Abstract
PML is a potent tumor suppressor and proapoptotic factor and is functionally regulated by post-translational modifications such as phosphorylation, sumoylation, and ubiquitination. Histone deacetylase (HDAC) inhibitors are a promising class of targeted anticancer agents and induce apoptosis in cancer cells by largely unknown mechanisms. We report here a novel post-transcriptional modification, acetylation, of PML. PML exists as an acetylated protein in HeLa cells, and its acetylation is enhanced by coexpression of p300 or treatment with a HDAC inhibitor, trichostatin A. Increased PML acetylation is associated with increased sumoylation of PML in vitro and in vivo. PML is involved in trichostatin A-induced apoptosis and PML with an acetylation-defective mutation shows an inability to mediate apoptosis, suggesting the importance of PML acetylation. Our work provides new insights into PML regulation by post-translational modification and new information about the therapeutic mechanism of HDAC inhibitors.
The promyelocytic leukemia protein PML controls cell cycle progression, senescence, and cell death (1, 2). Wild-type PML is a potent growth suppressor that, when overexpressed, can block cell cycle progression in a variety of tumor cell lines (1); conversely PML–/– mouse embryo fibroblasts (MEFs)2 replicate significantly faster than their PML+/+ MEFs (3). PML also plays an essential role in DNA damage or stress-induced apoptosis, and PML–/– cells are resistant to a variety of apoptotic signals (4). In normal cells, the PML protein is localized in, and essential for the biogenesis of, discrete subnuclear compartments designated as nuclear bodies (NBs) (5). In NBs, PML coaccumulates with more than 70 kinds of proteins that are involved in tumor suppression, apoptosis, regulation of gene expression, anti-viral response, and DNA repair. PML is thought to exert its function by regulating the function of binding partners as a core of NBs (6). Intriguingly, NBs are disrupted in human acute promyelocytic leukemia cells by PML-RARα, a oncogenic fusion protein of PML, and RAR-α, which is thought to be the mechanism of anti-apoptotic effect of PML-RARα (7–9).
It has been reported that NB formation requires PML to be conjugated to SUMO-1 (5, 10). SUMO-1 is an 11-kDa protein that is structurally homologous to ubiquitin (11). Sumoylation is thought to regulate the subcellular localization, stability, DNA binding, and/or transcriptional ability of its target proteins such as PML, Ran GTPase-activating protein (RanGAP)1, IκBα, and heat shock transcription factor 2 (11). Virtually As2O3, a chemotherapeutic agent clinically used in the treatment of acute promyelocytic leukemia cells, induces PML sumoylation. Increased PML sumoylation induced by As2O3 treatment leads to the restoration of NBs disrupted by PML-RARα and then is followed by apoptosis in acute promyelocytic leukemia cells, which results in prolonged remission of the disease (12–15). These findings underscore the importance of PML sumoylation and the integrity of NBs to tumor suppression.
Histone deacetylase (HDAC) inhibitors, a promising class of targeted anticancer agents, can block proliferation and induce cell death in a wide variety of transformed cells (16). HDAC inhibitors block the activity of class I and II HDACs and induce histone acetylation, which leads to the relaxation of chromatin structure, enhanced accessibility of transcription machinery to DNA, and increased gene transcription (17). HDAC inhibitors also induce acetylation of transcription factors, which alters their activities and the expression of their target genes (18). Recent studies demonstrated that p53 acetylation induced by a HDAC inhibitor leads to expression of proapoptotic proteins such as Bax, PIG3, and NOXA (19, 20). However, the mechanisms of p53-independent apoptosis by HDAC inhibitor remain largely unknown.
Here, we report PML acetylation as its novel post-transcriptional modification. PML acetylation is induced by trichostatin A (TSA), a HDAC inhibitor. This enhanced acetylation leads to increased PML sumoylation and may play a key role in TSA-induced apoptosis. This work provides new insights into the functional regulation of PML and the therapeutic mechanisms of treatment with HDAC inhibitors.
EXPERIMENTAL PROCEDURES
Antibodies, Plasmids, and Cell Culture—The sources of antibodies and plasmids and the cell culture conditions are detailed in the supplemental data.
Transient Transfection, Immunoprecipitation, Immunoblotting, Immunofluorescence, and in Vitro and in Vivo Acetylation Assays—These were performed as described previously (21, 28) except transient transfections into PML–/– MEFs were performed by nucleofection using the Nucleofector system (Amaxa Biosystems) according to the manufacturer's instructions.
Antibody Array Assay and Detection and Quantification of Apoptosis—These are also detailed in the supplemental data.
In Vitro Sumoylation Assay—The in vitro sumoylation assay was performed essentially as described previously (21), except recombinant SUMO E1 ligase purchased from BIOMOL International was used instead of HeLa cell lysate.
Cell Sorting—Cell sorting was performed using BD FACSAria Cell Sorter (BD Biosciences) according to the manufacturer's instructions.
RESULTS
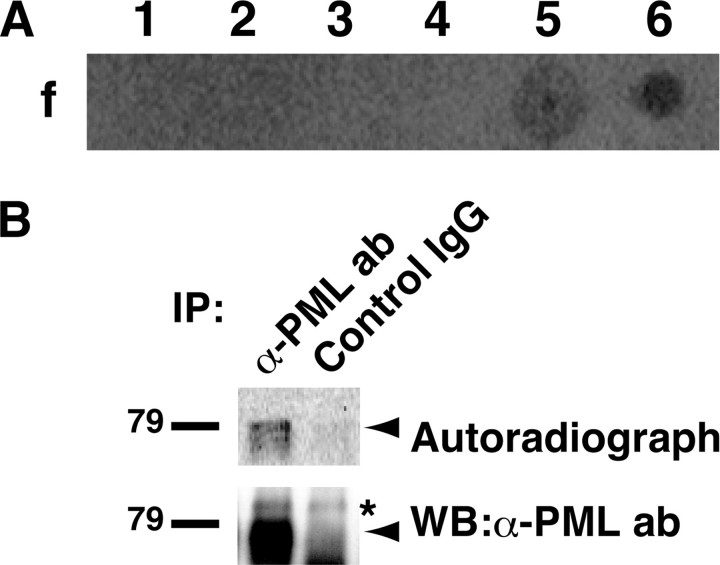
PML Exists as an Acetylated Protein in HeLa Cells Treated with Trichostatin A—HDAC inhibitors including TSA induce differentiation, growth arrest, and apoptosis of cancer cells. In addition to their effects on histones, HDAC inhibitors increase the acetylation level of several non-histone proteins, such as transcription factors, cytoskeletal proteins, and molecular chaperones, which are important for their effects on cancer cells (18). These observations prompted us to screen new acetylation targets of TSA with an antibody array assay combined with in vivo labeling of acetylated proteins with [14C]acetate. Seven spots indicating possible targets of TSA-induced acetylation were detected (supplemental Fig. S1 and Fig. 1A). We focused on one of these targets, PML, a multifunctional protein that is involved in apoptosis, tumor suppression, and cell cycle regulation (2). We set out to confirm whether PML was acetylated in vivo. The same anti-PML antibody as used in the antibody array assay immunoprecipitated an ∼79-kDa acetylated protein from lysates of TSA-treated HeLa cells (Fig. 1B), suggesting that PML existed as an acetylated protein in them. Of note, the antibody we used detected only a single band of PML, although PML has seven isoforms. The antibody was confirmed to be able to detect all PML isoforms, suggesting that this PML was the main one expressed in HeLa cells (supplemental Fig. S1).
FIGURE 1.
PML exists as an acetylated protein in HeLa cells treated with TSA. A, screening for acetylation targets of TSA by antibody array. Lysate from HeLa cells pulse-labeled with [14C]acetate and treated with TSA was incubated with a nitrocellulose array comprising 113 antibodies. After extensive washes, the array was subjected to autoradiography. Similar results were obtained from duplicated experiments. Representative spots are shown. The antibodies used for f-1 to f-6 are anti-MEK1/2 antibody, anti-phospho-MEK1/2 antibody, anti-MKP-1 (V-15) antibody, anti-PML (PG-M) antibody, anti-PML (H-238) antibody, and anti-acetylated lysine antibody, respectively. B, PML exists as an acetylated protein in TSA-treated HeLa cells. Pulse-labeled HeLa cells with TSA treatment were lysed as in A. The lysates were immunoprecipitated (IP) with PML antibody (ab) or control rabbit IgG. 90% of the immunoprecipitates were subjected to SDS-PAGE, autoradiography, and detection with phosphorimaging (upper panel). 10% of the immunoprecipitates were subjected to SDS-PAGE and immunoblotting with PML antibody (lower panel). The positions of PML and nonspecific band are indicated by an arrow and an asterisk, respectively. WB, Western blotting.
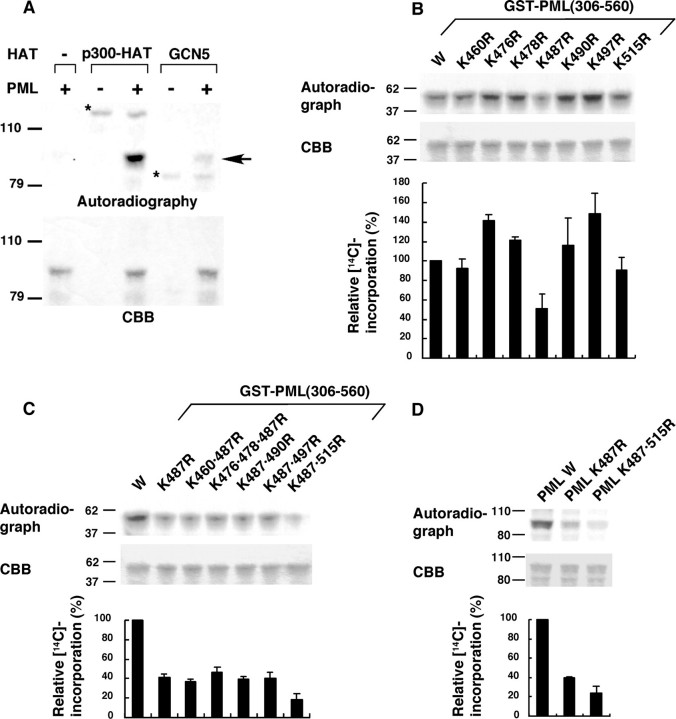
PML Is Acetylated by p300 and GCN5 in Vitro—To test whether known histone acetyltransferases (HATs), p300 and GSN5, can acetylate PML, we performed an in vitro acetylation assay using GST-PML as a substrate. Both HATs acetylated PML in vitro (Fig. 2A), and we focused on the acetylation by p300 that occurred with higher efficiency. Use of a series of PML subdomains in the in vitro p300 acetylation assay indicated that PML would be acetylated on the C-terminal domain, amino acids 448–560 (supplemental Fig. S2). Inspection of the PML sequence in this region revealed the presence of 7 lysine residues (supplemental Fig. S2); therefore, we introduced a series of arginine substitutions to map the acetylation sites. Within the C-terminal domain of PML, only the substitution of arginine for lysine 487 (K487R) measurably reduced acetylation of PML by p300 among all the individual substitutions (Fig. 2B; mutations are designated by the codon number between Lys and Arg). When arginine substitutions for other lysines were combined with K487R, a further reduction of acetylation was observed with K515R (Fig. 2C). Acetylation of full-length PML was impaired by substitutions of K487R and K487R/K515R similarly to that of the C-terminal domain of PML (Fig. 2D). Our results indicate that the principal sites of p300 acetylation in PML will be lysines 487 and 515.
FIGURE 2.
PML acetylation in vitro. A, PML is acetylated by p300 and GCN5. GST-PML was incubated with [14C]acetyl-CoA and the indicated HATs. The reaction mixtures were subjected to SDS-PAGE, Coomassie Brilliant Blue (CBB) staining, and autoradiography. The positions of acetylated PML and acetylases are indicated by an arrow and asterisks, respectively. B and C, identification of PML acetylation sites. Wild-type or mutant GST-PML (amino acids 306–560) were subjected to in vitro acetylation by p300-HAT as in A. 14C incorporation into each GST-PML construct was quantified using phosphorimaging. A representative phosphorimaging scan (top panel), the corresponding CBB-stained electrophoretogram (middle panel), and the quantitation of the 14C incorporation for each mutant, relative to the wild-type PML construct (bottom panel), are presented. The averages of three independent analyses and standard deviations are shown. D, mutation of lysines in full-length PML reduced acetylation by p300. Wild-type or mutant GST-PML (full-length) were subjected to in vitro acetylation assays as described in B.
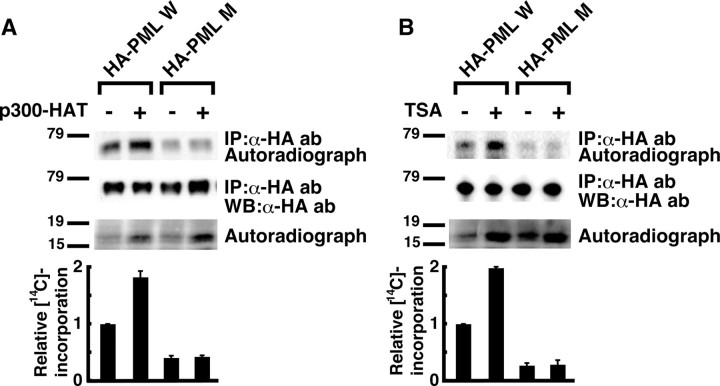
PML Acetylation Is Increased in Response to TSA Treatment—We examined whether PML acetylation by p300 occurred in vivo at the same sites as identified in vitro. Wild-type PML and PML with the K487R/K515R mutations were designated as PML W and PML M, respectively. Coexpression of p300 enhanced PML W acetylation, whereas acetylation PML M was weak in the basal state and showed no significant response to p300 coexpression (Fig. 3A, top panel). The efficiency of immunoprecipitation was the same for all samples (Fig. 3A, middle panel), and the increase in acetylation of a 17-kDa protein by coexpression of p300, which suggested the induction of histone acetylation, was equal between transfectants with PML W and PML M (Fig. 3A, bottom panel). These results suggest that p300 acetylates PML in vivo at the same sites as identified in vitro.
FIGURE 3.
PML acetylation in vivo. A, PML acetylation is induced by p300 cotransfection in vivo. HeLa cells were transfected with indicated expression vectors. PML acetylation was analyzed as in Fig. 1B except for the use of anti-HA antibody (ab) instead of anti-PML antibody for IP and IB. The lysates were also subjected to SDS-PAGE followed by autoradiography to confirm successful induction of histone acetylation by p300-HAT cotransfection. A representative autoradiograph of PML (top panel), the corresponding image of IB with anti-HA antibody (second panel from the top), and the autoradiograph of histone (third panel from the top) are presented. 14C incorporation into PML and the amount of immunoprecipitated (IP) PML protein were quantified using analyzer of each imaging system. Relative 14C incorporation adjusted by the efficiency of immunoprecipitation was calculated. The average and standard deviations for two independent analyses are presented (bottom panel). B, PML acetylation is increased in response to TSA treatment. HeLa cells were transfected with indicated expression vectors and treated with or without 10 μm TSA for 4 h. PML acetylation was analyzed and presented as in A. WB, Western blotting.
Next, we tested whether PML acetylation is induced by TSA. Similarly to the case of p300 coexpression, TSA treatment enhanced PML W acetylation, whereas acetylation of PML M was significantly reduced and showed no significant response to TSA treatment (Fig. 3B, top panel). Our results suggest that PML is an acetylation target of TSA and that PML acetylation in response to TSA largely occurs at the sites of acetylation by p300.
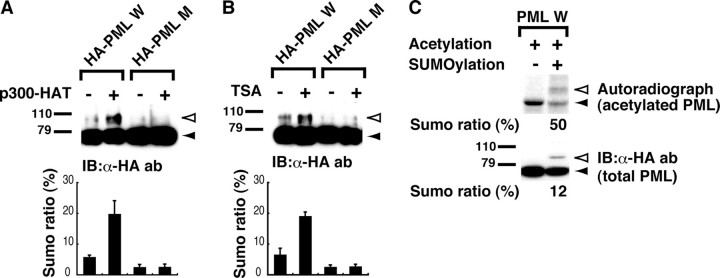
Acetylation of PML in Response to TSA Is Associated with Enhanced PML Sumoylation—Because one of the PML acetylation sites, lysine 487, is located in the putative nuclear localization signal (amino acids 476–490), we first examined whether PML acetylation affected its nuclear localization to see the effect of acetylation on PML function. However, TSA treatment and the acetylation-defective mutation did not obviously affect PML nuclear localization or accumulation to NBs in immunofluorescent staining (supplemental Fig. S3). We next investigated whether PML acetylation affected its sumoylation, which is required for PML to exercise many of its functions. We set up an in vivo sumoylation system in which conjugation of SUMO to PML could be detected by Western analysis, resulting in the appearance of a novel 100-kDa protein expected to be sumoylated PML (21). In this system, coexpression of p300 and exposure to TSA resulted in a significant increase in sumoylation of PML W, whereas sumoylation of PML M was weak and not obviously affected by these treatments (Fig. 4, A and B). These results suggest the possibility that PML acetylation at lysines 487 and 515 enhances its sumoylation. Next, to verify that acetylated PML is directly sumoylated, we set up an in vitro sumoylation system in which recombinant HA-tagged PML protein was incubated with recombinant SUMO E1 and E2 ligase and SUMO with or without a prior acetylation reaction using [14C]acetyl-CoA. Autoradiography visualizing only acetylated PML demonstrated that acetylated PML was efficiently sumoylated (Fig. 4C, upper panel). Of note, sumoylation efficiency observed in the autoradiograph was much higher than that in immunoblotting with anti-HA antibody where acetylated and nonacetylated PML were visualized (Fig. 4C, upper panel, lane 2 versus lower panel, lane 2). These results indicate that acetylated PML may be preferentially sumoylated in vitro.
FIGURE 4.
PML acetylation is associated with enhanced sumoylation. A, p300 coexpression enhances PML sumoylation. HeLa cells were transfected with the indicated expression vectors with cotransfection of those for SUMO and Ubc9. The cell lysates were subjected to SDS-PAGE followed by immunoblotting (IB) with anti-HA antibody (ab). The positions of sumoylated PML detected as an upper shifted band and nonsumoylated PML are indicated by white and black arrowheads, respectively. The ratios of sumoylated PML to total (sumoylated and unsumoylated PML) were calculated (SUMO ratio) and presented as bar charts. B, PML sumoylation increases in response to TSA treatment. PML sumoylation was examined as in A except that cells were treated with 10 μm TSA for 4 h instead of cotransfection of p300-HAT. C, acetylated PML is preferentially sumoylated in vitro. HA-tagged PML protein synthesized in vitro was immunoprecipitated with anti-HA antibody and incubated with p300-HAT and [14C]acetyl-CoA as in Fig. 2A. The protein was subjected to an in vitro sumoylation assay and SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Sumoylation of total (acetylated and nonacetylated) PML was visualized by IB with anti-HA antibody (lower panel). The same membrane was also subjected to an autoradiography to visualize sumoylation of acetylated PML (upper panel). Sumoylated and nonsumoylated PML are indicated as in A. SUMO ratio in each panel were calculated and presented at the bottom.
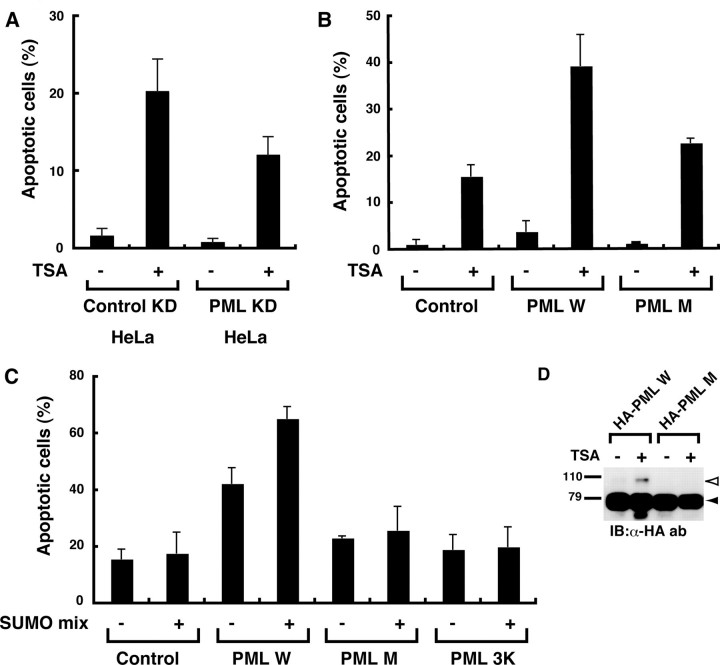
PML Acetylation May Play an Important Role in TSA-induced Apoptosis—TSA treatment induces apoptosis in HeLa cells by unknown mechanisms (16). In our previous study, PML sumoylation plays an important role in As2O3-induced apoptosis (21). Given our findings that PML acetylation is associated with increased PML sumoylation, this may represent one of the mechanisms of TSA-induced apoptosis. We first examined whether PML was involved in TSA-induced apoptosis. For this purpose, we established HeLa cells stably transfected with an expression vector for small hairpin RNA against PML and an empty control vector and designated them as PML KD HeLa and control KD HeLa, respectively. Successful knocking down of PML was confirmed by immunofluorescence analysis with an anti-PML antibody (supplemental Fig. S4). TSA treatment caused the appearance of a sub-G1 peak in cell cycle analysis, a marker of apoptosis, in both cells. However, the ratio of apoptotic cells was reduced by ∼60% in PML KD HeLa compared with control KD HeLa, suggesting the involvement of PML in TSA-induced apoptosis (Fig. 5A and supplemental Fig. S5). We examined further using PML–/– MEFs. Notably, overexpression of PML W in PML–/– MEFs substantially increased TSA-induced apoptosis relative to cells transfected with an empty vector, whereas, PML M displayed an impaired ability to mediate apoptosis in response to TSA (Fig. 5B and supplemental Fig. S6). An equal expression level of PML between PML W and PML M transfectants was confirmed (supplemental Fig. S6). These results further support the involvement of PML in TSA-induced apoptosis and suggest the importance of PML acetylation in conferring apoptosis by TSA.
FIGURE 5.
PML acetylation is important for apoptosis induced by TSA. A, PML knockdown reduces TSA-induced apoptosis in HeLa cells. HeLa cells whose PML was stably knocked down (PML KD HeLa) or control cells (Control KD HeLa) were treated with or without 1 μm TSA for 36 h. Apoptotic cells were detected and quantified as described under “Experimental Procedures.” The ratios of apoptotic cells are plotted on bar charts. The averages of two independent analyses and standard deviations are shown. B, an acetylation-defective mutant of PML displayed impaired ability to mediate TSA-induced apoptosis. PML–/– MEFs were transfected with a bicistronic expression vector for GFP alone (control) or GFP and the indicated PML. GFP+ cells were sorted and treated with or without 1 μm TSA for 48 h. Apoptotic cells were analyzed as in A. C, coexpression of Ubc9 and SUMO enhances PML-mediated apoptosis in response to TSA. The analyses of apoptosis were performed as in B except that cells were cotransfected with expression vectors for SUMO and Ubc9 (SUMO mix) or an empty vector as indicated. D, increased PML sumoylation induced by TSA in PML–/– MEFs. PML sumoylation in PML–/– MEFs was examined as in Fig. 4B. IB, immunoblotting; ab, antibody.
Next, we investigated the effect of PML sumoylation on PML-mediated apoptosis in response to TSA. Cotransfection of expression vectors for SUMO and Ubc9 further sensitized PML W transfectants to TSA-induced apoptosis (Fig. 5C, third and fourth lanes), whereas the proapoptotic effects of SUMO and Ubc9 were greatly reduced in control and PML M transfectants (Fig. 5C, first and second lanes and fifth and sixth lanes). Enhanced sumoylation of PML W induced by TSA and impaired sumoylation of PML M were observed also in PML–/– MEFs (Fig. 5D). These results suggest that sumoylation enhances PML-mediated apoptosis in response to TSA. To test this hypothesis, we created an expression vector for the PML-3K mutant, which had lysine-to-arginine mutations at all three PML sumoylation sites and cannot be sumoylated (22). Overexpression of PML-3K in either the presence or absence of SUMO and Ubc9 had little or no effect on TSA-induced apoptosis (Fig. 5C, seventh and eighth lanes), indicative of a requirement for PML sumoylation in TSA-mediated apoptosis. These results suggest the hypothesis that enhanced PML sumoylation through PML acetylation is one of the mechanisms of TSA-induced apoptosis.
Finally, to investigate the generality of the effects of TSA on PML among HDAC inhibitors, we used depsipeptide, another HDAC inhibitor that belongs to a different class. Depsipeptide enhanced acetylation and sumoylation of PML, and its apoptotic effect was increased by PML expression similarly to TSA (supplemental Fig. S8). These results further support the hypothetical importance of PML acetylation in HDAC inhibitor-induced apoptosis.
DISCUSSION
The data presented here demonstrate that acetylation of PML may enhance its sumoylation and play an important role in the control of PML-dependent apoptosis in response to TSA exposure. Sumoylation of PML is necessary for NB formation (5, 10), and the apoptotic effects of PML may be dependent on NB formation (see Introduction). Given our findings that PML acetylation is associated with increased PML sumoylation, this sumoylation-dependent NB formation may represent one of the mechanisms by which PML acetylation can enhance apoptosis. This hypothesis is supported by our findings that coexpression of SUMO and Ubc9 enhances PML-dependent apoptosis by TSA, that acetylation-defective mutants of PML exhibit defects in sumoylation and apoptosis in response to TSA treatment, and that a sumoylation-impaired PML mutant (PML-3K) is also defective in TSA-mediated apoptosis. It should be noted that PML–/– MEF cells are still sensitive to apoptosis by TSA, although at a much reduced level compared with cells expressing PML. There may also be PML-independent apoptotic mechanisms that respond to TSA. This is not surprising, considering that TSA alters the expressions of various genes by the acetylation of histones and many kinds of transcription factors such as p53 (see Introduction). More work will be required to determine the individual contributions of these different actions of TSA on the proliferation, survival, and differentiation of cells.
Acetylation of lysine leads to loss of its positive charge. In some cases, arginine, a positive charged amino acid, and glutamine, a noncharged one, are reported to mimic nonacetylated and acetylated lysine, respectively. We examined whether glutamine substitution at acetylation sites, lysines 487 and 515, had an enhancing effect on PML sumoylation. The PML mutant with glutamine substitution, however, showed impaired sumoylation in HeLa cells similarly to the one with arginine substitution (data not shown). Effects of acetylation other than the loss of positive charge or subtle differences of amino acid structure between lysine and glutamine may be important for PML sumoylation.
Recent studies reveal that increasing numbers of proteins are targeted by both acetylation and sumoylation. However, the correlation between these modifications has hardly been investigated except the cases where both modifications competitively target the same lysine residue such as the cases of HIC1 (hypermethylated in cancer 1) and MEF2 (myocyte enhancer factor 2) (23, 24). This is the first report that suggests acetylation-dependent enhancement of sumoylation. Phosphorylation has been reported to regulate sumoylation. Recently, the classical sumoylation consensus motif (ψKXE) with an adjacent proline-directed phosphorylation motif (SP), ψKXEXXSP motif where ψ is a large hydrophobic residue and X is any residue, has been proposed as a phosphorylation-dependent sumoylation motif (25, 26). Between the two acetylation sites, lysines 487 and 515, lysine 487 is the major acetylation site, and K487R affects sumoylation more than K515R (Fig. 2, B and C, and data not shown). PML sumoylation is reported to occur at three lysine residues, lysine 65, 160, and 490 (22). It would be interesting to discover whether acetylation at lysine 487 specifically affects sumoylation at any of these lysine residues, especially at the adjacent lysine 490. p53 also has a similar sequence where an acetylated lysine lies adjacent to a sumoylated lysine (supplemental Fig. S9), although the correlation between acetylation and sumoylation has not been investigated (27). KψψKXE might be a motif of acetylation-dependent sumoylation.
In summary, our studies provide evidence for a new post-transcriptional modification of PML and a new mechanism of regulation of PML sumoylation, and establish a novel relationship between PML and TSA-induced apoptosis. This work provides new insights into the regulation of PML function and the control of protein sumoylation. Considering the large number of binding partners of PML and the key contributions of PML to the stability and function of the NBs, PML acetylation is likely to modulate multiple cell activities beyond apoptosis through regulation of recruitment or release of NBs components.
Supplementary Material
Acknowledgments
We are very grateful to Ryouhei Tanizaki, Yuka Nomura, and Chika Wakamatsu for technical assistance.
This work was supported by grants-in-aid from the Uehara Memorial Foundation, the National Institute of Biomedical Innovation, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1–S9 and supplemental data.
Footnotes
The abbreviations used are: MEF, mouse embryo fibroblast; HDAC, histone deacetylase; TSA, trichostatin A; NB, nuclear body; RAR, retinoic acid receptor; SUMO, small ubiquitin-like modifier; HAT, histone acetyltransferase; GST, glutathione S-transferase; HA, hemagglutinin; GFP, green fluorescent protein.
References
- 1.Mu, Z. M., Chin, K. V., Liu, J. H., Lozano, G., and Chang, K. S. (1994) Mol. Cell. Biol. 146858 –6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomoni, P., and Pandolfi, P. P. (2002) Cell 108165 –170 [DOI] [PubMed] [Google Scholar]
- 3.Wang, Z. G., Delva, L., Gaboli, M., Rivi, R., Giorgio, M., Cordon-Cardo, C., Grosveld, F., and Pandolfi, P. P. (1998) Science 2791547 –1551 [DOI] [PubMed] [Google Scholar]
- 4.Wang, Z. G., Ruggero, D., Ronchetti, S., Zhong, S., Gaboli, M., Rivi, R., and Pandolfi, P. P. (1998) Nat. Genet. 20266 –272 [DOI] [PubMed] [Google Scholar]
- 5.Ishov, A. M., Sotnikov, A. G., Negorev, D., Vladimirova, O. V., Neff, N., Kamitani, T., Yeh, E. T., Strauss, J. F., III, and Maul, G. G. (1999) J. Cell Biol. 147221 –234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellaire, G., and Bazett-Jones, D. P. (2004) Bioessays 26963 –977 [DOI] [PubMed] [Google Scholar]
- 7.Dyck, J. A., Maul, G. G., Miller, W. H., Jr., Chen, J. D., Kakizuka, A., and Evans, R. M. (1994) Cell 76333 –343 [DOI] [PubMed] [Google Scholar]
- 8.Koken, M. H., Puvion-Dutilleul, F., Guillemin, M. C., Viron, A., Linares-Cruz, G., Stuurman, N., de Jong, L., Szostecki, C., Calvo, F., Chomienne, C., Degos, L., Puvion, E., and de Thé, H. (1994) EMBO J. 131073 –1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weis, K., Rambaud, S., Lavau, C., Jansen, J., Carvalho, T., Carmo-Fonseca, M., Lamond, A., and Dejean, A. (1994) Cell 76345 –356 [DOI] [PubMed] [Google Scholar]
- 10.Zhong, S., Muller, S., Ronchetti, S., Freemont, P. S., Dejean, A., and Pandolfi, P. P. (2000) Blood 952748 –2752 [PubMed] [Google Scholar]
- 11.Kim, K. I., Baek, S. H., and Chung, C. H. (2002) J. Cell. Physiol. 191257 –268 [DOI] [PubMed] [Google Scholar]
- 12.Muller, S., Matunis, M. J., and Dejean, A. (1998) EMBO J. 1761 –70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, J., Koken, M. H., Quignon, F., Chelbi-Alix, M. K., Degos, L., Wang, Z. Y., Chen, Z., and de The, H. (1997) Proc. Natl. Acad. Sci. U. S. A. 943978 –3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, G. Q., Shi, X. G., Tang, W., Xiong, S. M., Zhu, J., Cai, X., Han, Z. G., Ni, J. H., Shi, G. Y., Jia, P. M., Liu, M. M., He, K. L., Niu, C., Ma, J., Zhang, P., Zhang, T. D., Paul, P., Naoe, T., Kitamura, K., Miller, W., Waxman, S., Wang, Z. Y., de The, H., Chen, S. J., and Chen, Z. (1997) Blood 893345 –3353 [PubMed] [Google Scholar]
- 15.Niu, C., Yan, H., Yu, T., Sun, H. P., Liu, J. X., Li, X. S., Wu, W., Zhang, F. Q., Chen, Y., Zhou, L., Li, J. M., Zeng, X. Y., Yang, R. R., Yuan, M. M., Ren, M. Y., Gu, F. Y., Cao, Q., Gu, B. W., Su, X. Y., Chen, G. Q., Xiong, S. M., Zhang, T., Waxman, S., Wang, Z. Y., Chen, S. J., Hu, J., Shen, Z. X., and Chen, S. J. (1999) Blood 943315 –3324 [PubMed] [Google Scholar]
- 16.Marks, P. A., Richon, V. M., and Rifkind, R. A. (2000) J. Natl. Cancer Inst. 921210 –1216 [DOI] [PubMed] [Google Scholar]
- 17.Wolffe, A. P., and Pruss, D. (1996) Cell 84817 –819 [DOI] [PubMed] [Google Scholar]
- 18.Glozak, M. A., Sengupta, N., Zhang, X., and Seto, E. (2005) Gene (Amst.) 36315 –23 [DOI] [PubMed] [Google Scholar]
- 19.Bandyopadhyay, D., Mishra, A., and Medrano, E. E. (2004) Cancer Res. 647706 –7710 [DOI] [PubMed] [Google Scholar]
- 20.Terui, T., Murakami, K., Takimoto, R., Takahashi, M., Takada, K., Murakami, T., Minami, S., Matsunaga, T., Takayama, T., Kato, J., and Niitsu, Y. (2003) Cancer Res. 638948 –8954 [PubMed] [Google Scholar]
- 21.Hayakawa, F., and Privalsky, M. L. (2004) Cancer Cell 5389 –401 [DOI] [PubMed] [Google Scholar]
- 22.Kamitani, T., Kito, K., Nguyen, H. P., Wada, H., Fukuda-Kamitani, T., and Yeh, E. T. (1998) J. Biol. Chem. 27326675 –26682 [DOI] [PubMed] [Google Scholar]
- 23.Stankovic-Valentin, N., Deltour, S., Seeler, J., Pinte, S., Vergoten, G., Guerardel, C., Dejean, A., and Leprince, D. (2007) Mol. Cell. Biol. 272661 –2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregoire, S., and Yang, X. J. (2005) Mol. Cell. Biol. 252273 –2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hietakangas, V., Anckar, J., Blomster, H. A., Fujimoto, M., Palvimo, J. J., Nakai, A., and Sistonen, L. (2006) Proc. Natl. Acad. Sci. U. S. A. 10345 –50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, X. J., and Gregoire, S. (2006) Mol. Cell 23779 –786 [DOI] [PubMed] [Google Scholar]
- 27.Kwek, S. S., Derry, J., Tyner, A. L., Shen, Z., and Gudkov, A. V. (2001) Oncogene 202587 –2599 [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa, F., Towatari, M., Ozawa, Y., Tomita, A., Privalsky, M. L., and Saito, H. (2004) J. Leukocyte Biol. 75529 –540 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.