Abstract
The Escherichia coli chaperonin GroEL is a double-ring chaperone that assists in protein folding with the aid of GroES and ATP. It is believed that GroEL alternates the folding-active rings and that the substrate protein (and GroES) can bind to the open trans-ring only after ATP in the cis-ring is hydrolyzed. However, we found that a substrate protein prebound to the trans-ring remained bound during the first ATP cycle, and this substrate was assisted by GroEL-GroES when the second cycle began. Moreover, a slow ATP-hydrolyzing GroEL mutant (D398A) in the ATP-bound form bound a substrate protein and GroES to the trans-ring. The apparent discrepancy with the results from an earlier study (Rye, H. S., Roseman, A. M., Chen, S., Furtak, K., Fenton, W. A., Saibil, H. R., and Horwich, A. L. (1999) Cell 97, 325–338) can be explained by the previously unnoticed fact that the ATP-bound form of the D398A mutant exists as a symmetric 1:2 GroEL-GroES complex (the “football”-shaped complex) and that the substrate protein (and GroES) in the medium is incorporated into the complex only after the slow turnover. In light of these results, the current model of the GroEL-GroES reaction cycle via the asymmetric 1:1 GroEL-GroES complex deserves reexamination.
Chaperonins are a conserved class of molecular chaperones that assist in protein folding in the cell and are found in eubacteria, mitochondria, chloroplasts, archaea, and the eukaryotic cytosol (1, 2). The best characterized of these is the Escherichia coli chaperonin GroEL and its partner, GroES (3–5). Because GroEL is known to assist in the folding of hundreds of proteins, including those that are essential for cell growth (6–8), the molecular mechanism by which GroEL and GroES facilitate protein folding has been a key issue in the fields of chaperones and protein folding for almost 2 decades.
GroEL is a large cylindrical protein complex comprising two heptameric rings of identical 57-kDa subunits, and these rings are stacked back to back (9, 10). GroES is a single heptameric ring of identical 10-kDa subunits (11). A large conformational change induced by ATP binding to GroEL promotes the formation of the GroEL-GroES complex (10). The GroEL ring that binds ATP and GroES is called the cis-ring, and it has a cavity for the encapsulation of the substrate protein (the cis-cavity) (12, 13). The origin of the asymmetry in the GroEL-GroES complex is explained by the nested cooperativity in the ATP binding, the positive cooperativity within the same GroEL rings, and the negative cooperativity between the two rings (14).
The most efficient manner of GroEL-GroES-assisted folding involves the substrate protein (up to ∼57 kDa (15)) bound to GroEL being ejected into the cis-cavity upon the ATP-dependent formation of the GroEL-GroES complex. ATP hydrolysis in the cis-ring results in the formation of the asymmetric ADP-bound GroEL-GroES complex (called the “ADP bullet”). Subsequent ATP binding to the opposite side of the GroEL ring (the trans-ring) induces the release of GroES, ADP, and the encapsulated substrate (either folded or not (16–18)) from the cis-ring (19, 20). The ATP-bound trans-ring is then reoriented to a new cis-ring, thereby allowing the next ATPase cycle. Multiple rounds of the GroEL cycle are required for the productive folding of stringent substrates, such as rhodanese and ribulose-bisphosphate carboxylase/oxygenase (Rubisco)3 (16–18).
The key experiments to establish the above alternation model were a series of studies using an ATPase-deficient GroEL mutant in which Asp398 was replaced with Ala (EL398) (19, 20). EL398 is deficient in ATP hydrolysis (<2% of the wild type) but not in ATP binding and thus forms a long-lived ATP-bound GroEL-GroES complex (asymmetric GroEL-GroES complex, called the ATP bullet). Rye et al. (20) reported that the trans-ring of the ATP bullet cannot bind either GroES or the substrate protein. ATP hydrolysis in the cis-ring of the ATP bullet permits the binding of both ATP and the substrate to the trans-ring (19, 20).
Substrates can bind to both of the GroEL rings at the same time (a substrate-saturated GroEL) (21–24). The availability of the substrate-saturated GroEL prompted us to examine whether the ATP bullet, formed from the substrate-saturated GroEL, retains the bound substrate in the trans-ring because the previous experiment using EL398 started from the GroEL mutant without bound substrates. In this study, we addressed this question and found that the bound substrate in the trans-ring remained bound during the GroEL cycle. In addition, we reevaluated the mechanism of EL398 and unexpectedly found a discrepancy between our results and those from a previous study (20). We resolved the apparent discrepancy by a careful reexamination of EL398. Revisiting the GroEL cycle model implied an efficient two-stroke mechanism (25) via the symmetric football complex.
EXPERIMENTAL PROCEDURES
Proteins and Reagents—Hexokinase was from Sigma. Proteinase K, ATP, and ADP were obtained from Roche Applied Science. The trace amount of contaminating ATP in the ADP solution was eliminated by a hexokinase/glucose treatment (22). Cy3-N-hydroxysuccinimide (FluoroLink Cy3 monofunctional dye) was from GE Healthcare. The following proteins were purified and prepared as described previously: wild-type and mutant GroEL, GroES, and bovine mitochondrial rhodanese (26); Rubisco from Rhodospirillum rubrum (19); and Cy3-labeled GroES (Cy3-GroES) and Cy3-labeled substrate (Cy3-rhodanese and Cy3-Rubisco) (27).
SDS-PAGE Analysis of GroEL-GroES-Substrate Ternary Complexes—GroEL and EL398, which had been saturated with denatured rhodanese, were prepared as described previously (22). Briefly, rhodanese was heat-denatured at 60 °C for 15 min in HKM buffer (20 mm HEPES-KOH (pH 7.4), 100 mm KCl, and 5 mm MgCl2) containing GroEL. To initiate the GroEL ATPase cycle, the solution containing GroEL (or EL398), which was saturated with rhodanese, and GroES in HKM buffer was mixed with a 2-fold volume of the solution containing ATP and then incubated at 25 °C. The final concentrations of the components in the reaction mixtures were as follows: 1 mm ATP, 0.5 μm GroEL or EL398 saturated with rhodanese, 1.0 μm GroES, 200 mm glucose, 1 mm dithiothreitol, and 20 mm Na2S2O3. For the single turnover ATP hydrolysis experiment (denoted “ATPsingle”), the excess ATP was hydrolyzed to ADP by adding hexokinase (final concentration, 0.04 units/μl) to the reaction mixture at 3 s after the initiation of the reaction (22). To determine the amounts of GroES and rhodanese bound to GroEL or EL398, the released GroES and rhodanese were removed by ultrafiltration (Microcon YM-100, Millipore). Subsequently, proteinase K (final concentration, 1 μg/ml) was added to an aliquot of the solution. Following an incubation for 30 min at 25 °C, the components with molecular masses <100 kDa were removed by ultrafiltration (Microcon YM-100). The retained solution was analyzed by 13% SDS-PAGE. The intensity of the band staining was quantified using the ImageJ program and was calibrated using known protein concentrations. To quantify the amount of GroES bound to EL398, EL398-GroES complexes formed in the presence of ATP, ADP, ATP with 10 mm NaF and 1 mm BeCl2, or ADP with 10 mm NaF and 1 mm BeCl2 were also analyzed by 13% SDS-PAGE as described above.
Rhodanese Folding Assay—The folding assays were conducted in HKM buffer including 200 mm glucose, 1 mm dithiothreitol, and 20 mm Na2S2O3. The final concentrations were 0.5 μm GroEL or EL398 saturated with rhodanese and 1.5 μm GroES. Folding was initiated by the addition of either ATP or ADP at a final concentration of 1 mm. For the single turnover ATP hydrolysis reactions, the excess ATP was removed by adding hexokinase (final concentration, 0.04 units/μl) to the reaction mixture at 3 s after the initiation of the reaction. For the repeated single turnover experiment (see Fig. 1B), additional ATP (final concentration, 5 mm) was mixed in the reaction mixture at 25 min after the initiation of the first single turnover experiment. At the times indicated, the folding was quenched by mixing aliquots of the solution with 750 μl of a solution containing 100 mm KH2PO4, 150 mm Na2S2O3, and 1 mm EDTA. After the quench, the enzymatic assay was initiated by adding 250 μl of 0.25 m KCN at 25 °C and was stopped by adding 200 μl of 37% formaldehyde after 15 min. After the further addition of 0.5 ml of ferric nitrate reagent (100 g of Fe(NO3)3·9H2O and 200 ml of HNO3, brought to 1000 ml with H2O), the rhodanese activity was measured colorimetrically by the absorbance at 460 nm (28), indicating the formation of a complex between the ferric ions and the thiocyanate reaction product.
FIGURE 1.
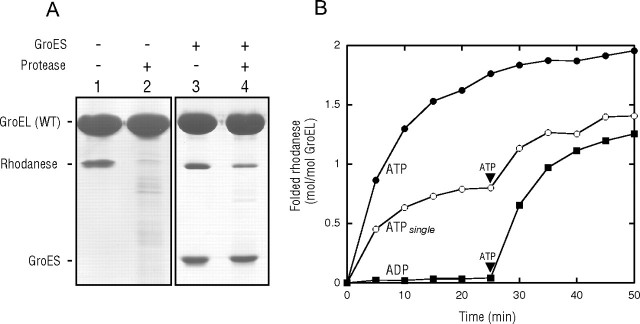
Retention of rhodanese bound to the trans-ring of the GroEL-GroES complex during the ATPase cycle. A, sensitivity of rhodanese bound to the GroEL complex to protease digestion. ATP was added to the mixture containing 200 mm glucose, GroES, and GroEL saturated with heat-denatured rhodanese for a single reaction cycle. After 3 s, hexokinase was added to the solution to hydrolyze all free ATP. After 60 min, when the folding of rhodanese in the cis-ring was saturated during the single turnover conditions (22, 23), the GroEL-GroES complexes were isolated by ultrafiltration (100-kDa cutoff) and then subjected to SDS-PAGE analysis with (lane 4) or without (lane 3) proteinase K treatment. As a control experiment, the rhodanese-saturated GroEL was also subjected to SDS-PAGE analysis with (lane 2) or without (lane 1) proteinase K treatment. WT, wild-type. B, recoveries of rhodanese that had bound to the trans-ring during the single turnover experiment. Recovered yields are expressed as moles of recovered enzyme/mol of GroEL. At time 0, ATP or ADP was added to the mixtures containing the rhodanese-saturated GroEL and GroES. In the ATPsingle experiment, the mixture also contained 200 mm glucose, and then hexokinase was added 3 s after the addition of ATP. The rhodanese activity at a 2:1 molar ratio of native rhodanese to GroEL was taken as 2.0 mol/mol of GroEL.
Binding Assay Using Gel Filtration—The ATP bullet and the ATP football complexes of EL398 were formed by mixing 1.5 μm EL398 with 1.5 μm and 3 μm GroES, respectively, 1 mm dithiothreitol, and 1 mm ATP in HKM buffer to initiate the ATP hydrolysis reaction (t = 0). Following an incubation for 30 s at 25 °C, the complexes were isolated by gel filtration (PD-10 column, GE Healthcare) in the same buffer. The capacity of binding of the complexes or unliganded EL398 to GroES was then examined by mixing 0.5 μm complexes or unliganded EL398 with 1 μm Cy3-GroES in 1 mm ATP at the indicated times following the initiation of ATP hydrolysis. The capacity of binding of the complexes or unliganded EL398 to denatured substrates was examined by mixing 0.5 μm complexes or unliganded EL398 with either 0.25 μm urea-denatured Cy3-rhodanese or 0.25 μm acid-denatured Cy3-Rubisco at the indicated times following the initiation. After a 5-min incubation at 25 °C, the samples were analyzed with a gel filtration HPLC column (G3000SWXL, Tosoh Corp.). Aliquots were loaded onto the column, which was equilibrated with HKM buffer containing 50 mm Na2SO4. The flow rate was 0.5 ml/min, and the elution profile was monitored for the Cy3 fluorescence using an in-line fluorometer (excitation at 550 nm and emission at 570 nm).
Quantification of Bound Nucleotides—To analyze the bound nucleotides in the EL398-GroES complex, we mixed 2 μm EL398, 4 μm GroES, 1 mm dithiothreitol, 1 mm ATP, 20 mm HEPES-KOH (pH 7.4), 50 mm KCl, and 5 mm MgCl2. After 5 min, aliquots were rapidly subjected to gel filtration using three TSK-GEL guard columns (Tosoh Corp.) connected in series and equilibrated with buffer containing 25 mm HEPES-KOH (pH 7.0), 100 mm Na2SO4, and 5 mm MgSO4. The isolated EL398 complexes were treated with perchloric acid (final concentration, 1.0%), and the supernatant was neutralized with K2CO3. The supernatants were applied to a reverse-phase HPLC column (ODS-80Ts, Tosoh Corp.) for the separation of ATP and ADP by monitoring the absorbance at 260 nm (29). The amount of nucleotide was calculated by the integrated peak area and was calibrated using known nucleotide concentrations.
RESULTS
Denatured Rhodanese Bound to the trans-Ring Remains Bound during GroEL-GroES Cycling—We have developed a method to prepare substrate-saturated GroEL, in which the substrates are bound to both of the GroEL rings (22–24). The addition of ATP and GroES to the substrate-saturated GroEL results in the formation of an ATP-bound asymmetric GroEL-GroES complex (22, 23), leading to the encapsulation of one substrate bound to the cis-ring within the cis-cavity. What is the fate of the other substrate bound to the trans-ring? Although an earlier key experiment in which the ATP-bound asymmetric GroEL-GroES complex (ATP bullet) could not bind the substrate protein in the trans-ring (20) suggested that the other substrate in the trans-ring should be released, no experiments were performed to clarify this point. To address this question, we prepared the substrate-saturated GroEL. The saturation protocol reproducibly resulted in the binding of 2.5∼3 mol of denatured rhodanese/mol of GroEL tetradecamer (Fig. 1A) (22, 23). We note that both of the rhodanese-bound GroEL rings efficiently bound GroES as evident from our previous studies (22, 23). The rhodanese bound to GroEL was completely digested by proteinase K (Fig. 1A, lanes 1 and 2), indicating that the bound rhodanese was in a protease-susceptible state. ATP was then added in the presence of GroES to initiate the GroEL ATPase cycle. After the reaction proceeded for 3 s, hexokinase was added to the solution (containing glucose) to hydrolyze all of the free ATP to prevent a second GroEL ATPase cycle. After the single turnover experiment (referred to as ATPsingle (22)), the GroEL complexes were treated with proteinase K to digest the exposed denatured rhodanese and then subjected to ultrafiltration (100-kDa cutoff membrane) to separate the digested/released rhodanese or GroES. Quantification of GroES based on the band densities revealed that 1.0 mol of GroES/mol of GroEL was retained in the isolated GroEL-GroES complex (Fig. 1A, lanes 3 and 4), confirming the efficient formation of the asymmetric GroEL-GroES complex in the single turnover experiment using the rhodanese-saturated GroEL. The release of bound rhodanese from the trans-ring, as predicted from the current GroEL model, should result in no difference in the band intensity of rhodanese with or without the protease treatment. However, we found that the 2.8 mol of rhodanese preloaded with the GroEL-GroES complex had been reduced to 1.2 mol after the protease treatment (Fig. 1A, lanes 3 and 4). Because 1.0 mol of GroES was retained in the isolated ternary complex, the protease-protected rhodanese is considered to have been encapsulated in the cis-cavity (22–24). Therefore, the presence of the protease-susceptible rhodanese would indicate the retention of the substrate by the trans-ring after the completion of the single turnover experiment.
Next, we tested whether the folding of the trans-retained rhodanese is still assisted by GroEL-GroES. As shown in our previous studies (22, 23), ∼2 mol of rhodanese/mol of GroEL tetradecamer was recovered when ATP and GroES were added to the rhodanese-saturated GroEL. In contrast, ADP and GroES did not assist in the folding of rhodanese. The ATPsingle condition resulted in a gradual recovery of the rhodanese activity until it reached a value corresponding to ∼0.8 mol of rhodanese/mol of GroEL (Fig. 1B), showing that the rhodanese encapsulated in the cis-cavity was folded under the ATPsingle condition as reported previously (22, 23). At 25 min under the ATPsingle condition, ATP was added to the mixture to trigger a second GroEL cycle. The second addition of ATP re-induced the folding of rhodanese, reaching a value of ∼1.4 mol of rhodanese/mol of GroEL, which was almost twice the recovery compared with that before the addition of the second ATP. The second recovery of rhodanese was not inhibited by the inclusion of a trap-GroEL, which binds the substrate but does not release it even in the presence of ATP (12), in the solution (data not shown), indicating that the trans-bound substrate was not released into the bulk solution during ATP hydrolysis in the cis-ring. Therefore, we concluded that the trans-ring of the cis-ATP complex retains the substrate that is available for GroEL-GroES-dependent folding.
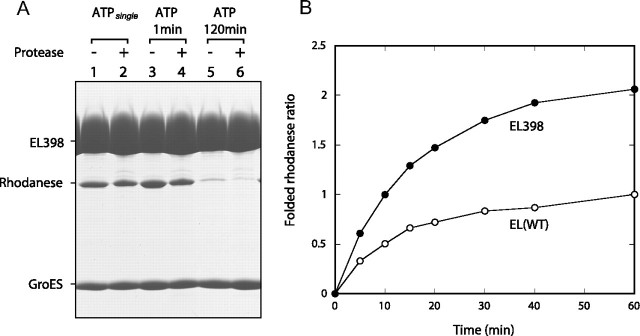
The GroEL(D398A) Mutant Binds Two GroES Heptamers in the Presence of ATP, Producing a Symmetric “ATP Football” Complex—Because the ATPsingle condition experiment was conducted with wild-type GroEL, it was possible that the trans-bound substrate might have been transiently released from the ATP bullet but then bound again to the trans-ring after the formation of the ADP bullet as a result of ATP hydrolysis. To rule out this possibility, we conducted the above protease protection experiment using EL398 because this mutant remains in the ATP-bound form with a half-life of ∼30 min (19, 20), which is long enough to isolate the ATP-bound EL398-GroES complex in our protocol. The release of the trans-bound rhodanese by the ATP-bound EL398 complex should result in the isolation of a trans-free EL398-GroES complex after ultrafiltration. At first, we conducted the ATPsingle condition experiment using EL398 as a control (Fig. 2A). We expected that about half of the bound rhodanese would be digested by proteinase K because the ATPsingle condition should produce the asymmetric ATP bullet complex, which has protease-susceptible rhodanese in the trans-ring. However, we found only a slight reduction in the amount of rhodanese after the protease treatment (Fig. 2A, lanes 1 and 2). Quantification of the retained rhodanese revealed that 2.7 mol of rhodanese was protected after the protease treatment (Fig. 2A, lane 2). In addition, we noticed that ∼2 mol of GroES/mol of GroEL was bound to the EL398 complex after the completion of a single turnover (Fig. 2A, lanes 1 and 2), suggesting the formation of a symmetric 1:2 EL398-GroES complex (ATP football). Because the formation of the stable ATP football complex would result in the encapsulation of rhodanese in both cavities, we then compared the recovery of the rhodanese activity using EL398 with that using wild-type GroEL under the ATPsingle condition in the presence of GroES. Strikingly, the recovery of the rhodanese activity assisted by EL398-GroES was almost double that assisted by wild-type GroEL-GroES (Fig. 2B). This means that the EL398-GroES complex has two folding chambers to assist in the folding of ∼2 mol of rhodanese/mol of EL398 tetradecamer, further supporting the formation of the ATP football complex.
FIGURE 2.
Encapsulation of rhodanese bound to both rings of EL398 within the cavities. A, protection of encapsulated rhodanese from proteinase K. Mixtures of EL398 saturated with heat-denatured rhodanese, GroES, 200 mm glucose, and ATP were incubated for 3 s (lanes 1 and 2), 1 min (lanes 3 and 4), or 120 min (lanes 5 and 6). In the experiment denoted as ATPsingle (lanes 1 and 2), hexokinase was added to the aliquot after 3 s, and the reaction was left for 60 min longer as described for Fig. 1A. Aliquots underwent one of the two following treatments: ultrafiltration (100-kDa cutoff) and SDS-PAGE (lanes 1, 3, and 5) or ultrafiltration (100-kDa cutoff), proteinase K treatment, a second ultrafiltration (100-kDa cutoff), and SDS-PAGE (lanes 2, 4, and 6). Gels were stained with Coomassie Brilliant Blue. B, GroEL (wild-type (WT))- or EL398-assisted recovery of rhodanese activities for a single reaction cycle. A single round of folding was initiated by the addition of ATP to the mixtures containing 200 mm glucose, GroEL saturated with denatured rhodanese, and GroES. Hexokinase was then added after 3 s of the initiation. For a comparison, the ratio of the recovered activity to that at 60 min assisted by GroEL (wild-type) is shown. EL(WT), wild-type EL398.
We next examined whether the substrates are released by the ATP-bound EL398 complex. Separation of the EL398-GroES complex at 1 min after the addition of ATP resulted in the retention of ∼2 mol of the protease-protected rhodanese (Fig. 2A, lanes 3 and 4). In addition, ∼2 mol of GroES was retained. The retention of both cis- and trans-bound substrates was interpreted as an encapsulation of both substrates in two cis-cavities of the ATP football. The retention of rhodanese was reduced to <1 mol after 120 min (Fig. 2A, lanes 5 and 6). This reduction in the amount of retained rhodanese was interpreted as being the consequence of several rounds of EL398 ATPase turnover during the 120-min reaction, resulting in the release of native rhodanese from the EL398-GroES complex.
Taken together, our experiments using EL398 strongly suggested the formation of the ATP football complex, which was not observed in previous studies using EL398 (19, 20). These unexpected results prompted us to carefully reevaluate EL398.
Reevaluation of EL398: The Asymmetric ATP Bullet Complex Binds a Second GroES or Denatured Substrate to the trans-Ring—To compare the results reported by Rye et al. (20) with ours, EL398 without bound rhodanese was used. Because our previous experiment using fluoroberyllate (BeFx) revealed that the incubation of GroEL (wild-type) and GroES in the presence of ATP + BeFx and ADP + BeFx resulted in the formation of a stable football (Fig. 3A, lane 4) and a bullet (lane 5), respectively (23), we used the football and bullet as references for the following experiment. When we mixed EL398 and GroES at a molar ratio of 1:2 in the presence of ATP, the isolation of the EL398-GroES complex resulted in the retention of ∼2.0 mol of GroES/mol of EL398 (Fig. 3A, lane 2), which was the same as the reference for the ATP/BeFx football complex (lane 4). The mixing of EL398 and GroES at a molar ratio of 1:1 in the presence of ATP resulted in the retention of ∼1.3 mol of GroES/mol of EL398 (Fig. 3A, lane 1), which is almost the same as the reference for the ADP/BeFx bullet (lane 5). The half-occupancy of GroES to EL398 in the ATP-bound form reflects the apparent formation of a bullet complex, i.e. an ATP bullet. Note that the mixing of EL398 and GroES at a molar ratio of 1:2 in the presence of ADP resulted in an ADP bullet with the retention of ∼1.4 mol of GroES/mol of EL398 (Fig. 3A, lane 3).
FIGURE 3.
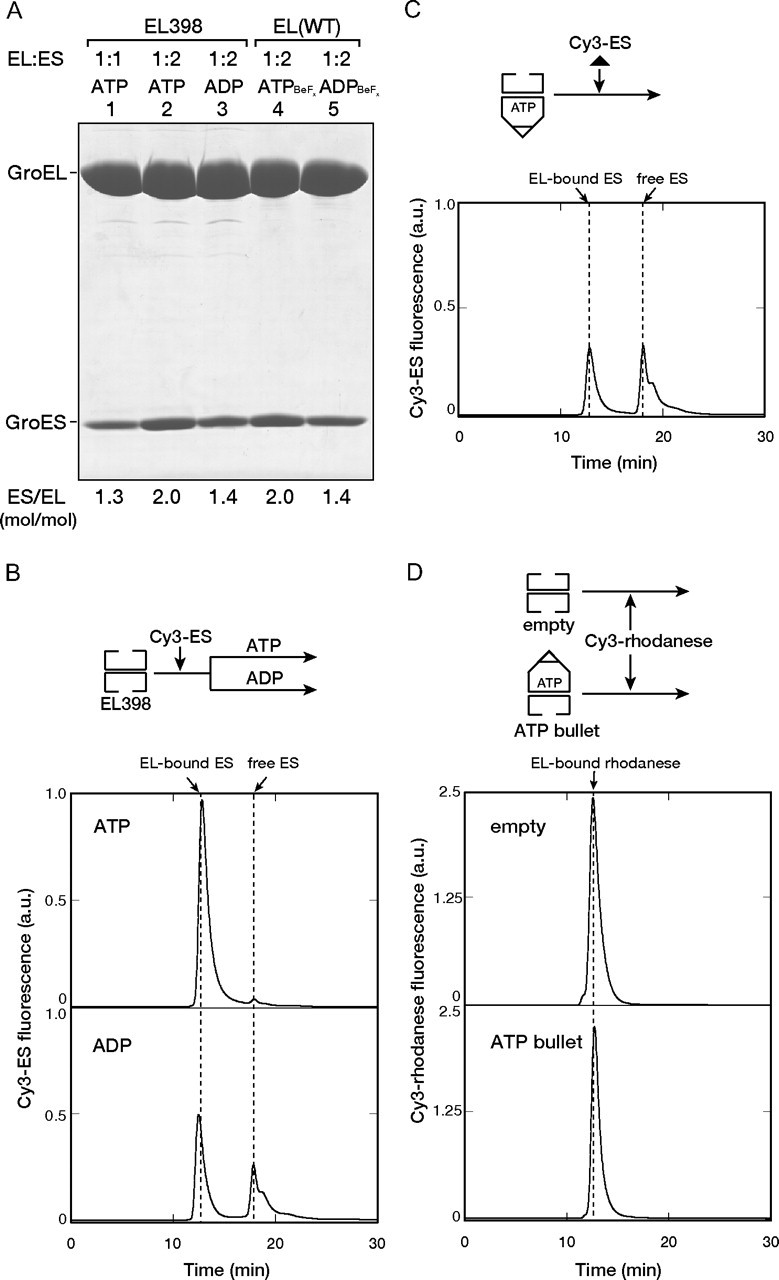
Binding of GroES and the denatured substrate to the trans-ring of the EL398-GroES ATP bullet complex. A, SDS-PAGE analysis of the isolated EL398-GroES complex formed in the presence of ATP (lanes 1 and 2) or ADP (lane 3). EL398 and GroES were mixed at 1:1 (lane 1) and 1:2 (lanes 2 and 3) ratios. For comparison, the wild-type GroEL-GroES complexes formed in the presence of either ATP + BeFx (lane 4) or ADP + BeFx (lane 5) were also analyzed. The GroES/GroEL (ES/EL) molar ratios indicated at the bottom were determined by the band intensities. EL(WT), wild-type EL398. B and C, binding of Cy3-GroES to the empty EL398 (B) or the EL398-GroES ATP bullet formed using unlabeled GroES (C). Cy3-GroES was mixed with EL398 (B) or the EL398-GroES ATP bullet (C) at a 2:1 ratio in the presence of either ATP or ADP (B) or ATP (C). The mixtures were applied to a gel filtration HPLC column. Cy3 fluorescence was monitored in-line. The scale of the y axis is the same in B and C. a.u., arbitrary units. D, binding of denatured Cy3-rhodanese to the empty EL398 or the EL398-GroES ATP bullet. Denatured Cy3-rhodanese (0.15 μm) was diluted in buffer containing the empty EL398 or the EL398-GroES ATP bullet (0.3 μm) and was incubated for 5 min. The mixtures were subjected to gel filtration as described above. A schematic view of the experiments is shown above each graph.
The formation of the ATP football complex was further confirmed by gel filtration analysis using GroES labeled with fluorescent Cy3 (Cy3-GroES), which has been shown to function normally (27, 30). Almost 2 mol of Cy3-GroES bound to 1 mol of EL398 in the presence of ATP, whereas only half of the Cy3-GroES bound to EL398 in the presence of ADP (Fig. 3B).
The EL398-GroES ATP bullet, which was prepared by mixing GroES and EL398 at a molar ratio of 1:1, was used to reexamine the binding of GroES to the trans-ring. We prepared the EL398-GroES ATP bullet without the fluorescent dye and tested whether the trans-ring binds a second GroES. When 2 mol of Cy3-GroES/mol of ATP bullet was added to a freshly prepared EL398 ATP bullet, stoichiometric binding of Cy3-GroES to the ATP bullet was observed (Fig. 3C). Furthermore, we tested the binding of denatured rhodanese to the trans-ring of the ATP bullet. When we added the stoichiometric amount of Cy3-labeled rhodanese to the EL398-GroES ATP bullet, we observed significant binding of Cy3-rhodanese to the ATP bullet, which was lower than but comparable with that to the empty GroEL ring (Fig. 3D). These results were unexpected because Rye et al. (20) showed that the trans-ring of the EL398 ATP bullet cannot bind either GroES or the substrate in the ATP-bound form.
Reevaluation of EL398: The Symmetric ATP Football Complex Does Not Bind a Second GroES or the Substrate but Binds Them after ATP Hydrolysis—To explain the apparent discrepancy between the results reported by Rye et al. (20) and our findings, we next tested the behavior of the EL398 ATP football complex. After the formation of the ATP football complex, we added Cy3-GroES (Fig. 4A) or Cy3-labeled substrates (rhodanese (Fig. 4B) and Rubisco (Fig. 4C)) and then analyzed the complexes by gel filtration HPLC. Soon after the initiation of the reactions, insignificant binding of Cy3-GroES and the Cy3-substrates to the ATP football complex was detected. Prolonged incubation gradually led to the binding of Cy3-GroES or the Cy3-substrate up to a stoichiometric amount. Because these results are indistinguishable from those reported by Rye et al. (20), we suspect that the ATP bullet assumed in the work by Rye et al. was actually an ATP football. Their interpretation that the trans-ring of the ATP bullet does not bind GroES or the substrates but binds them after ATP hydrolysis should be revised. Instead, GroES or the substrates cannot access the ATP football complex due to the full occupancy of the binding sites by two GroES heptamers.
FIGURE 4.
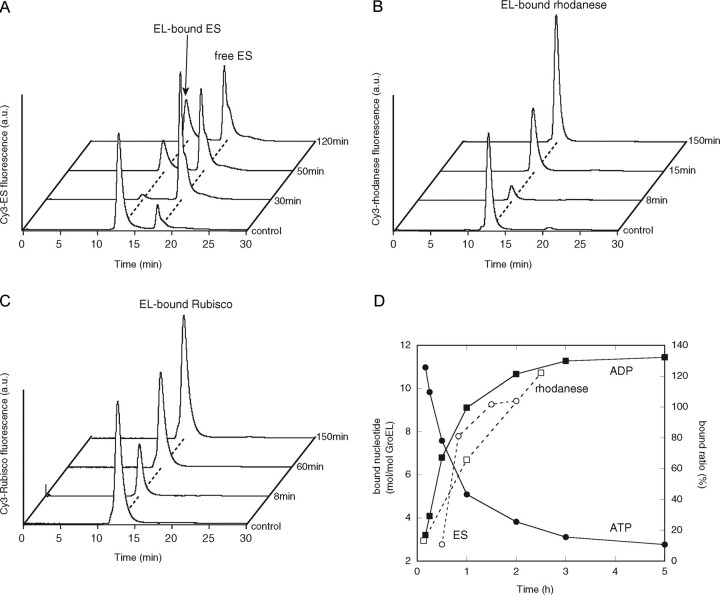
Binding of GroES and the denatured substrate to the symmetric GroES-EL398-GroES ATP football complex. A, binding of Cy3-GroES to the EL398 ATP football complex formed using unlabeled GroES. The stable EL398 ATP football complex was generated by mixing EL398 (EL) with GroES (ES) at a 1:2 molar ratio in the presence of ATP (t = 0 min) and incubating the mixture for 5 min. The mixture was rapidly applied to a gel filtration column to isolate the ATP football complex. The isolated ATP football complex (0.5 μm) was mixed with Cy3-GroES (1 μm) and ATP (1 mm) at the indicated times and then separated by gel filtration HPLC with fluorescence detection. B and C, binding of denatured Cy3-labeled substrates to the EL398 ATP football complex. The ATP football complexes (0.5 μm) were mixed with either Cy3-rhodanese (0.25 μm)(B) or Cy3-Rubisco (0.25 μm)(C) at the indicated times and then separated by gel filtration with fluorescence detection. The trace labeled control represents the direct mixing of the empty EL398 (0.5 μm) with 1 μm Cy3-GroES and 1 mm ATP (A), 0.25 μm Cy3-rhodanese (B), or 0.25 μm Cy3-Rubisco (C). a.u., arbitrary units. D, time course of ATP hydrolysis by the isolated EL398 ATP football complex and quantification of the GroES and rhodanese bound to the EL398 ATP football complexes. The hydrolysis of ATP in the isolated ATP football complexes was determined as described under “Experimental Procedures.” The determination of the amounts of bound GroES and rhodanese was performed as follows. The integrated peak areas in the traces obtained by the binding assay of Cy3-GroES (A) and Cy3-rhodanese (B) were calculated. The amounts of GroES and rhodanese bound to unliganded EL398 (control in A and B) were taken as 200 and 100%, respectively.
Finally, we quantitated the time course of ATP hydrolysis by EL398 and compared it with that by GroES or the substrate binding to the ATP-bound EL398-GroES complex as shown in Fig. 4 (A and B). Extrapolation of the bound ATP to 0 h yielded a value of ∼14 mol of ATP/mol of EL398 tetradecamer (Fig. 4D), confirming that EL398 and GroES form a symmetric ATP football in which both of the EL398 rings are occupied by ATP. The time course of the ATP hydrolysis correlated with the increased binding of GroES or the substrates (Fig. 4D), suggesting that EL398 releases GroES from the ATP football complex upon ATP hydrolysis, leading to the exchange of GroES or the substrates.
DISCUSSION
All known chaperonin family members, including the eukaryotic group II chaperonin, consist of double-ring structures (5). How the double ring functions during the chaperonin cycle has been one of the key issues in the field. Extensive studies on the E. coli chaperonin GroEL have revealed an outline of the reaction cycle using the double ring. Briefly, GroEL alternates the functional ring in the presence of ATP and GroES by coordinating the binding of GroES and the substrate protein (19, 20). Our results presented here suggest that some modifications of the prevailing model are necessary, as discussed below.
Retention of the trans-Bound Substrate during the ATPase Cycle—One of the main findings in this work is the demonstration of the retention of the trans-bound substrate during the GroEL ATPase cycle. Several previous studies have already described that GroEL can bind substrate proteins on both sides of the rings (21, 22, 31–33). ATP-dependent GroES binding to one of the GroEL rings triggers the encapsulation of one of the substrates in the cis-cavity (22, 23). However, it has been unclear whether the other substrate is retained on the trans-ring after the initiation of the GroEL ATPase cycle.
Our previous study on the effect of fluoroberyllate, which mimics the γ-phosphate part of ATP, suggested the retention of both substrates after the single turnover experiment because BeFx + ATP generates a GroEL-GroES football complex, in which the bound substrates are encapsulated in both cavities of the football complex (23). We showed here that the GroEL-bound substrate that was destined to the trans-side was retained by the trans-ring after the single ATP turnover. The finding that EL398 in the ATP-bound form also retained the trans-bound substrate (Fig. 2A) supports the retention of the trans-bound substrate during the GroEL ATPase cycle. The binding of the substrate to an “empty” trans-ring is known to stimulate the GroEL ATPase cycle (20). The retention of the substrate during the GroEL cycle should ensure more efficient cycling, which should be independent of the rate constant for the association of the substrate with the GroEL ring.
Reevaluation of EL398—Another important point in this study is the reevaluation of the well known GroEL mutant, EL398. The role of EL398 in the elucidation of the GroEL mechanism has been critically important (19, 20). Because of a slow ATP hydrolysis rate, the extremely long-lived ATP-bound EL398 complex (t½ ∼30 min) has facilitated investigations of the intermediate complex during the GroEL cycle. By using EL398, Rye et al. revealed that ATP binding to the trans-ring of the ADP bullet triggers the release of cis-bound GroES, the substrate, and ADP (19) and that the trans-ring of the ATP bullet cannot bind either GroES or the substrate (20). Although their investigations were based on the premise that EL398 forms the ATP bullet complex in the presence of ATP, we found that EL398 stably bound two GroES heptamers to both GroEL rings in the presence of ATP, producing the ATP football complex (Figs. 2A and 3). In addition, we revealed that both of the cavities in the football complex are active to assist in the folding of a stringent substrate, rhodanese (Fig. 2B). The stable formation of the ATP football should be associated with the prolonged ATP-bound form in EL398. Indeed, we reported previously another stable football complex when the ATP-bound form of wild-type GroEL was mimicked by ATP + BeFx (23).
In addition to the isolation of the ATP football, we have also shown the isolation of the ATP bullet using EL398 with the limited addition of GroES (Fig. 3A). Specifically, the addition of 1 mol of GroES heptamer/mol of EL398 tetradecamer resulted in the quantitative isolation of the asymmetric ATP bullet. The EL398 ATP bullet was able to bind a second GroES or the substrate to the trans-ring, in sharp contrast to the earlier report by Rye et al. (20). The difference can be rationalized if Rye et al. (20) actually used the ATP football in their work because the ATP football accepts neither GroES nor the substrate, as shown in Fig. 4 (A–C). We also demonstrated that the GroES in the ATP football was exchangeable with free GroES in the bulk solution after ATP hydrolysis. The time course of the exchange of GroES (Fig. 4A) closely resembles that reported by Rye et al. (20), which has been interpreted as the binding of GroES to the trans-ring after ATP hydrolysis, suggesting again that Rye et al. actually used the EL398 ATP football complex in their work.
Revision of the GroEL-GroES Cycling Model—Although our data on the formation of the ATP football were obtained with the GroEL mutant EL398, several lines of evidence from studies on wild-type GroEL strongly suggested the formation of the symmetric ATP football during the functional cycle. First, there have been many reports on the observation of the football complex in electron microscopic analyses (see below for further discussion). Second, we observed previously a symmetric football complex in the presence of ATP/BeFx (23). Third, in the accompanying article, Funatsu and co-workers (41) have shown the transient accumulation of the symmetric football complex of wild-type GroEL and GroES under equilibrium conditions by a fluorescence resonance energy transfer experiment as well as by electron microscopic observations of the symmetric football complex of EL398, GroES, and ATP. Finally, even Rye et al. (20) did not rule out the possibility of the ATP football complex in their discussion. Thus, the formation of the football complex by wild-type GroEL seems to be feasible, although the lifetime of the ATP football formed by wild-type GroEL should be transient.
The prevailing model predicts the binding of a second GroES to the trans-ring after the hydrolysis of cis-bound ATP. In the modified model, the second GroES binds the trans-ring even without the hydrolysis of cis-bound ATP, forming the symmetric football complex. The modified model is based on the prevailing alternation model, in which the functional GroEL ring alternately switches during the ATPase cycle. The differences between the previous model and the revised model proposed here are the incorporation of the trans-bound substrate in the ATP-bullet and the subsequent formation of the football intermediate.
Implication for the Two-stroke Mode via the Symmetric Football Complex—Previous studies, including electron microscopy, chemical cross-linking, and several biochemical analyses, clearly showed the accumulation of the symmetric football-shaped GroEL-GroES complex in the presence of ATP (21, 31, 34–39). However, there have been debates as to whether the GroEL-GroES football complex is critical to the productive folding assisted by GroEL-GroES. An in vitro analysis revealed that the symmetric intermediate is not required for chaperonin function, and its presence does not significantly increase the rate of chaperonin-assisted folding (39). In addition to the debates on the possible necessity of the symmetric football complex, the previous findings that ATP binding to the trans-ring of the ATP bullet triggers the release of GroES in the cis-ring play a decisive part in the debates because the findings easily predict that the release of GroES occurs before the binding of the second GroES to the trans-ring (the dissociative mechanism) (19, 20). However, our revised cycle model strongly suggests that the release of GroES occurs after the binding of the second GroES to the trans-ring (the associative mechanism). A previous evaluation of the dissociative and associative mechanisms favored the associative mechanism, although ADP was used in the study (40). Our results strongly suggest that the associative mechanism can be applied, even in the presence of ATP.
In conclusion, the consequences of our findings favor the model in which GroEL and GroES function via the symmetric football complex as an unavoidable intermediate. The combination of the symmetric complex and the retention of the trans-bound substrate reminds us of the “two-stroke engine” mode (25) to explain chaperonin function. Our study again raises the long-standing question about the role of the football intermediate during the functional GroEL-GroES cycle. In particular, in vivo analyses of the roles of the symmetric and asymmetric complexes would be worth pursuing.
Supplementary Material
This work was supported by Grant-in-aids for Scientific Research on Priority Areas 17049009, 19037007, and 19058002 (to H. T.) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article was selected as a Paper of the Week.
Footnotes
The abbreviations used are: Rubisco, ribulose-bisphosphate carboxylase/oxygenase; HPLC, high pressure liquid chromatography; BeFx, fluoroberyllate.
References
- 1.Hartl, F. U., and Hayer-Hartl, M. (2002) Science 295 1852–1858 [DOI] [PubMed] [Google Scholar]
- 2.Young, J. C., Agashe, V. R., Siegers, K., and Hartl, F. U. (2004) Nat. Rev. Mol. Cell Biol. 5 781–791 [DOI] [PubMed] [Google Scholar]
- 3.Thirumalai, D., and Lorimer, G. H. (2001) Annu. Rev. Biophys. Biomol. Struct. 30 245–269 [DOI] [PubMed] [Google Scholar]
- 4.Taguchi, H. (2005) J. Biochem. (Tokyo) 137 543–549 [DOI] [PubMed] [Google Scholar]
- 5.Horwich, A. L., Fenton, W. A., Chapman, E., and Farr, G. W. (2007) Annu. Rev. Cell Dev. Biol. 23 115–145 [DOI] [PubMed] [Google Scholar]
- 6.McLennan, N., and Masters, M. (1998) Nature 392 139. [DOI] [PubMed] [Google Scholar]
- 7.Kerner, M. J., Naylor, D. J., Ishihama, Y., Maier, T., Chang, H. C., Stines, A. P., Georgopoulos, C., Frishman, D., Hayer-Hartl, M., Mann, M., and Hartl, F. U. (2005) Cell 122 209–220 [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara, K., and Taguchi, H. (2007) J. Bacteriol. 189 5860–5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braig, K., Otwinowski, Z., Hegde, R., Boisvert, D. C., Joachimiak, A., Horwich, A. L., and Sigler, P. B. (1994) Nature 371 578–586 [DOI] [PubMed] [Google Scholar]
- 10.Xu, Z., Horwich, A. L., and Sigler, P. B. (1997) Nature 388 741–750 [DOI] [PubMed] [Google Scholar]
- 11.Hunt, J. F., Weaver, A. J., Landry, S. J., Gierasch, L., and Deisenhofer, J. (1996) Nature 379 37–45 [DOI] [PubMed] [Google Scholar]
- 12.Weissman, J. S., Hohl, C. M., Kovalenko, O., Kashi, Y., Chen, S., Braig, K., Saibil, H. R., Fenton, W. A., and Horwich, A. L. (1995) Cell 83 577–587 [DOI] [PubMed] [Google Scholar]
- 13.Mayhew, M., da Silva, A. C., Martin, J., Erdjument-Bromage, H., Tempst, P., and Hartl, F. U. (1996) Nature 379 420–426 [DOI] [PubMed] [Google Scholar]
- 14.Horovitz, A., and Willison, K. R. (2005) Curr. Opin. Struct. Biol. 15 646–651 [DOI] [PubMed] [Google Scholar]
- 15.Sakikawa, C., Taguchi, H., Makino, Y., and Yoshida, M. (1999) J. Biol. Chem. 274 21251–21256 [DOI] [PubMed] [Google Scholar]
- 16.Todd, M. J., Viitanen, P. V., and Lorimer, G. H. (1994) Science 265 659–666 [DOI] [PubMed] [Google Scholar]
- 17.Weissman, J. S., Kashi, Y., Fenton, W. A., and Horwich, A. L. (1994) Cell 78 693–702 [DOI] [PubMed] [Google Scholar]
- 18.Taguchi, H., and Yoshida, M. (1995) FEBS Lett. 359 195–198 [DOI] [PubMed] [Google Scholar]
- 19.Rye, H. S., Burston, S. G., Fenton, W. A., Beechem, J. M., Xu, Z., Sigler, P. B., and Horwich, A. L. (1997) Nature 388 792–798 [DOI] [PubMed] [Google Scholar]
- 20.Rye, H. S., Roseman, A. M., Chen, S., Furtak, K., Fenton, W. A., Saibil, H. R., and Horwich, A. L. (1999) Cell 97 325–338 [DOI] [PubMed] [Google Scholar]
- 21.Sparrer, H., Rutkat, K., and Buchner, J. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motojima, F., and Yoshida, M. (2003) J. Biol. Chem. 278 26648–26654 [DOI] [PubMed] [Google Scholar]
- 23.Taguchi, H., Tsukuda, K., Motojima, F., Koike-Takeshita, A., and Yoshida, M. (2004) J. Biol. Chem. 279 45737–45743 [DOI] [PubMed] [Google Scholar]
- 24.Koike-Takeshita, A., Shimamura, T., Yokoyama, K., Yoshida, M., and Taguchi, H. (2006) J. Biol. Chem. 281 962–967 [DOI] [PubMed] [Google Scholar]
- 25.Lorimer, G. (1997) Nature 388 720–721 [DOI] [PubMed] [Google Scholar]
- 26.Motojima, F., Makio, T., Aoki, K., Makino, Y., Kuwajima, K., and Yoshida, M. (2000) Biochem. Biophys. Res. Commun. 267 842–849 [DOI] [PubMed] [Google Scholar]
- 27.Taguchi, H., Ueno, T., Tadakuma, H., Yoshida, M., and Funatsu, T. (2001) Nat. Biotechnol. 19 861–865 [DOI] [PubMed] [Google Scholar]
- 28.Sörbo, B. H. (1953) Acta Chem. Scand. 7 1129–1136 [Google Scholar]
- 29.Hisabori, T., Muneyuki, E., Odaka, M., Yokoyama, K., Mochizuki, K., and Yoshida, M. (1992) J. Biol. Chem. 267 4551–4556 [PubMed] [Google Scholar]
- 30.Ueno, T., Taguchi, H., Tadakuma, H., Yoshida, M., and Funatsu, T. (2004) Mol. Cell 14 423–434 [DOI] [PubMed] [Google Scholar]
- 31.Llorca, O., Marco, S., Carrascosa, J. L., and Valpuesta, J. M. (1994) FEBS Lett. 345 181–186 [DOI] [PubMed] [Google Scholar]
- 32.Corrales, F. J., and Fersht, A. R. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 5326–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, J., and Wang, C. C. (1999) J. Biol. Chem. 274 10790–10794 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt, M., Rutkat, K., Rachel, R., Pfeifer, G., Jaenicke, R., Viitanen, P., Lorimer, G., and Buchner, J. (1994) Science 265 656–659 [DOI] [PubMed] [Google Scholar]
- 35.Azem, A., Kessel, M., and Goloubinoff, P. (1994) Science 265 653–656 [DOI] [PubMed] [Google Scholar]
- 36.Azem, A., Diamant, S., Kessel, M., Weiss, C., and Goloubinoff, P. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 12021–12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrales, F. J., and Fersht, A. R. (1996) Folding Des. 1 265–273 [DOI] [PubMed] [Google Scholar]
- 38.Ben-Zvi, A. P., Chatellier, J., Fersht, A. R., and Goloubinoff, P. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 15275–15280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayer-Hartl, M. K., Ewalt, K. L., and Hartl, F. U. (1999) Biol. Chem. 380 531–540 [DOI] [PubMed] [Google Scholar]
- 40.Horowitz, P. M., Lorimer, G. H., and Ybarra, J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 2682–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sameshima, T., Ueno, T., Iizuka, R., Ishii, N., Terada, N., Okabe, K., and Funatsu, T. (2008) J. Biol. Chem. 283 23765–23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.