observed by electron microscopy (8, 9) and chemical cross-linking experiments (10, 11). However, it is undeniable that electron microscopy and chemical cross-linking studies do not always reflect the situation under physiological conditions due to the fixation procedure by negative staining or glutaraldehyde cross-linking.
Does football-shaped complex exist during the chaperonin reaction cycle? To clarify this issue, we attempted to detect the football-shaped complex in the chaperonin reaction cycle in real-time without chemical cross-linking. We utilized a fluorescence resonance energy transfer (FRET)5 assay to monitor GroEL-GroES interaction during the reaction cycle. The result showed that about half of the GroEL molecules form football-shaped complexes during the GroEL-GroES reaction cycle. Using a FRET assay, we found that the ratio of football-shaped complex was regulated by the amount of ADP in solution. In addition, we confirmed that an ATPase-deficient mutant of GroEL (D398A, termed EL398), which has been believed to form a bullet-shaped complex (12), formed the football-shaped complex in the presence of ATP. These findings lead us to reconsider the two-stroke model of the GroEL-GroES interaction cycle and to take into account the folding mechanism including symmetric football-shaped complex.
EXPERIMENTAL PROCEDURES
Reagents and Proteins—ATP, ADP, malate dehydrogenase (MDH) from porcine hearts, phosphoenolpyruvate, and pyruvate kinase were purchased from Roche Diagnostics (Mannheim, Germany). MDH was denatured by HKM buffer (25 mm HEPES-KOH, pH 7.4, 100 mm KCl, 5 mm MgCl2) containing 6.4 m urea. BeSO4, bovine serum albumin (BSA), and hexokinase were obtained from Sigma-Aldrich. NaF was purchased from Wako (Osaka, Japan). ADP was treated with hexokinase in the presence of glucose to remove contaminating ATP as described previously (13). The E315C variant of GroEL (termed EL315) replaces the 315th glutamate with cysteine without substitutions of endogenous cysteine residues. The ES98C version of GroES adds a single cysteine at the C terminus of each GroES subunit (14). The D398A variant of GroEL replaces the 398th aspartate with alanine (15). Wild-type and mutant GroEL and GroES were expressed in E. coli cells and purified as described previously (16). Purified proteins were stored in a 65% saturated ammonium sulfate suspension until use.
Fluorescence Labeling of Proteins—For the FRET analysis, EL315, and E315C/D398A GroEL mutant (EL315/398) were labeled with Cy3-maleimide (GE Healthcare UK Ltd., Bucking-hamshire, UK) or tetramethylrhodamine (TMR)-5′-maleimide (Invitrogen), and ES98C was labeled with Cy5-maleimide (GE Healthcare UK Ltd.) in HKM buffer for 90 min at room temperature. The labeled protein was separated from unreacted reagents using an NAP-5 column or PD-10 column (GE Healthcare UK Ltd.).
Protein and fluorescent dye concentrations were determined by absorption spectroscopy using the following extinction coefficients: GroEL tetradecamer, 130,480 m–1 cm–1 at 280 nm; GroES heptamer, 8,960 m–1 cm–1 at 280 nm; Cy3, 150,000 m–1 cm–1 at 553 nm; TMR, 95,000 m–1 cm–1 at 555 nm; Cy5, 250,000 m–1 cm–1 at 649 nm. The concentration of Cy3-EL315 or TMR-EL315 was determined by correcting for the 280 nm absorbance of the conjugated dye. The concentration of Cy5-ES98C was determined using the Lowry method (37) (DC protein assay; Bio-Rad). The protein concentration was expressed as the oligomer (GroEL, tetradecamer; GroES, heptamer). The molar ratio of Cy3 or TMR to the EL315 (tetradecamer) was 0.5–1.0, and that of Cy5 to the ES98C (heptamer) was 2.5–4.0 throughout this study. The Förster distance was estimated to be 5.3 nm for Cy3 and Cy5 and 6.1 nm for TMR and Cy5, respectively. The average distance between the donor and acceptor in the GroEL-GroES complex was about 6.7 nm. The FRET efficiencies with a single donor in EL315 and with three acceptors in ES98C were calculated to be 0.72 (Cy3 as donor) and 0.82 (TMR as donor).
FRET Experiments—HKM buffer containing EL315, GroES (Cy5-ES98C or wild-type GroES (wtES)), and denatured malate dehydrogenase (dMDH) with or without beryllium fluoride (BeFx) (1 mm BeSO4 and 10 mm NaF) was preincubated at 23 °C. Subsequently, ATP or ADP was added to the solution, and the fluorescence spectrum was measured. All experiments were carried out in the presence of 1.0 mg/ml BSA to avoid adsorption of GroEL and GroES to a tube or cuvette. Fluorescence spectra were measured at 23 °C with a spectrofluorometer (FP-6500; Jasco, Tokyo, Japan) using a microcuvette (3 × 3 × 37 mm; Jasco) except for the experiment shown in Fig. 3, where a 10 × 10 × 45-mm cuvette was used instead. The excitation wavelength was 520 nm (Cy3) or 525 nm (TMR). The FRET efficiency was determined by Equation 1.
 |
(Eq. 1) |
where FDA is the fluorescence intensity of donor-labeled EL315 in the presence of acceptor-labeled ES98C, and FD is the fluorescence intensity of donor-labeled EL315 in the presence of unlabeled wtES.
FIGURE 3.
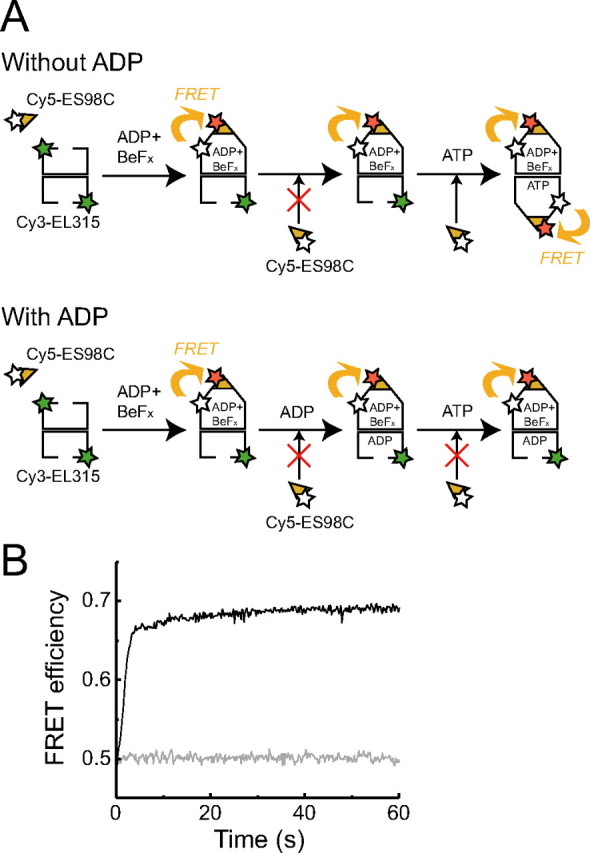
ADP prevents the binding of the second GroES to the trans-ring of GroEL. A, schematic illustration measuring the association rate of a second GroES with the trans-ring of bullet-shaped GroEL preformed by ADP and BeFx. Unfolded protein substrates are not included in this figure because we could not determine whether dMDH attached to the cis-ring or trans-ring or both rings in our study. B, the change in FRET efficiency by the association of the second GroES with the ADP + BeFx bullet-shaped complex in the presence (gray line) or absence (black line) of 1 mm ADP. The ADP + BeFx bullet-shaped complex was formed in a solution containing 500 nm Cy3-EL315, 2 μm GroES (Cy5-ES98C or wtES), 2 μm dMDH, 1.0 mg/ml BSA, 5 mm DTT, and BeFx (1 mm BeSO4, and 10 mm NaF). Subsequently, this mixture was diluted 50-fold in HKM buffer containing 500 nm GroES (Cy5-ES98C or wtES), 500 nm dMDH, 5 mm DTT, and 1.0 mg/ml BSA in the presence or absence of 1 mm ADP. 1 mm ATP was added to the mixture, and the change in donor fluorescence (excitation at 520 nm, emission at 570 nm) was monitored. 0.48 mol of Cy3 and 3.3 mol of Cy5 were attached to 1 mol of EL315 and ES98C, respectively.
All experiments were respectively repeated three times. The molar ratio of fluorescence dye to GroEL (tetradecamer) or GroES (heptamer) was kept constant since it affected the FRET efficiency. The detailed experimental procedures and ratios of dye per protein in each experiment are described in the legends for Figs. 1, 2, 3, 4.
FIGURE 1.
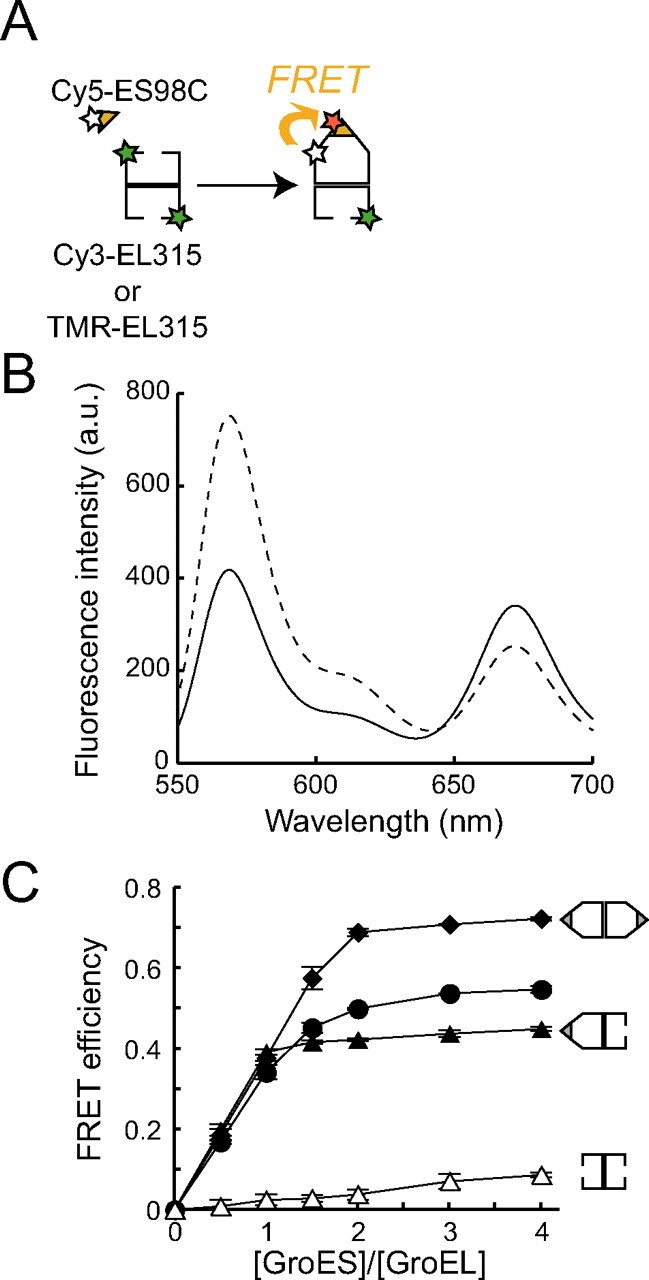
Detection of football-shaped complexes during the chaperonin cycle by FRET assay. A, schematic illustration of the FRET assay. The donor chromophore (Cy3 or TMR) and acceptor chromophore (Cy5) are attached to EL315 and ES98C, respectively. FRET occurs when GroES binds to GroEL. Unfolded protein substrates are not included in this figure because we could not determine whether dMDH attached to the cis-ring or trans-ring or both rings in our study. B, typical fluorescence spectra of the solution containing 500 nm Cy3-EL315, 2μm Cy5-ES98C, 2μm dMDH, 1.0 mg/ml BSA, and 5 mm DTT in the presence (solid line) or absence (broken line) of 1 mm ATP. a.u., arbitrary units. C, FRET efficiency at different concentrations of GroES. HKM buffer containing 500 nm Cy3-EL315, 2 μm dMDH, 1.0 mg/ml BSA, 5 mm DTT, and 250 nm–2 μm Cy5-ES98C or wtES was preincubated at 23 °C in the presence or absence of BeFx (1 mm BeSO4 and 10 mm NaF). BeFx and dMDH were added to the solution in sequence before the measurement. Subsequently, 1 mm nucleotide was added to the solution, and fluorescence spectra were measured after 1 min. Open triangles, closed circles, closed triangles, and closed diamonds represent the FRET efficiency in the absence of nucleotide and in the presence of 1 mm ATP, 1 mm ADP + BeFx (1 mm BeSO4 and 10 mm NaF), and 1 mm ATP + BeFx, respectively. 0.74 mol of Cy3 and 3.8 mol of Cy5 were attached to 1 mol of EL315 (tetradecamer) and ES98C (heptamer), respectively. Data represent the mean ± S.E. (n = 3).
FIGURE 2.
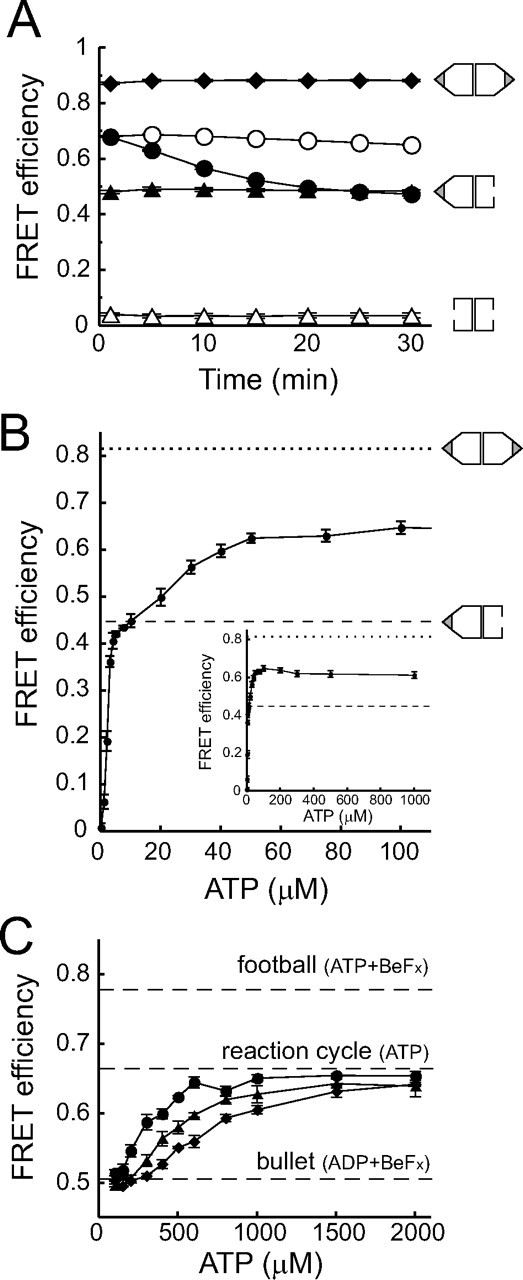
ADP prevents the formation of football-shaped complexes. A, time dependence of the FRET efficiency of the GroEL-GroES complex. 50 nm TMR-EL315, 500 nm Cy5-ES98C or wtES, 2 μm dMDH, and 1.0 mg/ml BSA were preincubated at 23 °C in HKM buffer. The values of FRET efficiency were measured at various time points after the addition of 200 μm nucleotides. Open triangles, closed triangles, and closed diamonds represent the FRET efficiency in the absence of nucleotide and in the presence of 200 μm ADP + BeFx (1 mm BeSO4 and 10 mm NaF) and 200 μm ATP + BeFx, respectively. Closed circles and open circles represent measurements in the absence or presence of an ATP regeneration system (5 mm phosphoenolpyruvate, 10 μg/ml pyruvate kinase). 0.74 mol of TMR and 3.6 mol of Cy5 were attached to 1 mol of EL315 and ES98C, respectively. Data represent the mean ± S.E. (n = 3). B, FRET efficiency of GroEL-GroES interaction at different ATP concentrations. 50 nm TMR-EL315, 500 nm Cy5-ES98C or wtES, 500 nm dMDH, and 1.0 mg/ml BSA were preincubated at 23 °C in HKM buffer with ATP regeneration system (5 mm phosphoenolpyruvate, 10 μg/ml pyruvate kinase). Subsequently, various concentrations of ATP were mixed 2 min before measuring fluorescence spectra. Dotted or broken lines represent the FRET efficiency obtained from a solution containing 50 nm TMR-EL315, 500 nm GroES (Cy5-ES98C or wtES), 500 nm dMDH, 1.0 mg/ml BSA, BeFx (1 mm BeSO4, 10 mm NaF), and 1 mm ATP or ADP, respectively. 0.84 mol of TMR and 2.9 mol of Cy5 were attached to 1 mol of EL315 and ES98C, respectively. Data represent the mean ± S.E. (n = 3). Inset, the data for high ATP concentration. C, FRET efficiency of GroEL-GroES interaction at different ATP concentrations in the presence of ADP. 50 nm TMR-EL315, 500 nm Cy5-ES98C or wtES, 500 nm dMDH, and 1.0 mg/ml BSA were preincubated at 23 °C in HKM buffer. Subsequently, various concentrations of ATP and a fixed amount of ADP (50 μm (closed circles), 100 μm (closed triangles), and 150 μm (closed diamonds)) were mixed 1 min before measuring fluorescence spectra. 0.93 mol of TMR and 2.4 mol of Cy5 were attached to 1 mol of EL315 and ES98C, respectively. Data represent the mean ± S.E. (n = 3).
FIGURE 4.
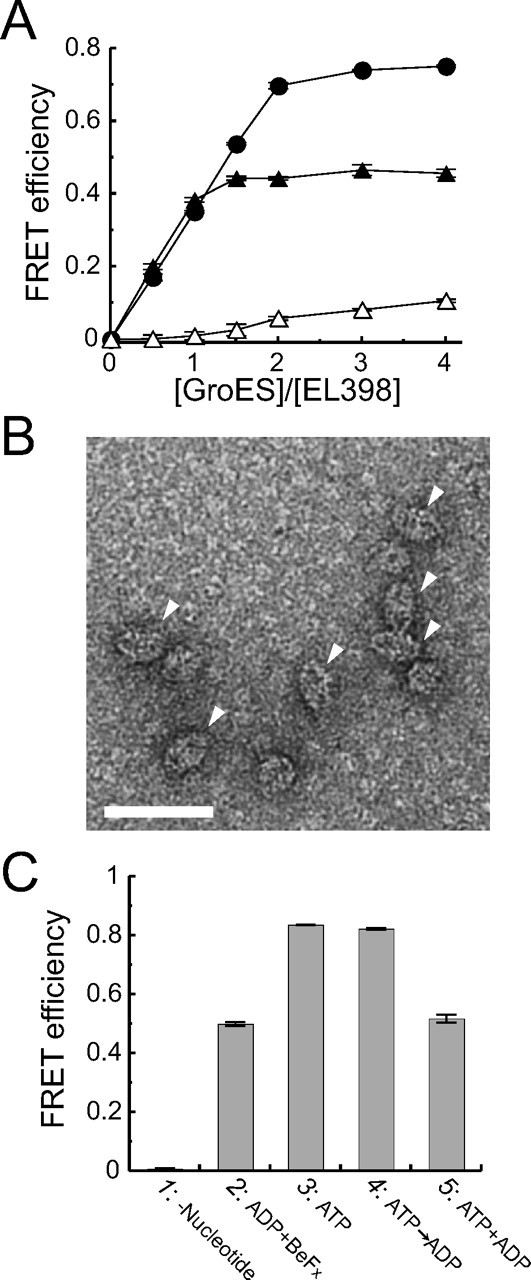
Football-shaped EL398-GroES complexes are formed in a solution containing ATP. A, the FRET efficiency of the EL398-GroES complex at different concentrations of GroES. HKM buffer containing 500 nm Cy3-EL315/398, 2 μm dMDH, 1.0 mg/ml BSA, 5 mm DTT, and 250 nm–2 μm Cy5-ES98C or wtES were preincubated at 23 °C in the presence or absence of BeFx (1 mm BeSO4 and 10 mm NaF). Subsequently, 1 mm nucleotide was added to the solution, and fluorescence spectra were measured after 1 min. Open triangles, closed circles, and closed triangles represent the FRET efficiency in the absence of nucleotide and in the presence of 1 mm ATP and 1 mm ADP + BeFx, respectively. 0.77 mol of Cy3 and 3.3 mol of Cy5 were attached to 1 mol of EL315/398 and ES98C, respectively. Data represent the mean ± S.E. (n = 3). B, electron micrograph of EL398-GroES complexes in the presence of 200 μm ATP. The sample was negatively stained with 1.0% uranyl acetate. Arrowheads represent the observed football-shaped complexes. The scale bar represents 50 nm. C, FRET assay of the EL315/398-GroES complex. 50 nm TMR-EL315/398, 500 nm GroES (Cy5-ES98C or wtES), 500 nm dMDH, and 1.0 mg/ml BSA were preincubated at 23 °C in HKM buffer with or without BeFx (1 mm BeSO4 and 10 mm NaF). Fluorescence spectra were measured after the addition of nucleotide. Column 1, nucleotide was not added to the solution. Column 2, 1 mm ADP was added to the solution in the presence of BeFx. Column 3, 1 mm ATP was added to the solution in the absence of BeFx. Column 4, 1 mm ADP was added to the solution 1 min after the addition of 1 mm ATP in the absence of BeFx. Column 5, 1 mm ATP and 1 mm ADP were added to the solution simultaneously in the absence of BeFx. 0.71 mol of TMR and 2.8 mol of Cy5 were attached to 1 mol of EL315/398 and ES98C, respectively. Data represent mean ± S.E. (n = 3).
Electron Microscopy—1 mm ATP was added to a solution containing 50 nm EL398, 500 nm GroES, and 500 nm dMDH in HKM buffer and incubated for 1 min at room temperature. Subsequently, an aliquot of the solution was applied to an electron microscope specimen grid covered with a carbon film. The specimen was stained with 1.0% uranyl acetate and observed with a transmission electron microscope (Tecnai F20; FEI Co., Hillsboro, OR) at an accelerating voltage of 120 kV. Images were recorded by a slow scan CCD camera (Getan retractable multiscan camera) at a magnification of 50,000.
Quantitative Determination of Nucleotides Bound to the EL398-GroES Complex—HKM buffer containing 500 nm EL398, 2 μm GroES, and 2 μm dMDH was incubated with 1 mm ATP or 1 mm ADP in the absence or presence of 1 mm BeSO4 and 10 mm NaF for 5 min at 23 °C. Then the mixtures were applied to a NAP-5 column equilibrated with HKM buffer to remove unbound nucleotides. The fractions containing proteins were treated with ice-cold perchloric acid at a final concentration of 1% and then neutralized with Na2CO3. This solution was centrifuged at 20,000 × g for 10 min, and the aliquots of the supernatant were analyzed by a reverse-phase column (TSKgel ODS-80Ts; Tosoh, Tokyo, Japan) with 30 mm sodium phosphate buffer, pH 6.8, containing 1% (v/v) methanol at a flow rate of 0.5 ml/min using an HPLC system (L-7100; Hitachi, Tokyo, Japan). The eluate was monitored with an on-line UV spectrophotometer (L-4000H; Hitachi) at 260 nm, and the amount of nucleotide was calculated using the peak area. The amount of EL398 was analyzed by SDS-PAGE. The densitometry of Coomassie Brilliant Blue-stained bands was analyzed using a molecular imager FX (Bio-Rad) and quantitated using Image J (National Institutes of Health).
RESULTS
FRET Assay Revealed That About Half of the GroEL-GroES Complexes Formed a Football-shaped Complex during the Functional Reaction Cycle—To analyze the ratio of bound GroES to GroEL during the reaction cycle, we employed a FRET assay in the presence of dMDH (Fig. 1A). We confirmed that 500 nm dMDH was sufficient to saturate the ATPase activity of GroEL. Therefore, it is reasonable to assume that a ring or both rings of GroEL are occupied by dMDH in our experimental conditions. However, dMDH is not shown in Fig. 1A because we could not determine whether dMDH attached to either or both of the rings in our study.
For the FRET analysis, we replaced a glutamate with a cysteine at the 315th amino acid of GroEL, which is located at the apical domain near the site of GroES and substrate binding (12), and one cysteine residue was added to the C terminus of GroES as described previously (12, 14, 17). In a previous study, three endogenous cysteine residues present in GroEL were replaced with alanine for site-specific labeling of fluorescence dye at the 315th cysteine (12, 17). However, this mutant is known to show a 30–40% reduction of ATPase activity when compared with the wild-type GroEL (wtEL) (12). Therefore, we constructed EL315 without replacing the endogenous cysteines. These mutants showed no reduction in ATPase activity and refolding activity in the presence of GroES (supplemental Figs. S1 and S2). These results indicated that EL315 and ES98C exhibit similar behavior to wtEL and wtES, respectively. Subsequently, a donor chromophore, Cy3 or TMR, was attached to the cysteines of EL315, and an acceptor chromophore, Cy5, was attached to the cysteines of ES98C. We confirmed that wtEL was less labeled with TMR-maleimide than EL315 (supplemental Fig. S3); therefore, we concluded that the 315th cysteine was labeled prior to intrinsic cysteines in GroEL. Fluorescent-labeled EL315 and ES98C also exhibit similar behavior to wtEL and wtES, respectively (supplemental Figs. S1 and S2). The association between acceptor-labeled ES98C and donor-labeled EL315 could be monitored as a decrease in donor fluorescence due to FRET (Fig. 1B). FRET efficiency was calculated using Equation 1 described under “Experimental Procedures.”
First, we monitored the GroEL-GroES interaction in the presence of BeFx to examine whether we could estimate the ratio of bound GroES to GroEL by FRET assay. BeFx, which is an inorganic phosphate analog, can mimic the phosphate of enzyme-bound nucleotides and stabilize transient complexes in nucleotide metabolization (18). In the case of GroEL, symmetric football-shaped complex is formed in the presence of ATP and BeFx (referred to as ATP + BeFx) (19). On the other hand, asymmetric bullet-shaped complex is formed in the presence of ADP and BeFx (referred to as ADP + BeFx) (19). BeFx is required to obtain stable bullet-shaped complex in the presence of substrate proteins (13, 19). Increasing concentrations of Cy5-labeled ES98C (Cy5-ES98C) or wtES were added to a solution containing 500 nm Cy3-labeled EL315 (Cy3-EL315), 2 μm dMDH, 1 mm ATP and BeFx (1 mm BeSO4 and 10 mm NaF), and then the FRET efficiency was calculated. We found that the FRET efficiency was saturated when a 2-fold amount of GroES was mixed with GroEL (Fig. 1C, closed diamonds). In contrast, in the presence of ADP + BeFx, the FRET efficiency was saturated when an equal amount of GroES was mixed with GroEL (Fig. 1C, closed triangles). These results agree with the previous report that GroEL forms a football-shaped or bullet-shaped complex in the presence of ATP + BeFx, or ADP + BeFx, respectively (19). In the absence of nucleotide, the apparent FRET efficiency increased slightly as the GroES concentration increased (Fig. 1C, open triangles). This may have been due to a reduction of donor fluorescence due to the inner filter effect of acceptors in a solution. In summary, these results show that we can determine the binding stoichiometry of GroEL and GroES using this FRET assay.
Subsequently, we observed a change in FRET efficiency in the presence of 1 mm ATP. We found that the value of FRET efficiency was saturated between those in the presence of ADP + BeFx (∼0.40) and ATP + BeFx (∼0.70) (Fig. 1C, closed circles). Based on the FRET efficiency, about 40% of the GroEL-GroES complexes were football-shaped complexes in the solution. To make sure that the change in FRET efficiency was due to the binding of GroEL and GroES and not the conformational change of GroEL, we performed fluorescence correlation spectroscopy analysis and determined the ratio of bound GroES to GroEL (supplemental text). As a result, we also determined that about half of the GroEL-GroES complexes were football-shaped complexes in the presence of ATP (supplemental text and Fig. S4).
Football-shaped Complexes Gradually Disappeared as ATP Was Hydrolyzed by GroEL—Next, we examined the time course of FRET efficiency in response to ATP hydrolysis by GroEL. We found that the FRET efficiency gradually decreased in a time-dependent manner (Fig. 2A, closed circles). About 20 min after the addition of 200 μm ATP, the FRET efficiency decreased to the same level as in the solution containing ADP and BeFx (Fig. 2A, closed triangles). The concentration of ATP and ADP were respectively about 160 and 40 μm at 20 min after the reaction (the rate constant for hydrolysis of the seven ATPs in the heptemeric ring was ∼0.1 s–1, supplemental Fig. S1). Subsequently, we carried out a similar experiment in the presence of an ATP regeneration system (5 mm phosphoenolpyruvate and 10 μg/ml pyruvate kinase) (Fig. 2A, open circles). The FRET efficiency remained nearly constant throughout the measurement and was intermediate between the FRET efficiencies in solutions containing ATP + BeFx (Fig. 2A, closed diamonds) and ADP + BeFx (Fig. 2A, closed triangles). From these results, we concluded that the football-shaped complexes disappeared as ATP was hydrolyzed by GroEL, and eventually, only bullet-shaped complexes were present in the reaction cycle.
ADP Accumulation, Not ATP Consumption, Decreased the Ratio of Football-shaped Complexes—There are two possible reasons for the disappearance of football-shaped complexes upon ATP hydrolysis, ATP consumption or ADP accumulation. First, we carried out a FRET assay at various concentrations of ATP to examine how great a concentration of ATP was required to form bullet-shaped complexes and football-shaped complexes. This assay was carried out in the presence of an ATP regeneration system to keep the ATP concentration constant. The FRET efficiencies were plotted as a function of ATP concentrations (Fig. 2B). Interestingly, the curve showed two plateau phases (first phase: 5–10 μm; second phase: >50 μm). We found that the FRET efficiency of the bullet-shaped complexes (0.45 ± 0.011, mean ± S.E., n = 3) (Fig. 2B, broken line) was very similar to that of the first plateau phase, suggesting that only bullet-shaped complexes were formed at low concentrations of ATP (<10 μm). On the other hand, the FRET efficiency of football-shaped complexes was determined to be 0.82 ± 0.0014 (mean ± S.E., n = 3), which was measured in the presence of ATP + BeFx (Fig. 2B, dotted line). The second plateau phase reflected the coexistence of bullet-shaped and football-shaped complexes in the solution. This result indicated that about 50 μm ATP is sufficient for GroEL and GroES to form a saturated amount of football-shaped complexes in solution. We concluded that the disappearance of football-shaped complexes in Fig. 2A is not due to ATP consumption because about 160 μm ATP remained in the solution even after 20 min of reaction. One possible reason for the multiphasic nature of FRET response is that ATP binds to the cis-ring of GroEL at low concentrations (5–10 μm) of ATP and to the the trans-ring at high concentrations (>50 μm) of ATP.
Next, we examined whether the presence of ADP affected the amount of football-shaped complexes in solution. A FRET assay at various concentrations of ATP was carried out in the presence of a fixed concentration of ADP. ADP was mixed with ATP before addition to the solution. We found that the ATP concentrations required to form half of the saturated amount of the football-shaped complexes were 290, 450, and 670 μm when 50, 100, and 150 μm ADP was present in solution, respectively (Fig. 2C). That is, the presence of ADP at a concentration ∼⅕ of the concentration of ATP decreases the ratio of football-shaped complexes to bullet-shaped complexes by half. From these results, we concluded that the disappearance of football-shaped complexes by ATP hydrolysis of GroEL was due not to ATP consumption but to ADP accumulation.
Binding of the Second GroES to the Trans-ring of the Bullet-shaped Complex Was Facilitated by ATP but Inhibited by ADP—To clarify the mechanism by which ADP prevents the formation of a football-shaped complex, we compared the association rate of the second GroES with the trans-ring of the bullet-shaped complex preformed by ADP + BeFx in the presence or absence of ADP (Fig. 3A). In the absence of ADP, the FRET efficiency increased as soon as ATP was added to the reaction mixture (Fig. 3B, black line). This result suggested that ATP bound to the trans-ring of the bullet-shaped complex preformed by ADP + BeFx, and the second GroES could also bind to the trans-ring. In contrast, when the bullet-shaped complex was preincubated with ADP, the addition of ATP induced no change in FRET efficiency (Fig. 3B, gray line). This result indicated that ADP inhibited the association of the second GroES with the trans-ring of the bullet-shaped complex, presumably due to binding of ADP to the trans-ring to inhibit binding of ATP.
An ATP Hydrolysis-defective Mutant, EL398, Formed a Football-shaped Complex in the Presence of ATP—It is widely known that inorganic phosphate analog BeFx mimics γ-phosphate in ATP prior to hydrolysis, and thus the structure of bullet-shaped complex formed in the presence of ADP + BeFx is considered to be similar to that of the ATP-bound bullet-shaped complex (18, 20). Therefore, we assumed that the second GroES can associate with the trans-ring of the ATP-bound bullet-shaped complex. To assess this hypothesis, we examined the behavior of an ATP hydrolysis-defective mutant, EL398, in the presence of ATP. EL398 has normal affinity for ATP but low ATPase activity corresponding to about 2% of that for wtEL (15). Upon binding of ATP, EL398 undergoes conformational change in the manner of wtEL to bind GroES with high affinity (15). First, we carried out a FRET assay using 500 nm Cy3-labeled EL315/398 and various concentrations of GroES (wtES or Cy5-ES98C). As in the case of Cy3-EL315 (Fig. 1C, closed triangles), FRET efficiency was saturated when an equal molar concentration of GroES was mixed with Cy3-EL315/398 in the presence of ADP and BeFx (Fig. 4A, closed triangles). This indicated that EL315/398, like EL315, formed a bullet-shaped complex in the presence of ADP + BeFx. In the presence of ATP, the FRET efficiency was saturated when a 2-fold molar ratio of GroES was mixed with Cy3-EL315/398 (Fig. 4A, closed circles). The result was quite different from that of Cy3-EL315 in the presence of ATP (Fig. 1C, closed circles) but similar to that of Cy3-EL315 in the presence of ATP + BeFx (Fig. 1C, closed diamonds).
Next, we observed EL398-GroES complexes formed in the presence of ATP using electron microscopy. We found that ∼86% (84 out of 98) of the identified side views of GroEL-GroES complexes were football-shaped complexes (Fig. 4B). From these results, we concluded that EL398 forms a football-shaped complex in the presence of ATP and that the second GroES associates with the trans-ring of the ATP-bound bullet-shaped complex.
To confirm that both rings of a football-shaped EL398-GroES complex were occupied with ATP, we examined the quantity of nucleotides bound to a football-shaped EL398-GroES complex (Table 1). The total number of nucleotide-binding sites of EL398 was estimated to be 14 (seven binding sites per ring). The EL398-GroES complex formed in the presence of ADP and BeFx contained 6.9 mol of ADP/mol of EL398 (Table 1, row 1), which is consistent with the amount of nucleotides bound to the one ring of GroEL. On the other hand, the EL398-GroES complex formed by ATP contained 11 mol of ATP/mol of EL398 (Table 1, row 2). 1.2 mol/mol of ADP was generated during the preparation for the HPLC analysis due to the hydrolysis of ATP by ATPase activity of EL398 or by the addition of perchloric acid. The reason why the sum of ATP and ADP (12 mol/mol) was smaller than the stoichiometric amount of 14 mol/mol is considered as follows. First, one of the GroES dissociated from a ring of EL398 football-shaped complex upon hydrolysis of ATP. Subsequently, ADP dissociated from the ring that was free from GroES. In fact, it has been reported that the affinity of nucleotide for GroEL decreases in the absence of GroES (14, 21). These results indicate that a EL398 football-shaped complex is formed when both rings of GroEL are occupied with ATP. In conclusion, binding of ATP to both rings of GroEL results in the formation of a football-shaped complex (Fig. 5, upper part).
TABLE 1.
Quantitation of nucleotides bound to the EL398-GroES complex
Data represent the mean ± S.E. (n = 3, independent experiments).
| Conditions | ATP/EL398 | ADP/EL398 |
|---|---|---|
| mol/mol | mol/mol | |
| ADP + BeFx | NDa | 6.9 ± 0.81 |
| ATP | 11 ± 0.51 | 1.2 ± 0.13 |
| ATP → ADPb | 10 ± 0.40 | 1.7 ± 0.081 |
| ATP + ADPc | 7.2 ± 0.80 | 2.8 ± 0.13 |
ND, not detected.
ATP → ADP: 1 mm ATP was added to the solution prior to 1 mm ADP.
ATP + ADP: 1 mm ATP and 1 mm ADP was added to the solution simultaneously.
FIGURE 5.
Schematic model for the reaction mechanism of GroEL and GroES. A football-shaped complex is formed when both rings of GroEL are occupied with ATP. However, in the presence of ADP, ADP inhibits the association of ATP with the trans-ring of bullet-shaped complex, and as a result, the second GroES does not bind to the trans-ring of GroEL. Unfolded protein substrates are not included in this figure because we could not determine whether dMDH attached to the cis-ring or trans-ring or both rings in our study.
ADP Prevents the Association of ATP to the Trans-ring of EL398 and Suppresses the Formation of Football-shaped Complexes—Subsequently, we examined the effect of ADP on the formation of football-shaped EL398-GroES complexes. First, we added 1 mm ADP to the solution 1 min after the addition of 1 mm ATP. The FRET efficiency did not show any difference when compared with that of the solution to which 1 mm
ADP was not added (Fig. 4C, compare columns 3 and 4). This indicated that ADP has no effect on the football-shaped EL398-GroES complexes preformed by ATP. Next, we added 1 mm ATP and 1 mm ADP to the solution simultaneously. Interestingly, the FRET efficiency was very similar to that of the bullet-shaped complex formed in the presence of 1 mm ADP + BeFx (Fig. 4C, compare columns 2 and 5). Subsequently, we carried out a similar experiment using a single-ring E315C/D398A GroEL mutant (SR1/315/398). ADP did not have any effect on the SR1/315/398-GroES complex (data not shown). Together with a previous report in which the association rate of GroEL and GroES in the presence of ATP (7.5×107 m–1 s–1) was much higher than that in the presence of ADP (4.9×105 m–1 s–1) (13), ADP does not affect the association of the first GroES. Therefore, ADP appears to inhibit the binding of ATP not to the cis-ring but to the trans-ring of GroEL.
To examine the effect of ADP on the binding of ATP to the trans-ring of GroEL more precisely, we measured the amounts of nucleotides bound to an EL398-GroES complex using HPLC analysis. First, 1 mm ATP was added to the solution 1 min prior to the addition of 1 mm ADP. In this condition, the EL398-GroES complex contained 10 mol of ATP and 1.7 mol of ADP/mol of EL398 (Table 1, row 3). The substoichiometric amount of ADP was ascribed to the hydrolysis of ATP because this value was similar to that shown in row 2. This result indicates that ADP does not dissociate bound ATP from the preformed EL398 football-shaped complex. In contrast, the EL398-GroES complex formed by the simultaneous addition of 1 mm ATP and ADP contained 7.2 mol of ATP and 2.8 mol of ADP/mol of EL398 (Table 1, row 4). This stoichiometric binding (7 mol/mol) of ATP to EL398 suggests that the cis-ring is occupied by ATP. Furthermore, this assumption is supported by a previous report showing that the formation rate of the cis-ring in the presence of ATP (7.5×107 m–1 s–1) was much higher than that in the presence of ADP (4.9×105 m–1 s–1) (13). On the other hand, it is expected that the trans-ring of EL398 is occupied with ADP. During preparation for the HPLC analysis, about 4 mol/mol of ADP dissociated from the trans-ring because of the weak affinity between nucleotides and the GroEL ring that was free from GroES (14, 21). These results suggest that ADP binds to the trans-ring and prevents ATP from binding to the trans-ring of GroEL. As a result, the second GroES was not able to bind to the trans-ring of GroEL (Fig. 5, lower part).
DISCUSSION
One of the controversial issues about the mechanism of GroEL-GroES interaction is the stoichiometry of the complexes involved. The current model of GroEL-mediated protein folding suggests that only asymmetric bullet-shaped complexes are of functional importance (12). Symmetric football-shaped complexes are regarded to occur under nonphysiological conditions or as transient facultative intermediates (6). In this report, we showed by directly measuring FRET between GroEL and GroES that about half of the GroEL form football-shaped complexes during the chaperonin cycle. This result supports the conclusions obtained by chemical cross-linking and electron microscopy (10, 11) or by the use of a hydrophobic fluorescence probe (22). Fluorescence anisotropy of GroES was measured for the real-time determination of the ratio of bullet-shaped and football-shaped complexes without chemical cross-linking (23). Although this method revealed the presence of football-shaped complexes, the experiments were performed in the absence of substrate proteins. Our study was thus the first to show that football-shaped and bullet-shaped complexes coexist in the functional chaperonin cycle without chemical cross-linking at physiological ionic strength and in the presence of denatured protein and ATP. Recently, Inobe et al. (7) reported that football-shaped complexes have not been detected by small angle x-ray scattering study under their experimental conditions. The discrepancy will be ascribed to the absence of denatured protein (supplemental text and Fig. S5) and accumulation of ADP (Fig. 2C) during the measurement.
We also showed that ADP acts as an inhibitor of the formation of a football-shaped complex during the chaperonin reaction cycle. This result supports the previous study using chemical cross-linking and electron microscopy (11) but also argues that ADP inhibition is about 5-fold greater than reported previously. It has been suggested that ADP prevents the ATP hydrolysis by GroEL (24), and a kinetic study of the conformational change in GroEL suggested that ADP prevents the association of ATP with the trans-ring of GroEL (25). We showed in this study that ADP-induced inhibition of the association of ATP with the trans-ring has great influence on the association of the second GroES with the trans-ring of GroEL (Fig. 3). Considering the ratio of GroES (5.1 μm)/GroEL (2.6 μm) (26) and ADP (1.04 mm)/ATP (7.90 mm) (27) in E. coli, football-shaped complexes are expected to exist in vivo (Fig. 5).
We showed that an ATP hydrolysis-defective mutant, EL398, forms a football-shaped complex in the presence of ATP. This result was surprising because EL398 has been previously thought to form an asymmetric bullet-shaped complex in the presence of ATP. Therefore, EL398 was commonly used to characterize the ATP-bound bullet-shaped complex (12, 28, 29). In these studies, GroES was considered not to bind to the trans-ring of the bullet-shaped complex (12, 28). As a result, studies of EL398-GroES complexes have often been carried out in a solution containing equal amounts of EL398 and GroES. For instance, a study using cryoelectron microscopy reported that 10–20% of football-shaped complexes were observed even under this experimental condition (28). It would not be surprising if a large population of EL398 formed football-shaped complexes in the presence of a GroES to EL398 ratio of 2:1 or higher (Fig. 4).
EL398 in which both rings were occupied by ATP formed football-shaped complexes (Table 1). However, it remains an open question whether football-shaped EL398 complexes of which two rings are occupied by ATP mimic the football-shaped wtEL complexes in the functional reaction cycle. Kinetic studies of ATPase activity and conformational change of GroEL using a series of different ATP concentrations have shown that ATP can bind to both rings of GroEL simultaneously at a concentration high enough to overcome the negative cooperativity between the rings of GroEL (30, 31). Therefore, it seems reasonable to assume that a football-shaped complex is formed when both rings are occupied by ATP. On the other hand, Gorovits et al. (23) reported that a football-shaped complex is favored by an asymmetric distribution of nucleotides; that is, when one ring is occupied by AMP-PNP, the other ring favors ADP and vice versa (23). The possibility still remains that when one ring of a football-shaped complex is occupied by ATP, the other ring is occupied by ADP-Pi or ADP during the functional reaction cycle.
Todd et al. (24) proposed that the football-shaped complex precedes the rate-limiting step in the chaperonin cycle. Recently, two rate-limiting steps were reported in the chaperonin reaction cycle (32–34). The ATP hydrolysis by GroEL was reported to precede the second rate-limiting step. Further studies will be required to determine the nucleotide state of football-shaped complexes during the functional reaction cycle and to figure out the relationship between these rate-limiting steps and formation of football or bullet-shaped complexes.
In this study, we showed the coexistence of football-shaped and bullet-shaped complexes under physiological conditions, suggesting that we should reconsider the protein-folding mechanism that involves the football-shaped complex. What is the physiological significance of the football-shaped complex? A correlation between the formation of a football-shaped complex and protein-folding activity has been reported using MDH or a mutant of maltose-binding protein (11, 35). These results suggest that binding of two substrate proteins established an efficient flip-flop mechanism of binding, i.e. release in the cavity and ejection into solution, with the two rings of GroEL being simultaneously active in protein folding (35).
It has been accepted that group II chaperonins found in archaea (thermosome) and eukaryotic cells (tailless complex polypeptide 1 (TCP-1) ring complex, TRiC; chaperonin containing TCP-1, CCT) form symmetric structure in the reaction cycle (20, 36). Our study indicated that group I chaperonins (GroEL) form symmetric football-shaped complexes in the reaction cycle. The similarity between group I and group II chaperonins revealed in this study will become a key to elucidation of the common principle that governs chaperonins of all species.
Supplementary Material
Acknowledgments
We thank Dr. Tomofumi Santa for the help with the HPLC analysis.
This work was supported by a grant from the Mitsubishi Foundation (to T. F.) and by Grant-in-Aid for Scientific Research (A) 17201031 (to T. F.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental text and five supplemental figures.
This article was selected as a Paper of the Week.
Footnotes
The abbreviations used are: FRET, fluorescence resonance energy transfer; EL398, D398A GroEL mutant; EL315, E315C GroEL mutant; ES98C, GroES mutant with a single cysteine added at the C terminus; EL315/398, E315C/D398AGroELmutant; SR1/315/398, a single-ring E315C/D398A GroEL mutant; MDH, malate dehydrogenase; dMDH, denatured MDH; TMR, tetramethylrhodamine; wtES, wild-type GroES; wtEL, wild-type GroEL; BeFx, beryllium fluoride; BSA, bovine serum albumin; HPLC, high pressure liquid chromatography; AMP-PNP, 5′-adenylyl-β,γ-imidodiphosphate; DTT, dithiothreitol.
References
- 1.Bukau, B., and Horwich, A. L. (1998) Cell 92 351–366 [DOI] [PubMed] [Google Scholar]
- 2.Hartl, F. U., and Hayer-Hartl, M. (2002) Science 295 1852–1858 [DOI] [PubMed] [Google Scholar]
- 3.Horwich, A. L., Farr, G. W., and Fenton, W. A. (2006) Chem. Rev. 106 1917–1930 [DOI] [PubMed] [Google Scholar]
- 4.Walter, S. (2002) CMLS Cell Mol. Life Sci. 59 1589–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayer-Hartl, M. K., Martin, J., and Hartl, F. U. (1995) Science 269 836–841 [DOI] [PubMed] [Google Scholar]
- 6.Engel, A., Hayer-Hartl, M. K., Goldie, K. N., Pfeifer, G., Hegerl, R., Muller, S., da Silva, A. C., Baumeister, W., and Hartl, F. U. (1995) Science 269 832–836 [DOI] [PubMed] [Google Scholar]
- 7.Inobe, T., Takahashi, K., Maki, K., Enoki, S., Kamagata, K., Kadooka, A., Arai, M., and Kuwajima, K. (2008) Biophys. J. 94 1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beissinger, M., Rutkat, K., and Buchner, J. (1999) J. Mol. Biol. 289 1075–1092 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt, M., Rutkat, K., Rachel, R., Pfeifer, G., Jaenicke, R., Viitanen, P., Lorimer, G., and Buchner, J. (1994) Science 265 656–659 [DOI] [PubMed] [Google Scholar]
- 10.Azem, A., Kessel, M., and Goloubinoff, P. (1994) Science 265 653–656 [DOI] [PubMed] [Google Scholar]
- 11.Azem, A., Diamant, S., Kessel, M., Weiss, C., and Goloubinoff, P. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 12021–12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rye, H. S., Roseman, A. M., Chen, S., Furtak, K., Fenton, W. A., Saibil, H. R., and Horwich, A. L. (1999) Cell 97 325–338 [DOI] [PubMed] [Google Scholar]
- 13.Motojima, F., and Yoshida, M. (2003) J. Biol. Chem. 278 26648–26654 [DOI] [PubMed] [Google Scholar]
- 14.Murai, N., Makino, Y., and Yoshida, M. (1996) J. Biol. Chem. 271 28229–28234 [DOI] [PubMed] [Google Scholar]
- 15.Rye, H. S., Burston, S. G., Fenton, W. A., Beechem, J. M., Xu, Z., Sigler, P. B., and Horwich, A. L. (1997) Nature 388 792–798 [DOI] [PubMed] [Google Scholar]
- 16.Motojima, F., Makio, T., Aoki, K., Makino, Y., Kuwajima, K., and Yoshida, M. (2000) Biochem. Biophys. Res. Commun. 267 842–849 [DOI] [PubMed] [Google Scholar]
- 17.Rye, H. S. (2001) Methods (Amst.) 24 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chabre, M. (1990) Trends Biochem. Sci. 15 6–10 [DOI] [PubMed] [Google Scholar]
- 19.Taguchi, H., Tsukuda, K., Motojima, F., Koike-Takeshita, A., and Yoshida, M. (2004) J. Biol. Chem. 279 45737–45743 [DOI] [PubMed] [Google Scholar]
- 20.Meyer, A. S., Gillespie, J. R., Walther, D., Millet, I. S., Doniach, S., and Frydman, J. (2003) Cell 113 369–381 [DOI] [PubMed] [Google Scholar]
- 21.Bochkareva, E. S., Lissin, N. M., Flynn, G. C., Rothman, J. E., and Girshovich, A. S. (1992) J. Biol. Chem. 267 6796–6800 [PubMed] [Google Scholar]
- 22.Torok, Z., Vigh, L., and Goloubinoff, P. (1996) J. Biol. Chem. 271 16180–16186 [DOI] [PubMed] [Google Scholar]
- 23.Gorovits, B. M., Ybarra, J., Seale, J. W., and Horowitz, P. M. (1997) J. Biol. Chem. 272 26999–27004 [DOI] [PubMed] [Google Scholar]
- 24.Todd, M. J., Viitanen, P. V., and Lorimer, G. H. (1994) Science 265 659–666 [DOI] [PubMed] [Google Scholar]
- 25.Cliff, M. J., Kad, N. M., Hay, N., Lund, P. A., Webb, M. R., Burston, S. G., and Clarke, A. R. (1999) J. Mol. Biol. 293 667–684 [DOI] [PubMed] [Google Scholar]
- 26.Lorimer, G. H. (1996) FASEB. J. 10 5–9 [DOI] [PubMed] [Google Scholar]
- 27.Lehninger, A. L., Nelson, D. L., and Cox, M. M. (eds) (1993) Principles of Biochemistry: Principle of Bioenergetics, 2nd Edition, pp. 364–399, Worth Publishers, New York
- 28.Ranson, N. A., Clare, D. K., Farr, G. W., Houldershaw, D., Horwich, A. L., and Saibil, H. R. (2006) Nat. Struct. Mol. Biol. 13 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranson, N. A., Farr, G. W., Roseman, A. M., Gowen, B., Fenton, W. A., Horwich, A. L., and Saibil, H. R. (2001) Cell 107 869–879 [DOI] [PubMed] [Google Scholar]
- 30.Danziger, O., Rivenzon-Segal, D., Wolf, S. G., and Horovitz, A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13797–13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yifrach, O., and Horovitz, A. (1995) Biochemistry 34 5303–5308 [DOI] [PubMed] [Google Scholar]
- 32.Ueno, T., Taguchi, H., Tadakuma, H., Yoshida, M., and Funatsu, T. (2004) Mol. Cell 14 423–434 [DOI] [PubMed] [Google Scholar]
- 33.Taguchi, H., Ueno, T., Tadakuma, H., Yoshida, M., and Funatsu, T. (2001) Nat. Biotechnol. 19 861–865 [DOI] [PubMed] [Google Scholar]
- 34.Yokokawa, M., Wada, C., Ando, T., Sakai, N., Yagi, A., Yoshimura, S. H., and Takeyasu, K. (2006) EMBO J. 25 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparrer, H., Rutkat, K., and Buchner, J. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iizuka, R., Yoshida, T., Ishii, N., Zako, T., Takahashi, K., Maki, K., Inobe, T., Kuwajima, K., and Yohda, M. (2005) J. Biol. Chem. 280 40375–40383 [DOI] [PubMed] [Google Scholar]
- 37.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265–275 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.