Abstract
Background
Decreased hippocampal volume is described in posttraumatic stress disorder (PTSD) and depression. However, it is not known whether it is a risk factor for the development of PTSD or a consequence of PTSD. We sought to determine the effects of PTSD and depressive symptoms on hippocampal volume.
Methods
Clinical and magnetic resonance imaging data were collected in a cross sectional study of 244 Gulf War veterans. Measures included lifetime and current Clinician Administered PTSD Scale, Hamilton Depression Scale, Life Stressor Checklist, and Lifetime Drinking History. Magnetic resonance imaging data were acquired with a 1.5-T scanner and analyzed with automated and semiautomated image processing techniques.
Results
Eighty-two veterans had lifetime PTSD, 44 had current PTSD, and 38 had current depression. In the linear regression analysis, current PTSD symptoms (standardized coefficient β = −.25, p = .03) but neither lifetime PTSD symptoms nor current depression were associated with smaller hippocampal volume. Gender, age, history of early life trauma, education, lifetime and current alcohol use, current marijuana use, and treatment with antidepressants did not have independent effects. Participants with chronic PTSD had, on average, a smaller hippocampus compared with those with remitted PTSD.
Conclusions
The finding that current but not lifetime PTSD symptom severity explains hippocampal size raises two possibilities: either a small hippocampus is a risk factor for lack of recovery from PTSD (trait) or PTSD effects on hippocampal volume are reversible once PTSD symptoms remit and the patient recovers (state).
Keywords: Depression, Gulf War veterans, hippocampus, magnetic resonance imaging, posttraumatic stress disorder
Magnetic resonance imaging (MRI) studies have shown that posttraumatic stress disorder (PTSD) is associated with a reduced size of the hippocampus (1–4), a brain structure known to be responsive to stress (5,6) and essential to learning and memory processing (7,8). However, the nature of the relation between PTSD and hippocampal size is poorly understood.
Two fundamentally different ideas about the relationship are considered. According to one, a small hippocampus is considered a sequel of chronic stress reactivity in PTSD, and hippocampal damage is either attributed to the toxic effects of glucocorticoids and glutamate or related to decreased brain trophic factors such as brain-derived neurotrophic factor (9–14). A causal model of stress exposure on hippocampal damage is largely supported by animal studies (15–19). It has further been shown that duration and severity of PTSD are inversely related with hippocampal volume (1,20) and, in one study, that pharmacological intervention can reverse the volume reduction (21), which implies hippocampal plasticity in humans.
The alternative view is that a preexisting small hippocampus increases the vulnerability to develop PTSD. This idea is supported by an elegant imaging study of homozygous twins discordant for trauma exposure (22) where PTSD symptom severity in combat-exposed veterans was predicted by hippocampal volume of the unexposed twins. A definite answer to the causality question requires longitudinal studies with imaging and clinical data before and after exposure to a traumatic event.
The question is even more complex, because both PTSD and depression can develop after trauma exposure (23) and are often comorbid. Data from the National Comorbidity Study indicate that 48% of individuals with PTSD have experienced major depressive disorder at some time in their lives (24). Depression is also thought to be associated with smaller hippocampal volume. However, not all studies have found that, and duration of illness as well as number of depressive episodes might play an important role (25–28).
With this background, we evaluated hippocampal volumes of veterans from the First Persian Gulf War who had participated in a large MRI study of Gulf War Illness. Although we have no longitudinal imaging data and therefore cannot determine causality, we were fortunate to have a relatively large sample size that allowed us to contrast the effects of current versus remitted PTSD on hippocampal volume. We sought to test the hypothesis that veterans with remitted as well as current PTSD would have smaller hippocampal volumes compared with veterans without PTSD, independent of the effects of depression. This hypothesis was predicated on the model suggested by the twin study—that is, smaller hippocampal volume is a trait vulnerability risk factor for the development of PTSD.
Methods and Materials
Study Design
We conducted a secondary analysis of data from a cross sectional study of the effects of service in the Persian Gulf War on the brain. The original study was designed to test the hypothesis that Gulf War Illness was associated with decreased N-acetyl aspartate, an in vivo marker of neuronal viability, in the basal ganglia and the pons. All research was approved by the University of California at San Francisco and Veterans Administration Committees on Human Research and the Department of Defense Human Subjects Research Review Board. All procedures were performed in accordance with relevant guidelines and regulations. All participants (n = 279) provided written informed consent. The design is described in detail elsewhere (29). The primary study, conducted between 2002 and 2007, did not find an association between Gulf War Illness and structural or spectroscopic MRI findings in the brain. Therefore, there was no need to control for the effects of Gulf War Illness on hippocampal volume.
Participants
Participants had been recruited as a convenience sample with a variety of methods, largely by advertisement, and also with a list of Gulf War Veterans provided by the Department of Defense. There was no effort to recruit specifically for subjects with PTSD or depression. However, because at the onset of the study it was recognized that PTSD, depression, and alcohol abuse/dependence might affect cognition, clinical symptoms and brain structure, or metabolism, quantitative measurements of these variables were obtained (see following). Inclusion criterion was being a US veteran of the First Persian Gulf War; exclusion criteria were severe physical impairment or medical illness, current or lifetime history of psychosis or of suicidal or homicidal ideation, history of neurological or systemic illness affecting central nervous system functioning, history of head injury with loss of consciousness for at least 10 min, presence of severe claustrophobia, ferro-metallic objects in the body, and evidence of cholesteatoma or tympanic membrane perforation. The Structured Clinical Interview for DSM-IV Diagnosis (SCID) was used to diagnose psychiatric disorders other than PTSD (30).
For the present analysis data from 35 participants (12.5%) had to be excluded because of insufficient image quality. We had complete datasets for the relevant variables from 244 participants.
Procedures
A trained Ph.D.-level clinical interviewer conducted the interview. In addition to standardized questionnaires, demographic data and data on medication including antidepressants, alcohol use, and drug use were collected. The diagnostic interviews were audio-taped. Fifteen percent of the interviews were randomly selected and reviewed by blinded raters, who made independent diagnostic decisions regarding Clinician Administered PTSD Scale (CAPS), Hamilton Depression Scale (HAM-D), and SCID-IV assessments to establish levels of inter-rater reliability. The inter-rater reliability showed a κ of .90 for the SCID, CAPS, and HAM-D categorical diagnoses. Discrepancies in the evaluation of subjects were resolved by a consensus meeting.
Measures
The CAPS is a measure that follows the DSM-IV criteria for PTSD (31). Each of the 17 PTSD symptoms is rated with regard to frequency and intensity, so that an overall severity score can be obtained. The continuous score on the CAPS was used for primary hypothesis testing. Following the definition of Weathers et al. (32), who defined a score of 40 or more as moderate PTSD, we chose this cut-off point as our criterion for a PTSD diagnosis in secondary analyses. The questionnaire allows the rating of PTSD symptoms during the last month (current PTSD symptoms) and for the month during lifetime when the symptoms were worst (lifetime PTSD symptoms) (32).
The HAM-D is a frequently used 17-item depression inventory. A score up to seven is seen as in the normal range; we defined participants with a score of ≥ 14 as meeting criteria for depression (33).
The Life Stressor Checklist-Revised is a 30-item questionnaire that asks about traumatic or stressful life events such as physical or sexual assault, death of a close person, or natural disasters, following a yes/no format. Severity, impact, and time frame of endorsed events are further clarified (34). We used a binary score of 14 traumatic life events to determine the presence of early life trauma before the age of 14 (35).
The Lifetime Drinking History (LDH) is a structured interview that obtains quantitative data on the amount, duration, and pattern of lifetime alcohol consumption. It provides quantitative indexes of the alcohol consumption patterns of an individual, including frequency, quantity, and type of drink, at different times of life. The LDH has excellent psychometric properties (36). For the purpose of this analysis we calculated average drinks/months over lifetime (i.e., since onset of first alcohol consumption) and current average drinks/month (defined as averaged over the last phase of drinking as operationalized in the LDH).
Imaging
Structural MRI data were acquired with a 1.5-T scanner (Vision, Siemens Medical Systems, Iselin, New Jersey) and a three-dimensional magnetization prepared T1-weighted gradient echo sequence with the following parameters: repetition time/spin-echo time/inversion time = 10/4/300 msec, 1 × 1 mm2 in-plane resolution, and 1.5-mm slab thickness, angulated perpendicular to the long axis of the hippocampus.
Semiautomated hippocampal volumetry was carried out as described in detail previously (37), with a commercially available high-dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, Colorado) that has been validated and compared with manual tracing of the hippocampus (37). Briefly, measurement of hippocampal volume is achieved first by manually placing 22 control points as local landmarks for the hippocampus on individual brain MRI data and second by applying fluid image transformations to match the individual brains to a template brain (38). The pixels corresponding to the hippocampus are then labeled and counted to obtain volumes (Figure 1). This method of hippocampal voluming has a documented reliability of an intraclass coefficient better than .94 (37) and has been used to measure hippocampal volume in alcohol-dependent individuals (39). Intracranial volume (ICV) was determined with FreeSurfer, an FSL-based software that uses an atlas based spatial normalization procedure on T1 weighted images (40,41).
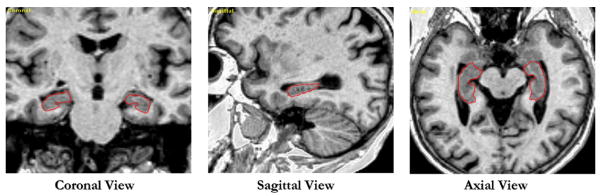
Figure 1.
Example of the semiautomatic hippocampal measurement with Surgical Navigation Technologies: coronal, sagittal, and axial views of the automatically set boundaries of the hippocampus.
Statistics
Bivariate correlation analyses were performed to determine the associations among clinical symptom scores. Because ICV is a significant determinant for regional brain volume including hippocampal volume, individual variations in ICV were accounted for by regressing hippocampal volumes against ICVs in the group and with the residuals of this regression as outcome variables for the following hierarchical linear regression analysis. Demographic factors, trauma experience, substance use, antidepressant treatment, and clinical symptom scores were entered as independent predictors in chronological order. Because the clinical symptom scores were highly correlated, they were entered into the regression equation individually and in different order. Group comparisons of the hierarchical models were tested with the F statistic. To simplify the model we performed a second regression analysis including only variables that had shown a p value of ≤ .1 in the first hierarchical regression analysis. This model was then used to test for interaction between PTSD symptoms/depression and depression/criterion A event. To determine whether hippocampal volumes differed among participants with chronic PTSD symptoms who were currently symptomatic, those with lifetime PTSD who have recovered, and those who never developed PTSD, a one-way analysis of variance was performed with posthoc testing to provide information about group differences. For each of the groups, mean values of the independent variables were calculated. The statistic package SPSS 16.0 (SPSS, Chicago, Illinois) was used for all these analyses. The level of significance was α = .05 in all tests.
Results
The demographic data are shown in Table 1. We had a predominantly male Caucasian population with a mean age of 45 years. A history of early life trauma was reported by 21% of the participants. Although all our participants were Gulf War veterans, only 61% fulfilled the criterion A of the DSM-IV PTSD diagnosis by reporting being exposed to, witnessing, or hearing about an event involving threat for life or physical integrity and with experience of intense fear, helplessness, or horror during or outside the military service. Figure 2 shows the types of trauma experienced: nearly 50% of the participants reported a combat trauma, and 10% reported an accident. Mean time since trauma exposure was 14.7 years with a range from 1 to 44 years. With the cut-off point of ≥40 on the CAPS for the diagnosis of PTSD, one-half of the 82 participants meeting criteria for lifetime PTSD had recovered at the time of the study. Thirty-eight participants had current depression on the basis of a HAM-D score of ≥ 14. Bivariate correlation analysis revealed strong correlations among the three clinical symptom scores, lifetime CAPS, current CAPS, and HAM-D (Spearman’s ρ between .49 and .88). Results of the linear regression analysis, in which hippocampal volume adjusted for ICV was used as outcome, are presented in Table 2. The demographic factors age, gender and education, lifetime alcohol use, current alcohol or marijuana use, treatment with anti-depressants, and history of early life trauma or of a traumatic event fulfilling the DSM-IV criterion for PTSD were not significantly associated with hippocampal volume. Lifetime PTSD symptom scores were also not significantly associated with hippocampal volume. In contrast, higher current PTSD symptom scores were associated with a smaller hippocampal volume. The result did not change significantly when current PTSD symptom scores were entered in the regression before lifetime scores. Depression scores were not significantly associated with hippocampal volume, whether they were entered before or after PTSD scores in the hierarchical model [ΔR2 = .0, ΔF (1,233) = .03, p = .8, and ΔR2 = .01, ΔF (1,231) = 1.7, p = .19, respectively].
Table 1.
Demographic Data and Test Results of 244 Gulf War Veterans
| n | Mean or Percentage | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age | 244 | 44.7 | 9.6 | 31 | 71 |
| Gender | |||||
| Female | 37 | 15% | |||
| Male | 207 | 85% | |||
| Ethnicity | |||||
| Caucasian | 151 | 62% | |||
| Afr. American | 38 | 16% | |||
| Latino | 21 | 9% | |||
| Other | 22 | 9% | |||
| Missing | 12 | 5% | |||
| Early Life Trauma Reported | 51 | 21% | |||
| Yrs of Education | 233 | 14.7 | 2.4 | 10 | 20 |
| Lifetime Average Drinks/Month | 241 | 31 | 40 | 0 | 256 |
| Current Average Drinks/Month | 226 | 12 | 28 | 0 | 300 |
| Current Marijuana Use | 17 | 7% | |||
| Prevalence of Trauma Exposure | 148 | 61% | |||
| Time of Trauma | |||||
| Premilitary | 11 | 5% | |||
| Military | 118 | 48% | |||
| Postmilitary | 19 | 8% | |||
| Duration of PTSD Symptoms (yrs) | 143 | 12.0 | 7.9 | 0 | 41 |
| Time Since Trauma (yrs) | 149 | 14.7 | 7.9 | 1 | 44 |
| CAPS Lifetime | 244 | 30.7 | 35.3 | 0 | 125 |
| CAPS Current | 244 | 16.8 | 24.9 | 0 | 108 |
| HAM-D | 244 | 6.7 | 6.3 | 0 | 28 |
| Antidepressant Use | 42 | 17% | |||
| ICV, mL | 244 | 1586 | 147 | 1217 | 2012 |
| Hippocampal Volume, mL | 244 | 5.27 | .66 | 3.57 | 7.18 |
PTSD, posttraumatic stress disorder; CAPS, Clinician Administered PTSD Scale; HAM-D, Hamilton Depression Scale; ICV, intracranial volume.
Figure 2.

Type of trauma reported by 244 Gulf War veterans. Multiple traumata are possible.
Table 2.
Summary of Hierarchical Regression Analysis on Hippocampal Volume Adjusted for ICV
| Independent Variables | ΔR2 | Adjusted R2 | ΔF | Standardized Coefficients β for Each Step
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | Step 6 | Step 7 | Step 8 | ||||
| Step 1 | .011 | .002 | 1.28 | ||||||||
| Age | −.027 | −.025 | −.045 | −.050 | −.056 | −.071 | −.081 | −.085 | |||
| Gender | −.096 | −.106 | −.116 | −.117 | −.100 | −.085 | −.095 | −.102 | |||
| Step 2 | .003 | .001 | .75 | ||||||||
| Early Life Trauma | .056 | .053 | .067 | .094 | .093 | .104 | .100 | ||||
| Step 3 | .010 | .003 | 1.19 | ||||||||
| Education | .060 | .055 | .050 | .054 | .035 | .041 | |||||
| Lifetime Alcohol Use | −.068 | −.065 | −.056 | .015 | −.005 | .000 | |||||
| Step 4 | .002 | .001 | .58 | ||||||||
| Criterion A Event | −.052 | .052 | .042 | .025 | .026 | ||||||
| Step 5 | .012 | .010 | 3.05 | ||||||||
| Lifetime CAPS Score | −.162 | −.171 | .041 | .043 | |||||||
| Step 6 | .020 | .018 | 1.64 | ||||||||
| Current Alcoholism Use | −.125 | −.126 | −.130 | ||||||||
| Current Marijuana Use | −.091 | −.065 | −.070 | ||||||||
| Antidepressant Use | .008 | .040 | .011 | ||||||||
| Step 7 | .019 | .033 | 4.67a | ||||||||
| Current CAPS Score | −.257a | −.317a | |||||||||
| Step 8 | .007 | .036 | 1.75 | ||||||||
| HAM-D Depression Score | .112 | ||||||||||
N = 244. The standardized regression coefficients β are provided for each step of the hierarchical regression.
Abbreviations as in Table 1.
p < .05.
The simplified regression model included only predictor variables with a significance level of p ≤ .1 in the first hierarchical regression model. In the final step, higher current alcohol use (β=−.14, p =.04) and higher current PTSD symptoms (β = −.29, p = .02) were associated with smaller hippocampal volume. Further exploration of the finding that current alcohol use correlated with smaller hippocampal volume showed that this relationship was driven by two individuals without trauma exposure who reported current average alcohol use of 180 and 300 drinks/month, more than 3 SDs outside the mean. Without these data points only current PTSD symptoms had a significant influence on hippocampal volume (Table 3). The simplified model was used to test whether the effect of predictor variables on hippocampal volume was moderated by other variables. We did not find a significant moderation of the effect of current PTSD symptoms by depression [F (1,239) = 3.2; p = .08] or lifetime PTSD symptoms by depression [F (1,239) = .06; p = .8] or of depressive symptoms by event criterion A [F (1,239) = .65; p = .4] or of current PTSD by current alcohol use [F (1,240) = .50; p = .5].
Table 3.
Summary of a Simplified Hierarchical Regression Analysis on Hippocampal Volume Adjusted for ICV
| Independent Variables | ΔR2 | Adjusted R2 | ΔF | Standardized Coefficients β
|
||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | ||||
| Step 1 | .010 | .002 | 1.2 | |||
| Gender | −.090 | −.075 | −.083 | |||
| Current Alcohol Use | −.050 | −.051 | −.063 | |||
| Step 2 | .012 | .010 | 3.0 | |||
| Lifetime CAPS Score | −.112 | .095 | ||||
| Step 3 | .020 | .026 | 4.8a | |||
| Current CAPS Score | −.250a | |||||
N = 242 after exclusion of two current alcohol use outliers. The standardized regression coefficients β are provided for each step of the hierarchical regression.
Abbreviations as in Table 1.
p < .05.
The difference in trauma exposure and PTSD symptoms led to four groups: participants without exposure to trauma, those with exposure who never developed PTSD, those with chronic PTSD (lifetime and current PTSD), and those who recovered from PTSD (lifetime but no current PTSD). Three participants who fulfilled criteria for current PTSD only were excluded from the following analyses. Table 4 shows the means of the predictor variables and hippocampal volume separately for the four groups. As expected, the group with chronic PTSD has more depression, uses more antidepressants, and has a higher fraction of individuals with early life trauma.
Table 4.
Comparison of the Groups
| No Trauma | Trauma, No PTSD | Remitted PTSD | Chronic PTSD | p Value of ANOVA | |
|---|---|---|---|---|---|
| Number of Participants | 95 (39%) | 64 (26%) | 41 (17%) | 41 (17%) | |
| Age | 46.4 | 44.4 | 43.0 | 42.1 | .094 |
| Female Gender | 15% | 9% | 20% | 22% | ns |
| Lifetime Alcohol Usea | 25.56 | 32.82 | 34.68 | 37.47 | ns |
| Current Alcohol Usea | 14.15 | 13.21 | 11.20 | 7.87 | ns |
| Current Marijuana Use | 6% | 5% | 2% | 17% | .041 |
| Early Life Trauma Reported | 7% | 21% | 24% | 47% | <.001 |
| Yrs of Education | 15.0 | 14.7 | 14.3 | 14.3 | ns |
| Use of Antidepressants | 12% | 11% | 20% | 39% | <.001 |
| ICV (mL) | 1582 | 1606 | 1583 | 1569 | ns |
| Depression Score (HAM-D) | 4.3 | 5.2 | 7.2 | 14.1 | <.001 |
| Current CAPS Score | 0 | 8.3 | 19.3 | 64.4 | <.001 |
| Lifetime CAPS Score | 0 | 20.7 | 63.3 | 84.9 | <.001 |
| BL Hippocampal Volumea (mL) | 5.292 | 5.343 | 5.367 | 5.006 | .040 |
| BL Hippocampal Volume, ICV-Adjusteda (mL) | 5.300 | 5.300 | 5.374 | 5.044 | .046 |
N = 241. The four groups were defined by trauma exposure and PTSD symptom severity. No Trauma: no Criterion A event reported; Trauma, no PTSD: Criterion A event reported, lifetime and current CAPS score < 40; Remitted PTSD: Criterion A event reported, lifetime CAPS score ≥ 40, current CAPS score < 40; Chronic PTSD: Criterion A event reported, lifetime and current CAPS score ≥ 40. Data is presented as means and percentages.
ANOVA, analysis of variance; BL, bilateral; other abbreviations as in Table 1.
Drinks/month.
Because ICV-adjusted hippocampal volume was identical for participants without trauma and without PTSD, these groups were combined. A one-way analysis of variance (Figure 3) showed a significant difference [F (2,238) = 4.1, p = .018] in ICV-adjusted hippocampal volume among participants with chronic PTSD symptoms, those who have recovered from PTSD, and those who never developed PTSD. Post hoc tests indicated that the group effect was attributable to the participants with chronic PTSD who met criteria at the time of imaging; their mean hippocampal volume was smaller than that of participants who recovered from PTSD (mean difference 6.5%, p < .05) and those who never developed PTSD (mean difference 5.1%, p <.05). There was no significant difference in mean adjusted hippocampal volume between participants who had recovered from PTSD and participants who had never developed PTSD (p =.7). When we replicated the analyses including only participants with trauma exposure, the results did not change in a significant way.
Figure 3.

Comparison of mean adjusted hippocampal volume in 241 Gulf War veterans. The error bars show the SD. The numbers at the base of the bars indicate the adjusted hippocampal volume in mm3. ICV, intracranial volume; PTSD, posttraumatic stress disorder.
Discussion
The main finding of this study is that current PTSD symptoms were associated with smaller hippocampal volume, whereas lifetime PTSD symptoms were not. Participants with chronic PTSD had on average a smaller hippocampus than those who recovered from PTSD or never developed PTSD. The finding remained significant after accounting for early life trauma, current and lifetime alcohol use, depression, and treatment with antidepressants. This result conflicts with our initial hypothesis that hippocampal volume is a vulnerability marker for PTSD and as such should be associated with both current and lifetime PTSD. Our results raise the possibility that hippocampal volume is state-dependent and might vary over time, consistent with findings in other pathologies.
This conclusion is supported by the aforementioned studies showing that duration and severity of PTSD symptoms were negatively correlated with hippocampal size (1,20) and that hippocampal size increased after long-term paroxetine therapy in PTSD patients (21). A growing literature in other diseases shows that the hippocampus can change in response to exercise, a variety of pharmacological interventions (42–50), and alcohol abstinence (39). The conclusion is also supported by our previous finding in PTSD patients of reduced volume in the hippocampal subfield CA3 and the dentate gyrus (51,52), areas that are known to undergo neurogenesis in adulthood (10,53,54). This would mean that the hippocampus can be damaged by pathophysiological processes in those with current PTSD and potentially recover from the associated volume loss, due to cellular plasticity including neurogenesis.
The alternative interpretation of our findings modifies the conclusion of Gilbertson et al. (22) that smaller hippocampal volume is a familial vulnerability factor for PTSD. Gilbertson et al. excluded twin pairs when the combat-exposed brother had past but no current PTSD; so they excluded recovered PTSD patients and compared only chronic PTSD patients with PTSD-resistant trauma-exposed veterans (55). Therefore, it is possible that PTSD symptoms develop independent of hippocampal volume; yet only patients with (familial) small hippocampal volume develop a chronic nonremitting form of the disorder. This model would suggest that patients with normal-sized hippocampus recover after some time and has implications for predicting treatment success in intervention studies. From this perspective a smaller hippocampal volume could be considered detrimental to recovery rather than a vulnerability factor for developing PTSD.
It seems reasonable to hypothesize that — considering the role of the hippocampus in learning, memory, and mood regulation—PTSD patients with normal hippocampal volume have a greater prospect of recovery than those with decreased volume before exposure. Pitman proposed that lower IQ, a known risk factor for PTSD (56), might be a mediating link leading to PTSD (57).
Because our data are from a cross sectional study with measurement of hippocampal volume at one time point only, we cannot determine which of these two interpretations is correct. However, the retrospective evaluation of PTSD symptoms allows differentiating the time course for the illness and points to the possibility that the vulnerability hypothesis might be too simple. In this context results of longitudinal studies in PTSD are of special interest. We previously mentioned the study by Vermetten et al. (21), which showed that smaller hippocampal size in patients with chronic PTSD increased after paroxetine treatment over 36–48 weeks, although there was no correlation with the improvement of the CAPS score. In a longitudinal study Bonne et al. (58) found neither a smaller hippocampus initially nor hippocampal volume loss over 6 months in 10 trauma survivors who developed PTSD relative to those who did not. The authors discussed short length of trauma exposure, older age at trauma exposure, and less chronicity of symptoms as possible explanations. A small study in outpatients demonstrated a smaller hippocampal volume in participants with PTSD compared with control subjects but did not find a change in volume after 4 months of clinically successful psychotherapy (59). In that study the sample size of nine patients receiving psychotherapy and the short time interval might have diminished the power to detect a longitudinal effect. Another longitudinal study in abused children by De Bellis et al. (60) could also not replicate previous cross sectional findings of hippocampal volume reduction. Recent studies suggest that, although a history of childhood trauma is associated with reduced hippocampal volume in adults, this does not become manifest until later in development (1,61,62). Interestingly, Cardenas-Nicholson et al. (63) found progressive brain atrophy in patients with chronic PTSD whose symptoms got worse, in contrast to those whose symptoms improved, although these atrophic changes did not involve the hippocampus. Taken together, the existing longitudinal studies have limitations for elucidating the role of hippocampal plasticity in PTSD, so that further research is needed.
Our study included several other predictors of hippocampal volume, of which only current alcohol use had a significant impact on first observation. However, closer inspection revealed that this relationship was driven by two participants with high current alcohol use. It has been described that chronic alcohol consumption to this degree is associated with significant hippocampal volume loss (64–66). Previously, we used the same methodology of measuring hippocampal volume as used in this report in alcohol-dependent individuals and found a smaller hippocampus as well as increases in hippocampal volume during abstinence from alcohol (39). In this cohort, however, the average for current drinks/month was 12 (SD 28) and the average for lifetime drinks/month was 31 (SD 40). There have been no reports of such an amount of alcohol consumption appreciably affecting hippocampal volume.
Furthermore, we did not find a significant effect of early life trauma on hippocampal size. This is discrepant with other studies that did find a negative effect of early life trauma on hippocampal volume (67–71). There are developmental periods during which areas of the brain are more sensitive; thus our variable “childhood trauma” covering up to the age of 14 might be too broad a time period (72).
Treatment with antidepressants for PTSD, anxiety, or depression was reported by 17% of all participants. Malberg et al. (73) showed in rodents that chronic administration of antidepressants increases hippocampal neurogenesis. This was shown for different classes of antidepressants (49). However, our analysis did not find an effect of antidepressant treatment on hippocampal size. Although the group of participants with chronic PTSD had a higher frequency of antidepressive treatment, their hippocampal volume was smaller compared with other groups. It is possible that the effect of chronic PTSD on hippocampal volume in this sample masks the effects of antidepressants.
Depression is another common sequela to traumatic stress exposure, and therefore we considered its potential impact in our multivariate analysis. We found that depression did not predict hippocampal volume, irrespective of whether we controlled for PTSD symptoms or not, which contrasts with meta-analysis studies that showed multiple episodes and severity of depression can influence hippocampal size especially at advanced age (25–28). In contrast to these studies, our study involved a relatively young population with relatively mild depression symptoms. This might have limited our ability to detect a link between depression and hippocampal volume.
The main limitation of our study is the cross sectional design, which limits our ability to determine causality with respect to hippocampal volume. Furthermore, we have limited generalizability by having a sample of Gulf War Veterans. Additionally, we did not measure family history of mood disorders and PTSD. Also history and clinical scores rely on the subjective report of the individual participant and may be biased by under- or over-reporting. It is likely that current symptom status influences estimates of past symptoms. However, the possible contamination effect of current symptom status on reports of lifetime severity in all likelihood would introduce a negative bias away from finding differences in the relationship of hippocampal volume to past versus current PTSD. In a previous study we showed that the volume loss in PTSD is primarily focused in the CA3 subfield (51); therefore measurements of the whole hippocampus might be less precise than measurements of hippocampal subfields. However, it remains unclear whether adult neurogenesis accounts for the whole change in hippocampal volume, because neurogenesis has been demonstrated in only a relatively small portion of the hippocampus and because there are many different cells in the hippocampus (74). In the future, well-designed longitudinal studies that follow high-risk populations before and after trauma exposure and follow fluctuations in symptom levels will be needed to establish a time course of possible hippocampal change in humans and specifically to advance our understanding of the biological processes underlying PTSD.
In conclusion, our data in more than 200 well-characterized participants suggest that current presence of chronic PTSD symptoms rather than remitted PTSD is associated with a smaller hippocampus. This raises the possibility of recovery from hippocampal volume loss in PTSD.
Acknowledgments
This study was supported by Department of Defense Grant DAMD17-01-1-0764, entitled, “Magnetic Resonance and Spectroscopy of the Human Brainin Gulf War Illness,” awarded to the Northern California Institute for Research and Education from the Department of Defense Gulf War Illnesses Research Program, US Army Medical Research and Materiel Command. This study was also supported by National Institutes of Health/National Institute of Environmental Health Sciences Grant ES09883 and the Mental Illness Research, Education, and Clinical Center of the Department of Veterans Affairs. Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco, California. Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs, and R01 AA10788 (DJM). We are grateful to Karl Friedl, Ph.D., for his support, and last but not least we thank Diana Truran and her staff for MRI scanning.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 4.Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry Suppl. 1992:18–23. [PubMed] [Google Scholar]
- 7.Dickerson BC, Eichenbaum H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitman R, Shalev A, Orr S. Posttraumatic stress disorder: Emotion, conditioning, and memory. In: Corbetta M, Gazzaniga M, editors. The New Cognitive Neurosciences. 2. New York: Plenum Press; 2000. pp. 687–700. [Google Scholar]
- 9.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 10.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 11.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 13.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 14.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons—a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 16.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20:1402–1406. doi: 10.1097/WNR.0b013e3283300fbc. [DOI] [PubMed] [Google Scholar]
- 21.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant RA, O’Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D. The psychiatric sequelae of traumatic injury. Am J Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 25.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, et al. The relationship between gulf war illness, brain N-acetyl aspartate and post-traumatic stress disorder. Mil Med. doi: 10.7205/milmed-d-10-00332. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis 1 Disorders—Non-Patient Edition (SCID-I/NP, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 31.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 32.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe J, Kimerling R, Brown PJ, Chresman KR, Levin K. Psychometric Review of the Life Stressor Checklist-Revised. Lutherville, Maryland: Sidran Publishing Group; 1996. [Google Scholar]
- 35.Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, editors. Assessing Psychological Trauma and PTSD. New York: Guilford; 1997. pp. 192–238. [Google Scholar]
- 36.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging. 1997;16:864–877. doi: 10.1109/42.650882. [DOI] [PubMed] [Google Scholar]
- 39.Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 42.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 44.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, Madsen TM, Wegener G, Nyengaard JR. Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression [published online ahead of print November 17] Hippocampus. 2009 doi: 10.1002/hipo.20718. [DOI] [PubMed] [Google Scholar]
- 46.Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 48.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 50.Koolschijn PC, van Haren NE, Cahn W, Schnack HG, Janssen J, Klumpers F, et al. Hippocampal volume change in schizophrenia. J Clin Psychiatry. 2010;71:737–744. doi: 10.4088/JCP.08m04574yel. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in post-traumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry. 2010;68:494–496. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 55.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, et al. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macklin ML, Metzger LJ, Litz BT, McNally RJ, Lasko NB, Orr SP, Pitman RK. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol. 1998;66:323–326. doi: 10.1037//0022-006x.66.2.323. [DOI] [PubMed] [Google Scholar]
- 57.Pitman RK. Posttraumatic stress disorder and dementia: What is the origin of the association? JAMA. 2010;303:2287–2288. doi: 10.1001/jama.2010.767. [DOI] [PubMed] [Google Scholar]
- 58.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: A MRI investigation. Psychol Med. 2005;35:1421–1431. doi: 10.1017/S0033291705005246. [DOI] [PubMed] [Google Scholar]
- 60.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 61.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 63.Cardenas V, Samuelson K, Lenoci M, Studholme C, Neylan T, Marmar C, et al. Changes in brain anatomy during the course of PTSD. Psychiatry Res. doi: 10.1016/j.pscychresns.2011.01.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 65.Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 66.Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Man-dyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 70.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 73.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balu DT, Lucki I. Adult hippocampal neurogenesis: Regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]