Abstract
The insulin-like growth factor-1 receptor (IGF-1R) and ErbB family of receptors are receptor tyrosine kinases that play important roles in cancer. Lack of response and resistance to therapies targeting ErbB receptors occur and are often associated with activation of the IGF-1R pathway. Combinations of agents that inhibit IGF-1R and ErbB receptors have been shown to synergistically block cancer cell proliferation and xenograft tumor growth. To determine the mechanism by which targeting both IGF-1R and ErbB receptors causes synergistic effects on cell growth and survival, we investigated the effects of combinations of selective IGF-1R and ErbB kinase inhibitors on proliferative and apoptotic signaling. We identified A431 squamous cell carcinoma cells as most sensitive to combinations of ErbB and IGF-1R inhibitors. The inhibitor combinations resulted in not only blockade of A431 cell proliferation, but also induced apoptosis, which was not seen with either agent alone. Upon examining phosphorylation states and expression levels of proteins in the IGF-1R and ErbB signaling pathways, we found a correlation between the ability of combinations to inhibit proliferation and to decrease levels of phosphorylated Akt and cyclin D1. In addition, the massive cell death induced by combined IGF-1R/ErbB inhibition was associated with Mcl-1 reduction and Bax activation. Thus, targeting both IGF-1R and ErbB receptors simultaneously results in cell cycle arrest and apoptosis through combined effects on Akt, cyclin D1, and Bax activation.
IGF-1R2 function is important for cellular processes that are activated in cancer cells, including cell proliferation, survival, metastasis, and invasion (1–4). Transformation of cells by several oncogenes has been shown to require IGF-1R function (2, 5), and anchorage-independent growth (3) and survival of cancer cells in response to cellular stress (5) can both be mediated by the IGF-1R. IGF-1R stimulates cell proliferation and survival through activation of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) and Akt pathways. Upon binding of IGF-1 or IGF-2 to the IGF-1R, the receptor becomes autophosphorylated on several tyrosine residues. These phosphotyrosines serve as binding sites for adaptor proteins, including insulin receptor substrate (IRS)-1–4 and Shc, which are then phosphorylated by the activated receptor. Phosphorylated IRS and Shc in turn recruit Grb2/SOS, which leads to activation of the MAPK pathway, and the p85 subunit of phosphatidylinositol 3-kinase, resulting in phosphatidylinositol 3,4,5-trisphosphate production and Akt activation (3, 6, 7). The MAPK and Akt pathways regulate levels of cell cycle proteins like cyclin D1 and p27 to cause increased cell proliferation (8) and decrease apoptosis by phosphorylating the proapoptotic protein, Bad, which results in its sequestration by 14-3-3 (9). Antibodies that target IGF-1R and one small molecule IGF-1R inhibitor are undergoing clinical trials, and several other IGF-1R small molecule inhibitors are in preclinical development (1, 4). Blockade of IGF-1R function by these inhibitors or through the use of antisense oligonucleotides or dominant negative forms of the receptor have been shown to block proliferation of many cancer cell lines, including breast, ovarian, prostate, lung, pancreatic, colon, multiple myeloma, and neuroblastoma cells (1, 5, 10, 11), as well as growth of tumors in mouse models (1–3, 12).
The ErbB family of receptor tyrosine kinases has also been shown to have important roles in cancer. The epidermal growth factor receptor (EGFR/ErbB1) and ErbB2 are overexpressed in many different types of cancer (13, 14), and activating mutations of EGFR have been identified in non-small cell lung cancer (NSCLC) and glioma (14). EGFR is also activated in several tumor types by the autocrine expression of its ligands (13–15). In 25–30% of breast cancers, ErbB2 is overexpressed, usually because of gene amplification (13). ErbB2 does not bind ligand and is activated by either homodimerization or heterodimerization with other ErbB family members, and overexpression of ErbB2 leads to constitutive activation of the receptor (13, 15). Activation of either EGFR or ErbB2 signaling results in proliferation, protection from cell death, migration, and invasion, which all have important roles in tumor formation and metastasis (13). Similar to IGF-1R, ErbB family receptors activate the Akt and MAPK pathways to cause cell proliferation and survival. Growth of cancer cells that depend on ErbB receptor signaling for these processes is inhibited by anti-ErbB therapies (15).
Considerable cross-talk occurs between the IGF-1R and ErbB receptors. As mentioned above, both receptors initiate signaling cascades that result in MAPK and Akt activation. In addition, IGF-1 treatment of breast cancer and COS7 cells leads to EGFR phosphorylation (16, 17). In other studies, amphiregulin activated IGF-1R phosphorylation in NSCLC cell lines (18), and epidermal growth factor treatment of the EGFR-positive breast cancer cell lines BT-20, MDA-MB-468, and T47D caused induction of IRS-1 and IRS-2 levels and resulted in potentiation of IGF-1-induced signaling in MDA-MB-468 cells (19). Increased IGF-1R signaling has also been implicated in resistance of NSCLC, breast cancer, and prostate cancer cells to the EGFR inhibitors, erlotinib and gefitinib (20, 21), and of breast cancer cells to the anti-ErbB2 antibody, trastuzumab (22). One mechanism of resistance to EGFR and ErbB2 inhibitors is increased levels of activated Akt even in the presence of ErbB inhibitors. In addition, it was recently shown that IGF-1R and ErbB2 co-immunoprecipitate in trastuzumab-resistant SKBR3 cells but not in the parental cells, and IGF-1 stimulation leads to phosphorylation of ErbB2 in the resistant cells (23). Similarly, IGF-1R was found in EGFR immunoprecipitates from NSCLC cells that were resistant to erlotinib and gefitinib (20, 24). Thus, inhibition of the IGF-axis may have clinical utility in the treatment of cancer patients who have become refractory to ErbB family receptor inhibitors.
Targeting both IGF-1R and ErbB receptors with dominant negative constructs, antibodies, or small molecule inhibitors has shown improved effects on inhibition of cell growth relative to single agents. In MCF7 cells overexpressing ErbB2, synergistic inhibition of cell growth was seen by the combination of trastuzumab with induction of a dominant negative form of IGF-1R (10). Combinations of anti-EGFR and anti-IGF-1R antibodies or a bispecific antibody that targeted both receptors exhibited significantly increased inhibition of BxPC3 cell proliferation compared with the individual antibodies (25). Combinations of IGF-1R and EGFR inhibitory antibodies have also shown improved activity in A549 xenografts relative to individual agents (26). In addition, a nonselective IGF-1R kinase inhibitor, AG1024, enhanced the ability of gefitinib or erlotinib to block proliferation and induce apoptosis of breast cancer (27), hepatoma (28), and NSCLC cell lines (20, 24).
Our goal in this study was to expand on the previous results seen with inhibition of IGF-1R and EGFR by determining the signaling mechanisms responsible for the combination effects of small molecule IGF-1R and ErbB receptor inhibitors on tumor cell growth and apoptosis. Thus, we examined the effects of combinations of known small molecule IGF-1R and ErbB kinase inhibitors on cell proliferation and apoptosis of cancer cell lines. The combination of the highly selective IGF-1R inhibitor, NVP-ADW742 (11), and gefitinib, which selectively blocks ErbB kinases, caused synergistic inhibition of cell proliferation and induction of apoptosis in A431 cells. The synergistic effects on inhibition of proliferation corresponded with a complete blockade of Akt phosphorylation and a decrease in cyclin D1 levels. Conversely, the increase in apoptosis was associated with decreased levels of the antiapoptotic protein, Mcl-1, as well as activation of the proapoptotic proteins Bax and caspase-3, suggesting the involvement of the intrinsic cell death pathway. Our data suggest that inhibiting both the IGF-1R and ErbB kinases may provide additional therapeutic efficacy, because blockade of both pathways results in complete inhibition of Akt-mediated proliferative and survival pathways.
EXPERIMENTAL PROCEDURES
Reagents and Cell Culture—Antibodies used included rabbit anti-EGFR Tyr(P)1068, mouse anti-pan-EGFR, rabbit anti-ERK1/2 Thr(P)185/Tyr(P)187 (BIOSOURCE, Camarillo, CA), rabbit anti-IGF-1Rβ, rabbit anti-cyclin D1, rabbit anti-p16, mouse anti-Bcl-2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-phospho-IGF-1Rβ (Tyr1135/1136)/IRβ (Tyr1150/1151), rabbit anti-phospho-Akt (Ser473), rabbit anti-Akt, rabbit anti-ERK1/2, rabbit anti-phospho-Rb (Ser807/811), mouse anti-Rb, rabbit anti-Bax (Cell Signaling Technology, Inc., Beverly, MA), mouse anti-Bax 6A7, mouse anti-actin (Sigma), mouse anti-p27, mouse anti-Bcl-XL (BD Transduction Laboratories), rabbit anti-Mcl-1, rabbit anti-Bim, and rabbit anti-Bcl-XL (Epitomics, Burlingame, CA). Gefitinib and NVP-ADW742 were prepared using published synthesis schemes (29, 30). pBABE-myr-Akt-puro and pBABE-puro constructs were kindly provided by Jameel Shah. A431 cells were obtained from ATCC (Manassas, VA). A431 cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. A431-pBABE control and A431-myr-Akt cell lines were generated by retroviral infection of A431 cells with pBABE-puro or pBABE-myr-Akt-puro retroviruses followed by treatment with puromycin, and resistant cells were pooled.
Cell Viability, Bromodeoxyuridine (BrdUrd) Incorporation, and Caspase-3 Assays—For viability assays, cells were seeded in 96-well plates in medium containing 10% fetal bovine serum and treated with different doses of compounds or DMSO as control for 72 h before an MTS assay (CellTiter Aqueous One, Promega, Madison, WI) was performed to determine viable cell numbers. Caspase-3 activation was measured by the cleavage of the fluorometric substrate Ac-DEVD-7-amino-4-methylcoumarin (Biomol Research Laboratories, Plymouth Meeting, PA), as described previously (31), after treatment of cells with compounds for 24 h. BrdUrd incorporation was measured using a BrdUrd enzyme-linked immunoassay kit (Roche Applied Science) after treatment of cells with compounds for 24 and 48 h.
Combination Index Determination—MTS assays were used to determine inhibition of cell survival after a 72-h treatment of multiple cell lines with different ratios of gefitinib and NVP-ADW742. Combination indices for the effects of gefitinib and NVP-ADW742 on cell survival for each cell line were calculated with CalcuSyn software (Biosoft, Cambridge, UK).
Analysis of Cell Cycle and Annexin V Staining by Flow Cytometry—A431 cells were treated with compounds in growth medium containing 10% fetal bovine serum for 24 or 48 h. At the end of the compound incubation, adherent cells were trypsinized and combined with any floating cells present and then washed with cold phosphate-buffered saline. Cells were then stained with nuclear isolation and staining solution-10 (NPE Systems, Pembroke Pines, FL), and DNA content per cell was measured by flow cytometry. Analysis of cell cycle distribution was determined with the ModFit LT model fitting program. Annexin V and propidium iodide (PI) staining were performed using the BD Pharmingen™ annexin V-fluorescein isothiocyanate apoptosis detection kit I (BD Biosciences), and flow cytometry was performed.
Compound Treatment, Cell Lysis, and Immunoblotting—To analyze effects of compound combinations on signaling and protein levels downstream of IGF-1R and EGFR in A431 cells, cells were treated with compounds in growth medium containing 10% fetal bovine serum for 6 h or overnight before lysis in CHAPS buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 1% CHAPS). Samples were analyzed for levels of phosphorylated and total signaling proteins by Western blotting using the NuPAGE electrophoresis and transfer systems (Invitrogen) and enhanced chemiluminescence or near infrared detection.
Determination of Levels of Activated Bax—A431 cells were treated with compounds for 16 h and then lysed in CHAPS buffer. Active Bax was immunoprecipitated with the 6A7 monoclonal antibody (Sigma) coupled to agarose beads. Levels of Bax in whole cell lysate samples or 6A7 immunoprecipitates were determined by Western blotting with an antibody that recognizes both activated and inactivated Bax.
Co-immunoprecipitation of Bim and Mcl-1—A431 cells were treated with compounds for 16 h and then lysed in CHAPS buffer. Mcl-1 was immunoprecipitated with a biotin-labeled mouse monoclonal antibody (Lab Vision, Fremont, CA) and streptavidin-agarose. Levels of Bim and Mcl-1 in whole cell lysate samples or immunoprecipitates were determined by Western blotting with rabbit antibodies.
RESULTS
Combinations of Small Molecule IGF-1R and ErbB Inhibitors Synergistically Block Cell Proliferation and Increase Cell Death in A431 Cells—To investigate whether combinations of EGFR and IGF-1R inhibitors have improved effects on proliferation and survival in multiple types of tumor cells, we performed MTS assays on several cell lines after treatment with different doses of the EGFR kinase inhibitor, gefitinib, and NVP-ADW742, which is a highly selective small molecule inhibitor of the tyrosine kinase activity of IGF-1R (11). Combination indices at the ED50 were calculated with Calcusyn software, which uses the median effect method described by Chou and Talalay (32). As shown in Table 1, synergistic effects on cell proliferation and survival were seen in five of seven tumor lines tested, as exhibited by combination index values much lower than 1 at the ED50.
TABLE 1.
Gefitinib and NVP-ADW742 combinations synergistically decrease cell numbers in multiple cell lines
Cells were grown for 3 days in medium with 10% fetal bovine serum in the presence of a dose response of these agents alone or in combination. Live cell numbers at the end of treatment were quantified with MTS reagents.
| Cell line | Cell type | Combination index at EC50a |
|---|---|---|
| A431 | Epidermoid carcinoma | 0.33 |
| BxPC3 | Pancreatic adenocarcinoma | 0.35 |
| MIA PaCa-2 | Pancreatic carcinoma | 0.34 |
| H1299 | Non-small cell lung carcinoma | 0.86 |
| A549 | Non-small cell lung carcinoma | 0.35 |
| MDA-435LM | Ductal breast carcinoma | 0.24 |
| Calu6 | Non-small cell lung carcinoma | 0.71 |
Combination indices for decreases in cell numbers caused by combinations of gefitinib and NVP-ADW742 were calculated using CalcuSyn. A combination index of <1 indicates synergism, combination index = 1 indicates additivity, and combination index of >1 indicates antagonism. Data are the average of two independent experiments with duplicate samples.
The most dramatic phenotypic effect of the combination of gefitinib and NVP-ADW742 was seen in the A431 epidermal squamous cell carcinoma cell line. A431 cells overexpress wild-type EGFR (33, 34), and their proliferation can be decreased by treatment of the cells with gefitinib, erlotinib, and antibodies targeting the EGFR (34, 35). To further characterize the effects of IGF-1R and ErbB inhibitor combinations in A431 cells, we treated cells grown in regular medium containing 10% fetal bovine serum with increasing concentrations of gefitinib alone or in the presence of 3 μm NVP-ADW742 and analyzed effects on cell number, BrdUrd incorporation, and caspase-3 activation. We chose 3 μm NVP-ADW742 to completely block IGF-1R activity, considering the cellular IC50 for inhibition of IGF-1R phosphorylation for this compound was 0.3 μm (data not shown). Treatment with gefitinib alone did inhibit growth of A431 cells, but the maximal decrease in cell number was only about 60%, and the maximal inhibition of BrdUrd incorporation was only about 20% even at concentrations of 3 and 10 μm (Fig. 1) (data not shown). Treatment of A431 cells with 3 μm NVP-ADW742 had little effect on decreasing cell numbers and inhibiting BrdUrd incorporation. Synergistic inhibition of cell proliferation and survival as well as BrdUrd incorporation was seen with combinations of gefitinib and 3 μm NVP-ADW742, which caused a nearly 100% decrease in cell number even at 1 μm gefitinib concentrations (Fig. 1). When fluorescence-activated cell sorting analysis of the cellular DNA staining was performed on A431 cells treated for 24 h with the different compounds, 3 μm gefitinib alone was able to induce some G1 arrest (Table 2). However, the combination of 3 μm gefitinib plus 3 μm NVP-ADW742 resulted in a higher proportion of the cells arresting in the G1 phase (88% versus 66% after treatment with gefitinib alone), and the percentage of cells in S phase was dramatically decreased. In the DMSO control, the percentage of S phase cells was 38%, and treatment with 3 μm gefitinib and the combination resulted in a decrease to 28 or 4%, respectively. Treatment of cells with 3 μm gefitinib for 48 h did result in an increased percentage of cells in G1 and a decreased percentage of cells in S phase, but the combination of gefitinib and NVP-ADW742 further increased the percentage of cells undergoing G1 arrest at this time point. Apoptosis of cells treated with the combination was also observed as indicated by an increase in the sub-G0 population of cells seen at both 24 and 48 h in the fluorescence-activated cell sorting analysis (Table 2).
FIGURE 1.
Combinations of gefitinib and NVP-ADW742 synergistically inhibit proliferation and survival of and increase caspase-3 activation in A431 cells. Top, decrease in cell number after 72 h of treatment; middle, inhibition of BrdUrd incorporation after 24 h of treatment; bottom, activation of caspase-3 after 24 h of treatment of A431 cells with 1 or 3 μm gefitinib and 3 μm NVP-ADW742 alone or in combination. Caspase-3 activation data are expressed as -fold level of caspase-3 activity in the DMSO-treated samples. *, combinations of 1 μm gefitinib and 3 μm NVP-ADW742 are statistically different from single agents with p ≤ 0.002. **, combinations of 3μm gefitinib and 3μm NVP-ADW742 are statistically different from single agentswithp≤0.001.***, combinations of 1 μm gefitinib and 3μm NVP-ADW742 are statistically different from single agents with p < 0.01. Data are the average ± S.D. of duplicate samples and are representative of two or three independent experiments.
TABLE 2.
Cell cycle analysis of A431 cells treated with combinations of gefitinib and NVP-ADW742
Data are the average of two independent experiments.
|
Treatment |
Percentage of viable
cellsa |
Percentage of total
cellsb |
||
|---|---|---|---|---|
| G1 | S | G2 | Sub-G0 | |
| % | % | % | % | |
| 24 h | ||||
| None | 50.3 | 40.2 | 9.5 | 0.7 |
| DMSO | 51.7 | 38.4 | 9.9 | 0.6 |
| Gefitinib 3 μm | 65.7 | 28.0 | 6.3 | 1.6 |
| NVP-ADW742 3 μm | 59.5 | 30.5 | 10.0 | 0.6 |
|
Gefitinib + NVP-ADW742
|
87.5
|
3.5
|
9.0
|
9.9
|
| 48 h | ||||
| None | 48.4 | 42.1 | 9.5 | 0.7 |
| DMSO | 44.9 | 49.3 | 5.8 | 0.7 |
| Gefitinib 3 μm | 81.7 | 11.6 | 6.7 | 2.1 |
| NVP-ADW742 3 μm | 56.3 | 35.6 | 8.1 | 0.9 |
| Gefitinib + NVP-ADW742 | 91.1 | 3.8 | 5.0 | 25.1 |
Data are expressed as the percentage of viable cells.
Data are expressed as the percentage of the total cell count.
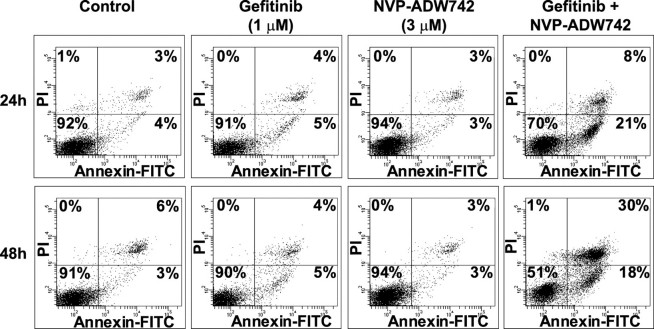
To confirm that the combinations of gefitinib and NVP-ADW742 induced apoptosis of A431 cells, we determined the effects of the compound combinations on caspase-3 activation and annexin V staining. The combinations of gefitinib and 3 μm NVP-ADW742 caused up to a 10-fold increase in caspase-3 activation relative to the DMSO-treated cells, whereas cells treated with either compound alone exhibited only a 2–3-fold increase in caspase-3 activation (Fig. 1, bottom). To quantify the effects of the compound combinations on apoptosis of A431 cells, cells treated with compounds for 24 or 48 h were stained with annexin V and PI and analyzed by flow cytometry. Annexin V binds to phosphatidylserine, which translocates from the inner leaflet to the outer leaflet of the plasma membrane in apoptotic cells, so cells that are positive for annexin V staining are undergoing apoptosis. PI staining provides a measure of cell viability and is used to distinguish between cells in early and late apoptosis. Greater than 90% of A431 cells treated for 24 or 48 h with DMSO as control were negative for both PI and annexin V (lower left quadrant) and thus viable (Fig. 2). Similar profiles were observed for cells treated with 1 μm gefitinib or 3 μm NVP-ADW742 alone. However, there was a large decrease in viable cells after treatment with the combination of gefitinib and NVP-ADW742 both at 24 and 48 h, with the percentages of viable cells decreasing to 70 and 51%, respectively. Accordingly, the number of cells in early apoptosis that are positive for annexin V but negative for PI (lower right quadrant) was increased to 20% in the cells treated with the combination at both time points. The number of cells that were positive for both annexin V and PI and undergoing late apoptosis or death (upper right quadrant) was slightly increased at 24 h and significantly increased at 48 h after the addition of the combination of gefitinib and NVP-ADW742 (Fig. 2). These data indicate that inhibition of EGFR and IGF-1R together can induce apoptosis in addition to G1 arrest of A431 cells.
FIGURE 2.
Combinations of gefitinib and NVP-ADW742 induce apoptosis of A431 cells. Shown is flow cytometry analysis of annexin V and PI staining of A431 cells treated with 1 μm gefitinib and 3 μm NVP-ADW742 alone or in combination. Cells were treated with compounds for 24 or 48 h before they were stained with annexin V-fluorescein isothiocyanate and PI. The percentages of cells in each quadrant are indicated. Data are representative of two independent experiments.
EGFR and IGF-1R Inhibitors Exhibit Combination Effects on Akt Phosphorylation and p27 and Cyclin D1 Expression—We wanted to determine mechanistically how the combinations of EGFR and IGF-1R kinase inhibitors exhibited synergistic effects on proliferation and apoptosis of A431 cells. Therefore, we investigated the effects of the inhibitor combinations on signaling downstream of both receptors. In the absence of compounds or stimulation, A431 cells exhibited easily detectable phosphorylation of EGFR, Akt, and ERK1/2, whereas phosphorylated IGF-1R was low (Fig. 3, A and B). Stimulation of the cells with IGF-1 resulted in a large increase in IGF-1R phosphorylation but had no effect on EGFR, Akt, or ERK phosphorylation (Fig. 3, A and B). Upon treatment of the cells with 1 or 3 μm gefitinib, the phosphorylation levels of EGFR were decreased, as expected (Fig. 3, A and B). In addition, levels of phosphorylated Akt and phosphorylated ERK1/2 were also decreased. Incubation of the cells with 3 μm NVP-ADW742 alone blocked IGF-1R phosphorylation and decreased phosphorylation of Akt but had no significant effect on EGFR or ERK phosphorylation (Fig. 3, A and B). Significantly, phosphorylation of Akt was completely abolished in the cells treated with the combinations of NVP-ADW742 and gefitinib, in contrast to the residual Akt phosphorylation seen following treatment with the individual IGF-1R and EGFR inhibitors (Fig. 3, A and B). However, when cells were treated with the compound combinations, phosphorylation of IGF-1R was decreased to the levels seen after treatment of the cells with NVP-ADW742 alone, whereas EGFR and ERK1/2 were decreased to the levels seen after treatment of the cells with gefitinib alone (Fig. 3, A and B).
FIGURE 3.

Effects of combinations of gefitinib and NVP-ADW742 on signaling and cell cycle proteins in A431 cells. A, levels of phosphorylated and total EGFR, IGF-1R, Akt, and ERK1/2 after a 6-h treatment of A431 cells with 1 or 3μm gefitinib and 3μm NVP-ADW742 alone or in combination followed by stimulation with 50 ng/ml IGF-1 for 10 min. Data in blots are representative of two independent experiments. B, quantification of effects of compounds on pEGFR, pIGF-1R, pAkt, and pERK from experiments performed as in A. The average ± S.E. for each analyte from densitometry measurements in the two different experiments are shown. Data for phosphorylation of EGFR, IGF-1R, Akt, and ERK were normalized to total protein for each analyte and expressed as -fold signal in the DMSO plus IGF-1 control. pEGFR levels in cells treated with the indicated compounds were statistically different from DMSO-treated cells with p < 0.02 (*). Levels of pIGF-1R in cells treated with 3 μm NVP-ADW742 alone or in combination with gefitinib were statistically different from DMSO- or gefitinib-treated cells with p < 0.001 (+). pAkt levels in cells treated with 1 μm gefitinib, 3 μm NVP-ADW742, or the combinations were statistically different from DMSO-treated cells with p < 0.005 (#), and pAkt levels in cells treated with the combinations were statistically different from levels in cells treated with the single agents with p ≤ 0.01 (▵). Levels of pERK in cells treated with gefitinib alone or in combination with NVP-ADW742 were statistically different from levels in cells treated with DMSO or NVP-ADW742 alone with p ≤ 0.002 (^). C, levels of phosphorylated and total EGFR, IGF-1R, Akt, ERK1/2, and Rb and levels of cyclin D1, p27, p16, and actin following overnight treatment of A431 cells with combinations of 1 or 3 μm gefitinib and 3 μm NVP-ADW742. Data are representative of four or five independent experiments. D, quantification of effects of compound combinations on phosphorylated Akt and Rb and total cyclin D1, p27, and p16 from experiments performed as in C. Data were normalized using phosphorylated protein/total protein for Akt and Rb and as total protein/actin for cyclin D1, p27, and p16. Data are expressed as the -fold DMSO control and are graphed as the average ± S.E. from the independent experiments. Levels of pAkt in cells treated with 1 μm gefitinib, 3 μm gefitinib, or 3 μm NVP-ADW742 were statistically different from DMSO-treated cells with p < 0.0001 (*), and pAkt levels in cells treated with combinations were statistically different from levels in cells treated with the single agents or DMSO with p ≤ 0.02 (**). Cyclin D1 levels in cells treated with both gefitinib concentrations were statistically different from DMSO-treated cells with p < 0.05 (+), and cyclin D1 levels in cells treated with the compound combinations were statistically different from levels in other treatment groups with p < 0.003 (++). Levels of p27 in cells treated with the compound combinations were statistically different from levels in other treatment groups with p < 0.05 (#). Levels of p16 in gefitinib- or combination-treated cells were statistically different from levels in DMSO or NVP-ADW742-treated cells with p < 0.05 (^). E, levels of phosphorylated and total EGFR, IGF-1R, Akt, ERK1/2, and Rb and levels of cyclin D1, p27, p16, and actin following 6- or 16-h treatment of A431 cells with combinations of 1 or 3 μm gefitinib and 3 μm NVP-ADW742. Data are representative of two independent experiments.
Because treatment of the cells with the IGF-1R and EGFR inhibitor combinations caused G1 arrest, we analyzed the expression levels of cyclin D1 and the cell cycle inhibitor proteins p16 and p27 as well as the levels of phosphorylated and total Rb. For these studies, cells were incubated overnight in growth medium containing 10% fetal bovine serum without IGF-1 stimulation to mimic the conditions for the cell cycle experiments. Similar effects of single agents on phosphorylation of EGFR, ERK, and Akt were observed in the absence of IGF-1 stimulation (Fig. 3C). Again, the combinations of gefitinib and NVP-ADW742 abolished Akt phosphorylation (Fig. 3, C and D). When levels of cell cycle proteins were examined, we found that treatment of A431 cells with gefitinib alone decreased cyclin D1 levels, slightly increased p27 levels, and significantly increased levels of p16 in the A431 cells. NVP-ADW742 treatment had no effect on these cell cycle proteins. Although there was no additional increase in p16 expression by the combinations of NVP-ADW742 and the EGFR inhibitor, there was a synergistic decrease in cyclin D1 levels and a further increase in p27 levels (Fig. 3, C and D). Treatment of A431 cells with the combinations also caused decreases in levels of both phosphorylated and total of Rb, whereas the single agents had no effect (Fig. 3C). When the levels of phosphorylated Rb were normalized to the total Rb levels, we found that relative Rb phosphorylation was not significantly inhibited compared with the effects on Rb expression (Fig. 3D).
To further characterize the effects of IGF-1R and EGFR combinations on the phosphorylation and levels of signaling and cell cycle proteins, A431 cells were treated with 1 and 3 μm gefitinib alone or in combination with 3 μm NVP-ADW742 for 6 h or overnight, and the Western analysis was performed (Fig. 3E). The effects of the compounds alone or in combination on levels of EGFR and ERK phosphorylation were already present at the 6 h time point. In addition, the synergistic decreases in Akt phosphorylation and cyclin D1 protein levels in cells treated with combinations of gefitinib and NVP-ADW742 was observed after both the 6- and 16-h treatments. There was also a slight decrease in phosphorylated and total Rb levels in the combination treated cells at 6 h, but the decrease in Rb expression was much greater after 16 h of compound treatment. Rb has been shown to be a substrate of caspase-3 (42), so the decreased levels of total Rb may be due to the elevated caspase-3/7 activity that resulted from the combined inhibition of IGF-1R/ErbB signaling (Fig. 1). The effects of the compounds on p27 and p16 levels were observed in samples from cells treated with compounds for 16 h but not earlier (Fig. 3E). These data suggest that the decrease in cyclin D1 is a direct consequence of blockade of both IGF-1R and ErbB signaling and that cyclin D1 degradation leads to G1 cell cycle arrest.
Expression of Activated Akt Prevents the Combination Effects of NVP-ADW742 and Gefitinib—To support our hypothesis that blockade of Akt phosphorylation is required for the synergistic effects of IGF-1R and EGFR inhibitors on cell proliferation and apoptosis, we determined cell numbers and caspase-3 activity following gefitinib and NVP-ADW742 treatment of A431 cells expressing myristoylated Akt, which is constitutively active (36). Similar to the parental A431 cells, A431-pBABE control cells treated with combinations of gefitinib and NVP-ADW742 exhibited synergistic decreases in cell number and induction of caspase-3 activation (Fig. 4A, left). However, treatment of A431-myr-Akt cells with the compound combinations only produced effects on cell numbers and caspase-3 activation similar to that seen following treatment of the cells with gefitinib alone (Fig. 4A, right). In addition, annexin V and PI staining showed that the number of viable A431-myr-Akt cells was not affected by treatment of the cells with gefitinib and NVP-ADW742, whereas treatment of the A431-pBABE control cells with this combination resulted in a large decrease in viable cell numbers and an increase in the number of cells undergoing apoptosis, as was seen in the parental A431 cells (Fig. 4B). A431-myr-Akt cells exhibited a large increase in both the expression and phosphorylation of Akt, as expected (Fig. 4C). Treatment of these cells with gefitinib alone or in combination with NVP-ADW742 did partially inhibit Akt phosphorylation in the A431-myr-Akt cells, but there was still a large amount of phosphorylated Akt present even in the cells treated with the compound combinations. The synergistic decreases in cyclin D1, phosphorylated Rb, and total Rb, but not the increase in p27 levels following treatment of the cells with gefitinib and NVP-ADW742, were also blocked by expression of constitutively active Akt (Fig. 4C).
FIGURE 4.

Expression of constitutively active Akt blocks the effects of gefitinib and NVP-ADW742 combinations on cell proliferation and apoptosis. A, decrease in cell number after a 72-h treatment and activation of caspase-3 after a 24-h treatment of A431-pBABE control (left) or A431-myr-Akt (right) cells with 1 or 3 μm gefitinib and 3μm NVP-ADW742 alone or in combination. Caspase-3 activation data are expressed as -fold caspase-3 activity in the DMSO-treated samples. Data are the average ± S.D. of triplicate samples and are representative of two independent experiments. B, flow cytometry analysis of annexin V and PI staining of A431-pBABE control (top) or A431-myr-Akt (bottom) cells treated with 1 μm gefitinib and 3 μm NVP-ADW742 alone or in combination. Cells were treated with compounds for 48 h before they were stained with annexin V-fluorescein isothiocyanate and PI. The percentages of cells in each quadrant are indicated. Data are representative of two independent experiments. C, levels of phosphorylated and total EGFR, IGF-1R, Akt, ERK1/2, and Rb and levels of cyclin D1, p27, p16, and actin following overnight treatment of A431-pBABE control or A431-myr-Akt cells with combinations of 1 or 3 μm gefitinib and 3 μm NVP-ADW742. Data are representative of two independent experiments.
NVP-ADW742 and Gefitinib Combinations Induce Bax Activation in A431 Cells—We next examined the mechanism responsible for the apoptotic effects of the IGF-1R and EGFR kinase inhibitor combinations in A431 cells. Growth factor withdrawal can produce apoptosis through the activation of the intrinsic or mitochondrial apoptosis pathway (37). In this pathway, the proapoptotic Bcl-2 family members, Bax and Bak, undergo conformational changes to active forms that disrupt the mitochondrial membrane to allow cytochrome c release into the cytosol and apoptosome formation. Activated caspase-9 in the apoptosome then cleaves procaspase-3, resulting in the formation of active caspase-3 (37, 38). In order to measure the amount of activated Bax present following compound treatment, A431 cells were incubated overnight with DMSO or with 1 or 3 μm gefitinib alone or in combination with 3 μm NVP-ADW742. Cells were then lysed, and Bax was immunoprecipitated with an antibody that specifically binds the active form of Bax (39). As seen in Fig. 5A, there was no activated Bax in untreated A431 cells or in cells treated with NVP-ADW742 alone and only a small amount of Bax activation in cells treated with gefitinib alone. However, there was a large increase in activated Bax levels in the cells treated with a combination of both compounds, corresponding to the levels of cleaved caspase-3 (Fig. 5A). These data indicate that the increase in caspase-3 activation seen upon treatment of the cells with EGFR and IGF-1R inhibitor combinations may be associated with activation of the intrinsic apoptosis pathway through activation of Bax.
FIGURE 5.
Effects of combinations of gefitinib and NVP-ADW742 on apoptotic proteins in A431 cells. A, levels of active and total Bax, total and cleaved caspase-3, and actin following overnight treatment of A431 cells with combinations of 1 or 3 μm gefitinib and 3 μm NVP-ADW742. Data are representative of three independent experiments. B, levels of Bcl-2, Bcl-XL, Mcl-1, and actin following overnight treatment of A431 cells with combinations of 1 or 3 μm gefitinib and 3 μm NVP-ADW742. Data are representative of three independent experiments. The average ± S.E. for Mcl-1 levels normalized to actin from densitometry measurements in the three experiments are shown in the graph below the blots. Mcl-1 levels in the combination-treated cells were statistically different from levels in DMSO- or NVP-ADW742-treated cells with p ≤ 0.003 (*), and Mcl-1 levels in the 3 μm gefitinib plus 3 μm NVP-ADW742-treated cells were statistically different from levels in cells treated with either gefitinib concentration with p ≤ 0.02 (**). C, levels of BimEL, BimL, BimS, and Mcl-1 in Mcl-1 immunoprecipitates (IP) or in whole cell lysate samples (WCL) from A431 cells treated with gefitinib and NVP-ADW742, alone or in combination. A431 cells were treated with compounds as in A. Data are representative of two independent experiments. The average ± S.E. for Bim levels normalized to Mcl-1 in the Mcl-1 immunoprecipitates are shown in the graph. Bim levels in immunoprecipitates from the 1 μm gefitinib- and the combination-treated cells were statistically different from levels in DMSO- or NVP-ADW742-treated cells with p ≤ 0.03 (*).
To determine how inhibition of IGF-1R and EGFR results in Bax activation, we examined the effects of gefitinib and NVP-ADW742 on levels of the antiapoptotic proteins Bcl-2, Bcl-XL, and Mcl-1 in the samples in which Bax was shown to be activated (Fig. 5B). When used alone or in combination, the IGF-1R and ErbB inhibitors did not affect the levels of Bcl-2 and Bcl-XL. However, levels of Mcl-1 were decreased in the samples treated with the inhibitor combinations (Fig. 5B).
Recently, multiple groups have showed that gefitinib induces apoptosis of highly sensitive cell lines containing mutated, constitutively activated EGFR by causing up-regulation of the proapoptotic protein, Bim (40, 41). Bim is also known to bind to Mcl-1, and this interaction can block inhibition of Bax and Bak by Mcl-1 (38, 42). When expression of the Bim isoforms, BimEL, BimL, and BimS, were examined in A431 cells treated with gefitinib, we found that BimEL levels were increased. This effect was maintained in cells treated with the combinations of gefitinib and NVP-ADW742 (Fig. 5C). We also analyzed Mcl-1 immunoprecipitates for the presence of Bim isoforms. Consistent with its increased expression levels, there were higher amounts of Bim associated with Mcl-1 in cells treated with gefitinib alone or the combination (Fig. 5C). Thus, decreased Mcl-1 and its association with Bim may contribute to Bax activation.
DISCUSSION
Cross-talk between IGF-1R and ErbB receptors has been shown to exist, and data from several studies have suggested that cancer cells resistant to treatment with ErbB inhibitors, such as gefitinib, erlotinib, and trastuzumab, continue to proliferate through activation of IGF-1R (20–22). Signaling through both receptor tyrosine kinases may also be simultaneously utilized by cancer cells to allow growth and survival. Synergistic effects on proliferation and apoptosis were seen in several different cancer cell lines treated with selective IGF-1R and ErbB small molecule inhibitors NVP-ADW742 and gefitinib.
We sought to determine what signaling mechanisms were responsible for the combination effects of highly selective small molecule IGF-1R and ErbB receptor inhibitors on proliferation and apoptosis. When the activation of signaling pathways downstream of the two receptors was examined in these cells, we found that there were combination effects on blockade of Akt phosphorylation. Following expression of constitutively activated Akt in A431 cells, combinations of gefitinib and NVP-ADW742 no longer exhibited synergistic inhibition of cell growth and induction of apoptosis, suggesting that inhibition of Akt activation is a key event required for the synergy. Treatment of A431 cells with the compound combinations caused decreased cyclin D1 and increased p27 levels. In contrast, levels of cyclin D1 expression were not decreased in gefitinib plus NVP-ADW742-treated A431-myr-Akt cells. Therefore, there appears to be a correlation between the ability of IGF-1R and ErbB inhibitor combinations to produce synergistic effects on growth inhibition and their ability to decrease cyclin D1 levels. Time course experiments indicated that cyclin D1 levels were decreased before p27 levels were significantly affected in A431 cells, suggesting that the loss of mitogenic signaling resulting in a decrease in cyclin D1 expression is an initial event that leads to inhibition of proliferation.
Cyclin D1 and p27 levels are regulated downstream of ErbB receptors and IGF-1R through both Akt- and ERK-mediated signaling (Fig. 6A). Cyclin D1 transcription and expression are increased following constitutive activation of ERK1/2 (43, 44), and Akt activation leads to stabilization of cyclin D1 protein (45, 46). The levels and activity of p27 can also be regulated by Akt and ERK. Expression levels of p27 are regulated by subcellular localization. In the G0 phase of the cell cycle, p27 is localized to the nucleus, but stimulation with mitogens leads to its redistribution to the cytoplasm, where it is degraded by the proteasome. Phosphorylation within the nuclear localization signal of p27 by Akt prevents its import into the nucleus and leads to its degradation (47). ERK is able to indirectly regulate p27 activity through transcriptional up-regulation of cyclin D1 (48). When cyclin D1 levels are decreased, p27 is available to bind to and inhibit cyclin E/Cdk2, preventing progression into S phase. Thus, it is probable that the G1 arrest seen in cells treated with NVP-ADW742 and gefitinib stems from effects of the combination on decreased cyclin D1 and increased p27 availability (Fig. 6B).
FIGURE 6.
Model illustrating how effects of combinations of IGF-1R and ErbB kinase inhibitors on signaling proteins could result in G1 arrest and apoptosis. A, IGF-1R and ErbB signaling results in proliferation and survival. Activation of IGF-1R and ErbB receptors by respective ligands or through receptor cross-talk leads to phosphorylation and activation of Akt and ERK. Activation of Akt and ERK results in increased cyclin D1 expression. Cyclin D1 sequesters p27, preventing it from inhibiting cyclin E/Cdk2 activity and allowing cell cycle progression and proliferation. Active ERK phosphorylates Bim and targets it for degradation. Active Akt causes stabilization of Mcl-1, so Mcl-1 is available to inhibit Bax activation and prevent apoptosis. B, inhibition of IGF-1R and ErbB receptor activity with small molecules causes cell cycle arrest and apoptosis. When cells are treated with the IGF-1R inhibitor, NVP-ADW742, and the EGFR inhibitor, gefitinib, activation and autophosphorylation of the two receptors are blocked. Akt and ERK are no longer phosphorylated and activated. As a result, levels of cyclin D1 are decreased and levels of p27 protein are increased. The increased p27 is available to bind to and inhibit cyclin E/Cdk2, leading to cell cycle arrest. In addition, when ERK is not activated, Bim remains stable and available to bind to Mcl-1. Mcl-1 expression is also lower because Akt is not active. Thus, the levels of Mcl-1 available to inhibit Bax are decreased, resulting in an increase in Bax activation and apoptosis.
The mechanism for the combination effects of IGF-1R and ErbB receptor inhibitors on caspase-3 activation and apoptosis induction appears to be through activation of the intrinsic apoptosis pathway, because there is a large increase in Bax activation following treatment with the combinations. One likely mechanism for activation of this apoptosis pathway is through the complete inhibition of Akt activation in the presence of IGF-1R and ErbB inhibitors, resulting in a decrease in prosurvival signaling downstream of Akt (Fig. 6A). Mcl-1, which is an antiapoptotic Bcl-2 family member, can prevent Bax and Bak from being activated in the intrinsic apoptosis pathway (38). Mcl-1 inhibits Bak through a direct association (37), whereas Mcl-1 indirectly inhibits Bax through a mechanism that remains to be elucidated (49). When Akt is active, Mcl-1 is expressed, because GSK3β, which can phosphorylate Mcl-1 and target it for degradation, is inactivated by Akt phosphorylation (50, 51). It has also been shown that activation of the phosphatidylinositol 3-kinase/Akt pathway can increase Mcl-1 levels by inducing its transcription (51). Consistent with the role of Akt in regulating Mcl-1 levels, in A431 cells treated with gefitinib and NVP-ADW742 combinations, Akt is inactivated and Mcl-1 levels are decreased. In addition, in untreated cells, the proapoptotic protein, Bim, is present at low levels, since it can be phosphorylated by ERK1/2 and targeted for degradation by the proteasome (38). When A431 cells are treated with gefitinib, BimEL migrates faster on SDS-polyacrylamide gels, consistent with lack of phosphorylation by ERK, and Bim levels are increased. The amount of Bim bound to Mcl-1 is also increased in cells in which EGFR is inactivated by gefitinib alone or in combination with NVP-ADW742 (Fig. 6B). Thus, the cells are primed for apoptosis due to the increase in Bim.
Based on the above described effects on Mcl-1 and Bim following IGF-1R/ErbB inhibition, we hypothesize that the intrinsic apoptosis pathway is activated following treatment of A431 cells with IGF-1R and ErbB kinase inhibitor combinations (Fig. 6, A and B). After treatment of cells with EGFR inhibitors, ERK is inhibited, and Bim is no longer phosphorylated and targeted for degradation. However, because Akt is still active, the levels of Mcl-1 do not change; therefore, apoptosis does not occur, because the balance of pro- and antiapoptotic factors favors survival. When IGF-1R inhibitors are combined with ErbB inhibitors, Akt activation is abolished, and Mcl-1 levels are decreased. The induction of Bim levels that occurs upon gefitinib treatment in the combination results in higher association of Bim to Mcl-1, thereby resulting in a net neutralization of Mcl-1 and subsequent activation Bax and of the apoptotic cascade (Fig. 6B).
We have shown that dual inhibition of IGF-1R and ErbB tyrosine kinase receptors produces synergistic blockade of cell proliferation and increases in apoptosis. Combinations of these inhibitors blocked Akt activation completely, whereas single compounds produced only partial blockade. The inhibition of cell proliferation appears to be due to G1 cell cycle arrest caused by decreased cyclin D1 levels. The induction of apoptosis seen with the IGF-1R and ErbB inhibitor combinations correlated with decreased levels of Mcl-1 and increased Bax activation, suggesting that inhibition of both receptors results in activation of the intrinsic apoptosis pathway.
Acknowledgments
We thank Zehan Chen for help with flow cytometry, Jameel Shah for providing the pBABE-myr-Akt-puro and pBABE-puro constructs, and Chris Tse, Jun Chen, and William Pappano for insights and helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IGF-1R, insulin-like growth factor-1 receptor; IGF, insulin-like growth factor; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; IRS, insulin receptor substrate; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; BrdUrd, bromodeoxyuridine; PI, propidium iodide; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.
References
- 1.Hofmann, F., and Garcia-Echeverria, C. (2005) Drug Discov. Today 10 1041–1047 [DOI] [PubMed] [Google Scholar]
- 2.Yakar, S., Leroith, D., and Brodt, P. (2005) Cytokine Growth Factor Rev. 16 407–420 [DOI] [PubMed] [Google Scholar]
- 3.Baserga, R. (2005) Expert Opin. Ther. Targets 9 753–768 [DOI] [PubMed] [Google Scholar]
- 4.Hubbard, R. D., and Wilsbacher, J. L. (2007) Chem. Med. Chem. 2 41–46 [DOI] [PubMed] [Google Scholar]
- 5.Bohula, E. A., Playford, M. P., and Macaulay, V. M. (2003) Anticancer Drugs 14 669–682 [DOI] [PubMed] [Google Scholar]
- 6.O'Connor, R. (2003) Horm. Metab. Res. 35 771–777 [DOI] [PubMed] [Google Scholar]
- 7.Pollak, M. N., Schernhammer, E. S., and Hankinson, S. E. (2004) Nat. Rev. Cancer 4 505–518 [DOI] [PubMed] [Google Scholar]
- 8.Liang, J., and Slingerland, J. M. (2003) Cell Cycle 2 339–345 [PubMed] [Google Scholar]
- 9.She, Q. B., Solit, D. B., Ye, Q., O'Reilly, K. E., Lobo, J., and Rosen, N. (2005) Cancer Cell 8 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camirand, A., Lu, Y., and Pollak, M. (2002) Med. Sci. Monit. 8 BR521–526 [PubMed] [Google Scholar]
- 11.Mitsiades, C. S., Mitsiades, N. S., McMullan, C. J., Poulaki, V., Shringarpure, R., Akiyama, M., Hideshima, T., Chauhan, D., Joseph, M., Libermann, T. A., Garcia-Echeverria, C., Pearson, M. A., Hofmann, F., Anderson, K. C., and Kung, A. L. (2004) Cancer Cell 5 221–230 [DOI] [PubMed] [Google Scholar]
- 12.Mitsiades, C. S., and Mitsiades, N. (2005) Expert Rev. Anticancer Ther. 5 487–499 [DOI] [PubMed] [Google Scholar]
- 13.Rabindran, S. K. (2005) Cancer Lett. 227 9–23 [DOI] [PubMed] [Google Scholar]
- 14.Normanno, N., De Luca, A., Bianco, C., Strizzi, L., Mancino, M., Maiello, M. R., Carotenuto, A., De Feo, G., Caponigro, F., and Salomon, D. S. (2006) Gene (Amst.) 366 2–16 [DOI] [PubMed] [Google Scholar]
- 15.Hynes, N. E., and Lane, H. A. (2005) Nat. Rev. Cancer 5 341–354 [DOI] [PubMed] [Google Scholar]
- 16.Gilmore, A. P., Valentijn, A. J., Wang, P., Ranger, A. M., Bundred, N., O'Hare, M. J., Wakeling, A., Korsmeyer, S. J., and Streuli, C. H. (2002) J. Biol. Chem. 277 27643–27650 [DOI] [PubMed] [Google Scholar]
- 17.Roudabush, F. L., Pierce, K. L., Maudsley, S., Khan, K. D., and Luttrell, L. M. (2000) J. Biol. Chem. 275 22583–22589 [DOI] [PubMed] [Google Scholar]
- 18.Hurbin, A., Coll, J. L., Dubrez-Daloz, L., Mari, B., Auberger, P., Brambilla, C., and Favrot, M. C. (2005) J. Biol. Chem. 280 19757–19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui, X., Kim, H. J., Kuiatse, I., Kim, H., Brown, P. H., and Lee, A. V. (2006) Cancer Res. 66 5304–5313 [DOI] [PubMed] [Google Scholar]
- 20.Morgillo, F., Woo, J. K., Kim, E. S., Hong, W. K., and Lee, H.-Y. (2006) Cancer Res. 66 10100–10111 [DOI] [PubMed] [Google Scholar]
- 21.Jones, H. E., Goddard, L., Gee, J. M., Hiscox, S., Rubini, M., Barrow, D., Knowlden, J. M., Williams, S., Wakeling, A. E., and Nicholson, R. I. (2004) Endocr. Relat. Cancer 11 793–814 [DOI] [PubMed] [Google Scholar]
- 22.Nahta, R., Yu, D., Hung, M. C., Hortobagyi, G. N., and Esteva, F. J. (2006) Nat. Clin. Pract. Oncol. 3 269–280 [DOI] [PubMed] [Google Scholar]
- 23.Nahta, R., Yuan, L. X., Zhang, B., Kobayashi, R., and Esteva, F. J. (2005) Cancer Res. 65 11118–11128 [DOI] [PubMed] [Google Scholar]
- 24.Morgillo, F., Kim, W. Y., Kim, E. S., Ciardiello, F., Hong, W. K., and Lee, H. Y. (2007) Clin. Cancer Res. 13 2795–2803 [DOI] [PubMed] [Google Scholar]
- 25.Lu, D., Zhang, H., Ludwig, D., Persaud, A., Jimenez, X., Burtrum, D., Balderes, P., Liu, M., Bohlen, P., Witte, L., and Zhu, Z. (2004) J. Biol. Chem. 279 2856–2865 [DOI] [PubMed] [Google Scholar]
- 26.Goetsch, L., Gonzalez, A., Leger, O., Beck, A., Pauwels, P. J., Haeuw, J. F., and Corvaia, N. (2005) Int. J. Cancer 113 316–328 [DOI] [PubMed] [Google Scholar]
- 27.Camirand, A., Zakikhani, M., Young, F., and Pollak, M. (2005) Breast Cancer Res. 7 R570–R579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desbois-Mouthon, C., Cacheux, W., Blivet-Van Eggelpoel, M. J., Barbu, V., Fartoux, L., Poupon, R., Housset, C., and Rosmorduc, O. (2006) Int. J. Cancer 119 2557–2566 [DOI] [PubMed] [Google Scholar]
- 29.Gibson, K. (October 31, 1996) Worldwide Patent WO96033980
- 30.Capraro, H., Furet, P., Garcia-Echeverria, C., and Manley, P. W. (November 21, 2002) Worldwide Patent WO02092599
- 31.Armstrong, R. C., Aja, T. J., Hoang, K. D., Gaur, S., Bai, X., Alnemri, E. S., Litwack, G., Karanewsky, D. S., Fritz, L. C., and Tomaselli, K. J. (1997) J. Neurosci. 17 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou, T. C., and Talalay, P. (1984) Adv. Enzyme Regul. 22 27–55 [DOI] [PubMed] [Google Scholar]
- 33.Merlino, G. T., Xu, Y. H., Ishii, S., Clark, A. J., Semba, K., Toyoshima, K., Yamamoto, T., and Pastan, I. (1984) Science 224 417–419 [DOI] [PubMed] [Google Scholar]
- 34.Yauch, R. L., Januario, T., Eberhard, D. A., Cavet, G., Zhu, W., Fu, L., Pham, T. Q., Soriano, R., Stinson, J., Seshagiri, S., Modrusan, Z., Lin, C. Y., O'Neill, V., and Amler, L. C. (2005) Clin. Cancer Res. 11 8686–8698 [DOI] [PubMed] [Google Scholar]
- 35.Matar, P., Rojo, F., Cassia, R., Moreno-Bueno, G., Di Cosimo, S., Tabernero, J., Guzman, M., Rodriguez, S., Arribas, J., Palacios, J., and Baselga, J. (2004) Clin. Cancer Res. 10 6487–6501 [DOI] [PubMed] [Google Scholar]
- 36.Edinger, A. L., and Thompson, C. B. (2002) Mol. Biol. Cell 13 2276–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooijman, R. (2006) Cytokine Growth Factor Rev. 17 305–323 [DOI] [PubMed] [Google Scholar]
- 38.Adams, J. M., and Cory, S. (2007) Oncogene 26 1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu, Y. T., and Youle, R. J. (1997) J. Biol. Chem. 272 13829–13834 [DOI] [PubMed] [Google Scholar]
- 40.Costa, D. B., Halmos, B., Kumar, A., Schumer, S. T., Huberman, M. S., Boggon, T. J., Tenen, D. G., and Kobayashi, S. (2007) PLoS Med. 4 1669–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cragg, M. S., Kuroda, J., Puthalakath, H., Huang, D. C., and Strasser, A. (2007) PLoS Med. 4 1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, L., Willis, S. N., Wei, A., Smith, B. J., Fletcher, J. I., Hinds, M. G., Colman, P. M., Day, C. L., Adams, J. M., and Huang, D. C. (2005) Mol Cell 17 393–403 [DOI] [PubMed] [Google Scholar]
- 43.Lavoie, J. N., L'Allemain, G., Brunet, A., Muller, R., and Pouyssegur, J. (1996) J. Biol. Chem. 271 20608–20616 [DOI] [PubMed] [Google Scholar]
- 44.Sherr, C. J., and Roberts, J. M. (1999) Genes Dev. 13 1501–1512 [DOI] [PubMed] [Google Scholar]
- 45.Knudsen, K. E., Diehl, J. A., Haiman, C. A., and Knudsen, E. S. (2006) Oncogene 25 1620–1628 [DOI] [PubMed] [Google Scholar]
- 46.Gladden, A. B., and Diehl, J. A. (2005) J. Cell. Biochem. 96 906–913 [DOI] [PubMed] [Google Scholar]
- 47.Viglietto, G., Motti, M. L., and Fusco, A. (2002) Cell Cycle 1 394–400 [DOI] [PubMed] [Google Scholar]
- 48.Philipp-Staheli, J., Payne, S. R., and Kemp, C. J. (2001) Exp. Cell Res. 264 148–168 [DOI] [PubMed] [Google Scholar]
- 49.Germain, M., Milburn, J., and Duronio, V. (2008) J. Biol. Chem. 283 6384–6392 [DOI] [PubMed] [Google Scholar]
- 50.Maurer, U., Charvet, C., Wagman, A. S., Dejardin, E., and Green, D. R. (2006) Mol. Cell 21 749–760 [DOI] [PubMed] [Google Scholar]
- 51.Mandelin, A. M., 2nd, and Pope, R. M. (2007) Expert Opin. Ther. Targets 11 363–373 [DOI] [PubMed] [Google Scholar]