Abstract
The dermis and the epidermis of normal human skin are functionally separated by a basement membrane but, together, form a stable structural continuum. Anchoring fibrils reinforce this connection by insertion into the basement membrane and by intercalation with banded collagen fibrils of the papillary dermis. Structural abnormalities in collagen VII, the major molecular constituent of anchoring fibrils, lead to a congenital skin fragility condition, dystrophic epidermolysis bullosa, associated with skin blistering. Here, we characterized the molecular basis of the interactions between anchoring fibrils and banded collagen fibrils. Suprastructural fragments of the dermo-epidermal junction zone were generated by mechanical disruption and by separation with magnetic Immunobeads. Anchoring fibrils were tightly attached to banded collagen fibrils. In vitro binding studies demonstrated that a von Willebrand factor A-like motif in collagen VII was essential for binding of anchoring fibrils to reconstituted collagen I fibrils. Since collagen I and VII molecules reportedly undergo only weak interactions, the attachment of anchoring fibrils to collagen fibrils depends on supramolecular organization of their constituents. This complex is stabilized in situ and resists dissociation by strong denaturants.
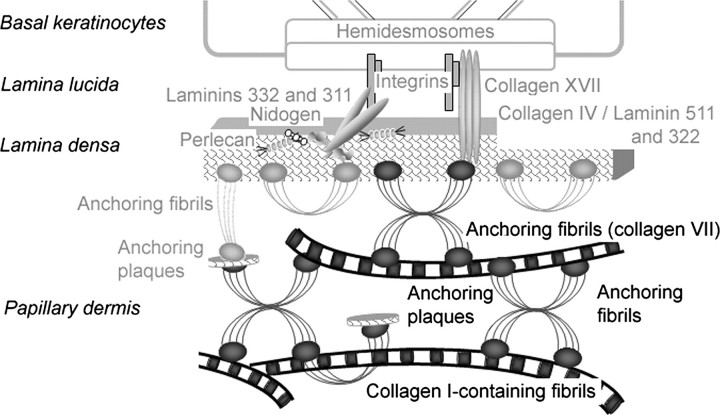
The functions and homeostasis of skin critically depend on the stable organization and cohesion between the epidermis and the dermis. These tissue layers are confined and interconnected by the dermo-epidermal junction zone (DEJZ)4, which comprises the basal keratinocytes, the dermo-epidermal basement membrane, and the uppermost, i.e. the papillary dermis. The suprastructural entity affording pivotal mechanical stability of the DEJZ is the anchoring complex, which sequentially consists of the hemidesmosomes at the basal surface of the keratinocytes, the anchoring filaments linking the hemidesmosomes to the basement membrane, and the anchoring fibrils connecting the basement membrane with the underlying dermal stroma (1). Anchoring fibrils are centro-symmetrically banded structures that originate in the basement membrane and either end in the papillary dermis or loop back into the basement membrane (2–4). Their calculated length is 785 nm (5), but they appear shorter in the tissue due to their insertion into the lamina densa (3, 6).
The quantitatively major molecular constituent of anchoring fibrils is collagen VII (7). The major component of D-periodically banded, dermal collagen fibrils, collagen I, copolymerizes with minor quantities of collagens III, V, XII, and XIV to form macromolecular alloys that vary in their composition and, because of this, also in their supramolecular organization. Therefore, the latter collagens may contribute only small mass fractions, yet critically determine the structural and functional properties of the fibrils (8–10).
Structural abnormalities of the anchoring complex lead to skin fragility, the landmark of epidermolysis bullosa, a group of heritable blistering skin diseases (11). The absence, scarcity, or structural abnormalities of anchoring fibrils underlie the dystrophic form of epidermolysis bullosa in which blister formation occurs in the uppermost papillary dermis. This, in turn, results in tissue repair by scar formation, which, in some cases, can be mutilating (12).
Although the importance of anchoring fibrils in the stability of skin and in the pathogenesis of dystrophic epidermolysis bullosa is well recognized, the precise nature of the link between the basement membrane and the dermal stroma mediated by anchoring fibrils remains incompletely understood. Previous studies addressing interactions between collagen VII and unpolymerized basement membrane molecules demonstrated that the amino-terminal, non-collagen-like domain 1 of collagen VII (NC-1(VII)) interacts with collagen IV and laminin 332 (13), components (14) of the basement membrane and of anchoring filaments, respectively (15).
Collagen IV and laminin 332 also reside in anchoring plaques, which are basement membrane-like patches interspersed into the banded fibril network of the papillary dermis (5, 15). As revealed by immunoelectron microscopy, anchoring plaques occur at the ends and at branching points of anchoring fibrils and, thus, extend the anchoring fibril network into deeper regions of the papillary dermis. The model derived from these observations proposed that a network of anchoring plaques and anchoring fibrils intertwines with dermal collagen fibrils, thereby achieving a stable connection between the basement membrane and the papillary dermis solely by entanglement (5). However, this model has been contested because anchoring plaques are relatively rare (4). Thus, there is the possibility that anchoring fibrils directly interact with collagen I-containing dermal fibrils and, indeed, weak in vitro-binding of collagen VII to monomolecular collagen I has been reported (14). However, when organized into fibrils, collagen I may offer multiple, possibly cooperative, binding sites for collagen VII, which may strengthen substantially the interaction between the two proteins.
Here, we have investigated interactions between anchoring fibrils and dermal collagen fibrils employing methods for isolation and separation of authentic fragments of suprastructures derived from the DEJZ. We also studied the interaction of reconstituted collagen I fibrils with recombinant forms of collagen VII, either the full-length protein or mini-collagens VII with large deletions leading to a loss of almost all of the triple helical and parts of the NC-1(VII) domain. We describe heterotypic interactions of these proteins, which depend on the aggregated state of collagen I, and represent a novel mechanism of dermal-epidermal adhesion.
EXPERIMENTAL PROCEDURES
Antibodies
Primary Antibodies—Rabbit polyclonal antibody to collagen type I R1038 and mouse monoclonal antibody to collagen type I AF5610 were from Acris Antibodies, Hiddenhausen, Germany. Mouse monoclonal antibody LH7.2 to the NC-1(VII) domain (16) was purchased from Sigma-Aldrich Chemie, Munich, Germany. Rabbit polyclonal antibodies NC-1-F3 to the NC-1(VII) domain and rabbit polyclonal antibodies NC-2–10 to the carboxyl-terminal, non-collagen-like domain 2 of collagen VII (NC-2(VII)) were described in Ref. 17.
Secondary Antibodies—Secondary antibodies were as follows: peroxidase-conjugated polyclonal goat anti-mouse immunoglobulins (Dako Cytomation); peroxidase-conjugated polyclonal anti-rabbit immunoglobulins (Sigma-Aldrich); 12- and 18-nm colloidal gold particles conjugated to anti-mouse or anti-rabbit immunoglobulins were from Jackson ImmunoResearch.
Gel Electrophoresis and Immunoblotting
SDS-PAGE was performed on 4.5%, 7.5% gels or 4.5–15% gradient gels according to established protocols. Separated proteins were either stained with Coomassie Brilliant Blue or transferred to nitrocellulose membrane (Schleicher & Schuell and Sigma-Aldrich) in a wet blotting procedure. After total protein staining (Pierce), immunodetection occurred following standard protocols. The signals were detected with a chemiluminescence substrate (ECL, Pierce).
Dermal Extracts
Normal human skin was obtained with informed consent from patients undergoing plastic surgery. The isolation of authentic supramolecular fragments from the DEJZ was achieved as reported previously (18). Briefly, the epidermis was removed after treatment of the skin with 1 m NaCl in a neutral buffer. The de-epithelialized tissue was then frozen, and a layer of 200 μm was shaved off using a dermatome. The tissue pieces were minced further on ice using a Polytron homogenizer (Kinematica, Luzern, Switzerland) in phosphate-buffered saline (2 mm phosphate buffer containing 150 mm NaCl, pH 7.4, PBS) and proteinase inhibitors at pH 7.4. Finally, the tissue debris was removed by low speed centrifugation, and the supernatants containing suprastructures from the DEJZ and the underlying papillary dermis were used for further analysis. In the following, this extract will be referred to as crude skin extract, and it is the starting material for further isolations.
Depletion of Collagen I-containing Fibrils and Separation of Individual Basement Membrane Networks
Superparamagnetic polystyrene beads covered with affinity-purified secondary antibodies (sheep anti-mouse immunoglobulins and sheep anti-rabbit immunoglobulins) were purchased from Invitrogen, Karlsruhe, Germany. To enhance separation efficiency, affinity-purified primary antibodies directed against target structures were covalently coupled to the bead surface. For this purpose, the beads were incubated with 2–3 μg of the antibodies against matrix macromolecules per 107 beads under continuous rotation overnight at 4 °C. For cross-linking, the coated beads were first resuspended in 0.2 m triethanolamine and then incubated for 30 min at room temperature with 20 mm dimethyl pimelimidate dihydrochloride (Pierce). After magnetic separation, the reaction was stopped by resuspending the bead pellet in 50 mm Tris, pH 7.5, for 15 min. Finally, the beads were washed with PBS and resuspended in PBS containing 0.1% serum albumin.
To mechanically isolate collagen I-containing fibrils, 50 μgof crude skin extract were incubated with 107 anti-collagen I (AF5610)-Immunobeads. The reaction volume was adjusted to 200 μl with PBS, 0.1% serum albumin, and 0.04% Tween 20, and the incubation was performed under gentle rotation for 2 h at 4 °C. Magnetic separation was achieved by placing the tube for 3 min on a standard permanent magnet. The bead pellet was washed five times with 10 mm Tris-HCl buffer, pH 7.4, containing 150 mm NaCl (TBS), before resuspension in an appropriate volume of TBS and adsorbing it to copper or nickel grids (400 mesh) for electron microscopy (EM grids) for morphological and compositional analysis with the transmission electron microscope. For biochemical analysis, the analogous procedure was applied. The amount of starting material was scaled up to 250 μg of crude extract, and the following steps were adjusted to this amount. For increased purity, the separation step was repeated three times. The bead pellets from each step were pooled and resuspended in SDS-PAGE loading buffer containing 5% (v/v) β-mercaptoethanol. For electrophoretic separation, the remaining supernatant was mixed with 5-fold concentrated SDS-PAGE loading buffer including β-mercaptoethanol and boiled at 100 °C for 5 min, and then the volume was reduced with high speed vacuum centrifugation.
Transmission and Immunogold EM
For EM analysis, aliquots of crude skin extracts or isolated material immobilized on the Immunobead surface were adsorbed for 10 min to nickel EM grids previously coated with a Formvar/carbon film. To analyze the morphology of the adsorbed material, the grids were washed with water (HPLC grade), and the material was stained either with 2% uranyl acetate for 10 min or with an organotungstate compound (NanoW, Biotrend, Cologne, Germany) two times for 1 min each. For additional compositional analysis, immunogold labeling of the sample was performed. After adsorption of the samples, the grids were washed with TBS, and unspecific binding sites were blocked with 2% bovine serum albumin (w/v) in TBS. For immunolabeling, the grids were put on drops of buffer containing 2% bovine serum albumin-c (Sigma-Aldrich) and 0.025% Tween 20 and the primary antibody. Incubation occurred for 2 h at room temperature. Immunodetection was by secondary antibodies conjugated to gold particles, which were of different sizes when double labeling experiments were performed. Finally, the grids were washed five times with distilled water (HPLC grade), and the material was contrasted with 2% uranyl acetate or NanoW (Biotrend). In control experiments, the primary antibodies were omitted. Also, when using magnetic Immunobeads, the beads only, without any biological material, were adsorbed to the grid to exclude artifacts introduced by the beads. Electron micrographs were taken at 80 kV with a CM-10 electron microscope (Philips).
Treatment with Denaturing Reagents of Collagen I-containing Fibrils
Immunogold Electron Microscopy—After adsorption, purified collagen I-containing fibrils bound to the Immunobeads were exposed to 2 m guanidinium chloride in TBS for 30 min at room temperature. After washing with TBS, the grids were processed for immunogold labeling as described above. Immunolabeling of collagen VII was performed with different antibodies to collagen VII (LH 7.2 at the dilution 1:25 or NC-2-10 at 1:100).
SDS-PAGE and Immunoblotting—Collagen I-containing fibrils were depleted from crude extracts with Immunobeads as described. The bead pellets containing the immobilized collagen I-containing fibrils were washed with TBS, resuspended in 200 μl of 0, 1, 2, 4, and 6 m guanidinium chloride or 8 m urea or 8 m urea containing 10 mm SDS, and incubated for 2 h at room temperature or overnight at 4 °C under continuous slow rotation. Subsequently, magnetic separation was carried out by exposure to a permanent magnet. The supernatant was dialyzed against distilled water at 4 °C for 24 h prior to gel electrophoretic analysis. The volume was reduced after denaturing and reduction of the sample by vacuum centrifugation. The bead pellets were washed five times with TBS, resuspended in reducing SDS-PAGE sample buffer, and separated on a 5% SDS gel before electrotransfer to nitrocellulose membranes for immunodetection. Immunolabeling was performed with an antibody to collagen VII (NC-2–10, 1:3000 in TBS, containing 0.04% Tween 20).
Recombinant Full-length and Truncated Collagen VII
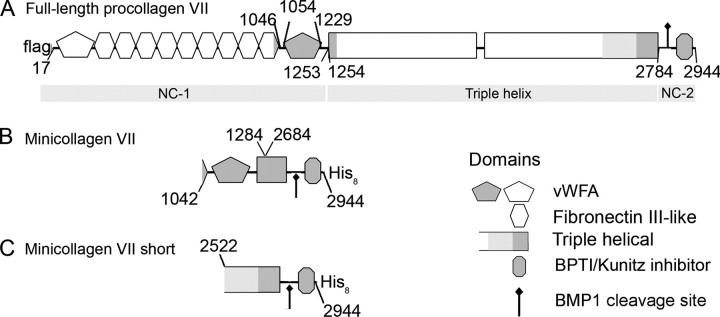
The human full-length collagen VII α1 chain contains 2944 amino acids. Collagen VII, a trimer of thee identical α1(VII) chains, is the quantitatively major component of anchoring fibrils. Each α1(VII) chain of collagen VII is composed of a very long triple helical region with several interruptions in the (Gly-Xaa-Yaa)n amino acid sequence. This flexible, rod-like domain is flanked by non-triple helical, amino-terminal NC-1 (145 kDa) and carboxyl-terminal NC-2 domains (34 kDa) (19–22). The NC-1 domain contains two von Willebrand factor-like domains separated by nine fibronectin type III-like repeats. The NC-2 domain is relatively small and contains a Kunitz inhibitor-like domain and the processing site for the conversion of procollagen VII into collagen VII by BMP-1 and related proteinases. After processing, antiparallel collagen VII dimers and other macromolecules associate into anchoring fibrils (23–25).
Recombinant truncated collagen VII fragments (mini-collagen VII and mini-collagen VII short, see Fig. 5) were produced and purified by procedures previously described (25). Briefly, truncated variants of the collagen VII protein were expressed with a carboxyl-terminal octahistidine tag and purified by Ni2+-chelate affinity chromatography. The purity and the molecular shapes of the recombinant mini-collagens were assessed by SDS-PAGE and rotary shadowing, respectively (25). To produce recombinant full-length collagen VII, a FLAG tag was introduced into the full-length collagen VII cDNA (generous gift of Y. Gache, INSERM U385, Nice, France) after amino acid 23 by overlap extension PCR (Expand high fidelity PCR system, Invitrogen). The resulting cDNA was cloned into the pcDNA3.1Zeo(–) expression vector (Invitrogen), and 10 μgof DNA were transfected into 70% confluent HEK293T cells using GeneJammer (Stratagene) as recommended by the manufacturer. Collagen VII-expressing cells were selected with 50 μg/ml phleomycin (Invitrogen) for 2 weeks, and single clones were isolated and tested for collagen VII expression by immunoblotting with a collagen VII-specific antibody (Calbiochem). Positive clones were expanded in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (all Invitrogen). For purification of full-length collagen VII, cells were shifted to serum-free Dulbecco's modified Eagle's medium supplemented with 50 μg/ml ascorbic acid. After 48 h, conditioned medium was collected, and proteins were precipitated with 40% ammonium sulfate overnight at 4 °C followed by centrifugation (15,000 × g, 1 h). The pellet was dissolved in TBS and dialyzed against TBS overnight at 4 °C to remove the ammonium sulfate. Collagen VII was affinity-purified from the medium concentrate using anti-FLAG-M2-Agarose (Sigma-Aldrich) and eluted with 5 μg/ml FLAG-peptide (Sigma-Aldrich).
FIGURE 5.
Schematic representation of the domain structures of recombinantly expressed full-length collagen VII and truncated collagen VII fusion constructs. The non-collagenous domains NC-1 and NC-2 are the amino- and the carboxyl-terminal globular domains, respectively, and separated by an interrupted triple helical domain. The NC-1 domain contains two von Willebrand factor A-like domains (vWFA) that are separated by nine fibronectin III-like repeats. The cleavage site for BMP-1 is located in the NC-2 domain. A, in the recombinant protein, a FLAG tag has been introduced at the amino terminus. B, mini-collagen VII results from the fusion between the amino acids 1042–1284 and 2684–2944. BPTI, bovine pancreatic trypsin inhibitor. C, mini-collagen VII short comprises the amino acids 2522–2944. Both fragments contain an octahistidine tag at their carboxyl terminus, which is used for purification.
In Vitro Binding of Full-length Collagen VII or Collagen VII Fragments to Reconstituted Collagen I Fibrils
Collagen I was extracted from embryonic chicken tendon and purified as described previously (26). Reconstitution of collagen I fibrils was induced by diluting solutions of 0.4 mg/ml collagen I in 0.4 m NaCl, 0.1 m Tris-HCl, pH 7.4, with an equal volume of water and by heating to 37 °C (27). The turbidity change of the solution was recorded with spectrophotometer at 313 nm. After 3 h, fibrillogenesis was complete since the turbidity curve reached plateau values. Aliquots of reconstituted collagen I fibrils were adsorbed for 10 min to nickel grids. Afterward, the grids were washed with TBS and blocked for 30 min with 2% bovine serum albumin (w/v) in TBS. In the next step, the grids were put on drops containing different concentrations of soluble full-length collagen VII or collagen VII fragments. After a 1-h incubation at room temperature, the grids were washed with TBS, and the complexes were immunogold-labeled with antibody NC-2–10 to the NC-2 domain. Binding of collagen VII ligands to collagen I fibrils was quantified by counting gold particles on 10 randomly selected electron micrographs and determining the fraction of total gold particles observed on fibrils. Values were given as gold particles per μm2 of fibril area, which was determined and analyzed by the ImageJ software. Average values were determined from three independent experiments. To estimate unspecific binding, background levels of gold particles were determined, omitting treatment with the antibodies to collagen VII epitopes.
In Vitro Cross-linking of Full-length Collagen VII and Collagen VII Fragments to Reconstituted Collagen I Fibrils
Sulfo-SBED (Pierce) was utilized as a chemical cross-linker. This multifunctional reagent reacts upon pretreatment with amino groups of one binding partner of aggregating proteins. Cross-linking after specific binding to the other binding partner is achieved after UV light irradiation of the agent, which then reacts with a random site in close proximity on the second binding partner. The cross-linker contains a disulfide bridge, which renders cross-linking reversible after treatment with reducing agents and which results in a transfer of a biotin label from the first to the second binding partner. Amino groups of collagen VII ligands in PBS were reacted with sulfo-SBED at molar excesses of 160 and 40 over full-length collagen VII and collagen VII fragments, respectively, by adding appropriate volumes of a stock solution (10 μg/ml dimethyl sulfoxide (DMSO)). The mixtures were incubated for 30 min at room temperature in the dark, and the reactions were terminated by adding 1 m Tris-HCl, pH 7.4. To remove unbound cross-linker, the solutions were extensively dialyzed against PBS at 4 °C in the dark. Collagen VII molecules carrying sulfo-SBED were added to collagen I fibrils reconstituted in vitro as described above, and the mixture was incubated for 1 h at room temperature in the dark. Subsequently, reaction mixtures were irradiated with UV light (365 nm) for 15 min in the cold. Samples were then denatured with SDS-PAGE sample buffer under reducing conditions and were subjected to electrophoretic separation and electrotransfer to nitrocellulose membranes. Detection of the species carrying the biotin label was carried out with ExtrAvidin conjugated to horseradish peroxidase (1:1000, Sigma-Aldrich) or of collagen VII ligands by immunoblotting with antibodies to collagen VII (NC-2–10, 1:3000).
RESULTS
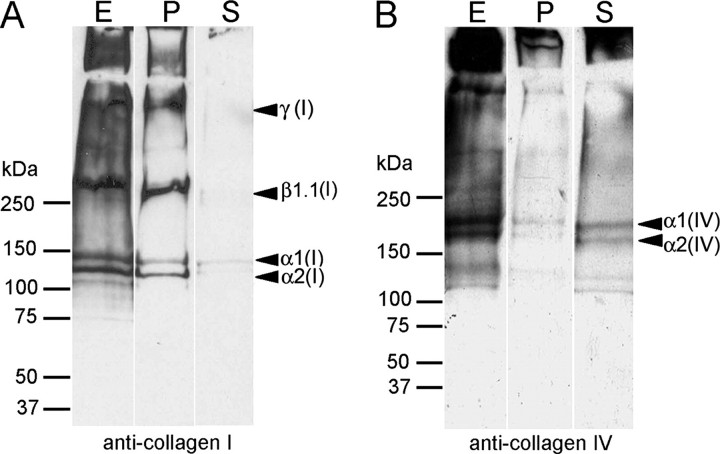
This study was undertaken to determine the functional relationship between anchoring fibrils and banded dermal collagen fibrils. Authentic suprastructural fragments from the DEJZ were isolated from normal human skin by the following procedure. After removing the epidermis after incubation with buffers of high salinity, dermal tissue was frozen, and thin superficial dermal layers containing the dermo-epidermal basement membrane and the papillary dermis were removed from the underlying tissue with a dermatome. The shavings were homogenized in buffered saline to generate fragments of papillary dermal suprastructures, including basement membrane networks, the anchoring complex, and banded collagen fibrils. Coarse tissue debris was eliminated by centrifugation at low gravitational forces, and fragments of distinct suprastructures were recovered from the supernatants for examination by transmission electron microscopy (Fig. 1A). Subsequently, banded fibrils, hereafter called the fibril fraction, were effectively separated from the crude mixtures by adsorption to immunomagnetic beads coated with antibodies to collagen I (Fig. 1B). Network-like suprastructures remained in the supernatant. As shown by immunoblotting (Fig. 2A, lane S), these supernatants were essentially devoid of collagen I and, thus, banded collagen fibrils. Conversely, material bound to the anti-collagen I beads contained only traces of collagen IV (Fig. 2B, lane P).
FIGURE 1.
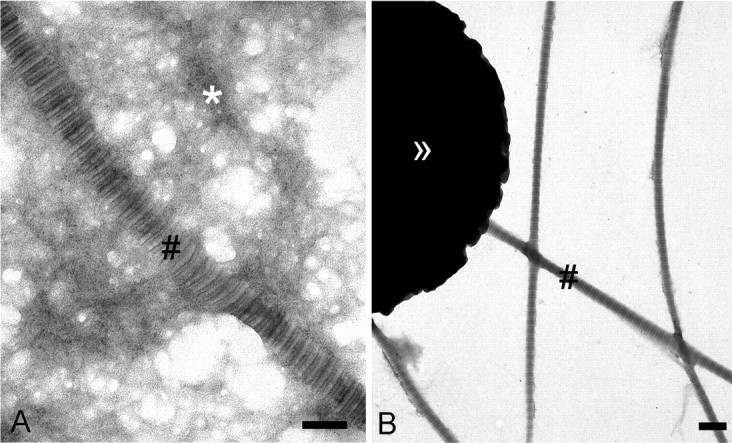
Collagen I-containing fibrils are mechanically separable from basement membrane networks. A, ultrastructural analysis by transmission electron microscopy of a crude skin extract obtained after tissue homogenization and differential centrifugation. The extract contains banded fibrils (#) and electron-dense network-like material. B, the crude extract was fractionated by immunomagnetic beads (>>) coated with antibodies to collagen I. D-periodically banded collagen I-containing fibrils (#) are separated from most of the network/like material. Bars, 200 nm.
FIGURE 2.
Collagen I-containing fibrils are mechanically separable from basement membrane networks. Immunoblots are shown of the fraction containing banded fibrils attached to immunomagnetic beads coated with antibodies to collagen I and of unbound material in the supernatants. The blots were reacted with antibodies to collagen I, the major component of banded fibrils (A) or basement membrane collagen IV (B). Lanes E, crude extract before separation; lanes P, material attached to immunomagnetic beads; and lanes S, supernatants after reaction with immunomagnetic beads.
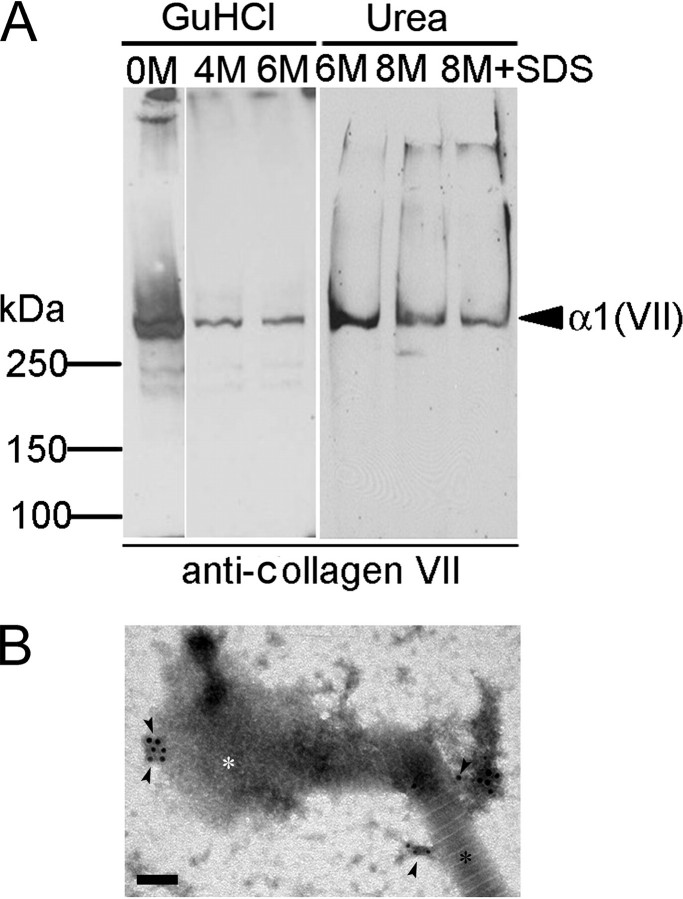
Next, we examined possible interactions between anchoring fibrils and collagen I-containing fibrils within these extracts. Filamentous structures immunogold-labeled with antibodies to collagen VII were frequently observed in association with banded fibrils irrespectively of their diameter (Fig. 3). The suprastructures containing collagen VII often had a semicircular appearance (a gallery of examples is shown in Fig. 3, B–I). Immunoblotting of such complexes treated with guanidinium chloride or urea at high concentrations revealed that the interaction between anchoring and banded fibrils was resistant to strong denaturing agents (Fig. 4A). These results were consistent with the notion that interactions of the filamentous structures with banded fibrils were stabilized in situ by covalent cross-links between collagens VII and I, respectively. Immunoelectron microscopy of fibril fractions treated with denaturants also revealed a tight association of collagen VII-containing material with banded fibrils that resisted disintegration by urea or guanidinium chloride (Fig. 4B).
FIGURE 3.
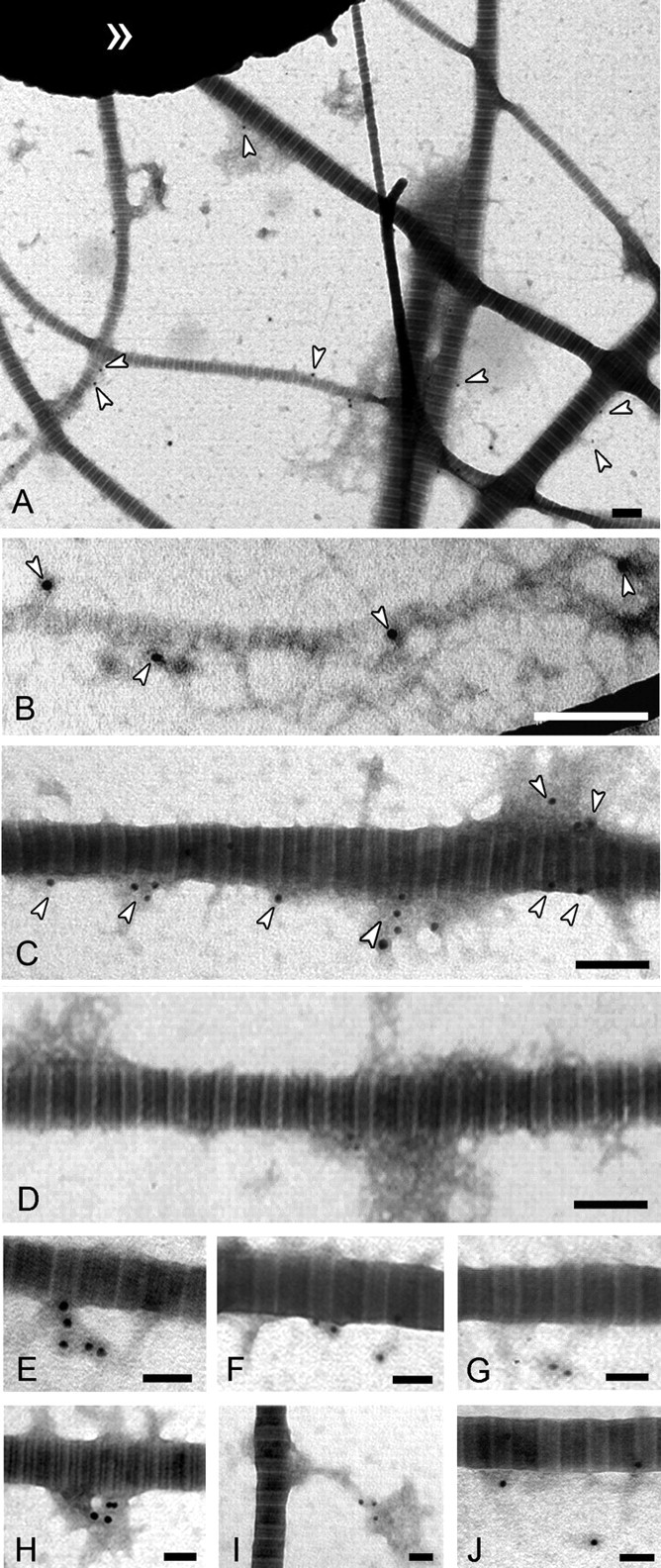
Anchoring fibrils are co-localized with D-periodically banded fibrils. Indirect immunogold electron microscopy of D-periodically banded fibrils sequentially treated with antibodies to collagen VII and gold-conjugated antibodies to immunoglobulins (white arrowheads) is shown. A, overview of the isolated collagen I-containing fibril fraction. Anchoring fibrils are associated with both small diameter (B) and large diameter (C) fibrils. D, negative control without treatment with antibodies to collagen VII. Bars, 200 nm. A gallery of immunogold-labeled structures is shown at higher magnification (E–J). Bars, 100 nm.
FIGURE 4.
D-periodically banded fibrils and anchoring fibrils interact with high affinity. A, fibril fractions isolated with anti-collagen I-immunomagnetic beads were treated and washed with denaturants as indicated, and the extracts were analyzed by immunoblotting with antibodies to collagen VII. Proteins were separated by SDS-PAGE on a 4.5% gel under reducing conditions. Note: Treatment of collagen I-containing fibrils with guanidinium chloride and urea at high, denaturing concentrations does not eliminate collagen VII (α1(VII): 290 kDa). B, immunogold electron microscopy using antibodies to collagen VII (black arrowheads) reveals suprastructures containing collagen VII connected to banded fibrils after the partial denaturation with 2 m guanidinium chloride. Black asterisk, intact fibril section; white asterisk, fibril section disintegrated by the denaturant. Bar, 100 nm.
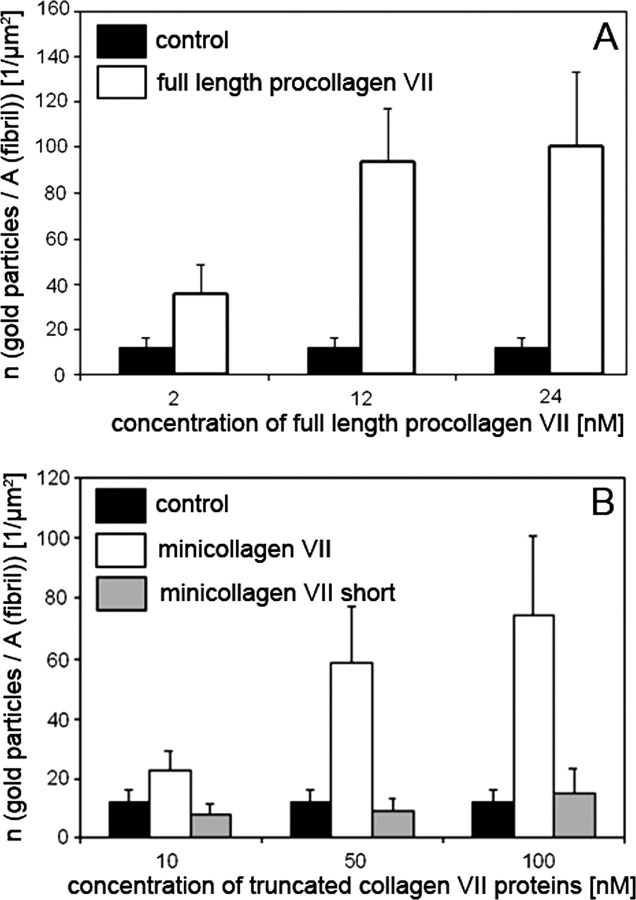
To provide further proof for the specific interaction between anchoring and banded fibrils, complex suprastructures were reconstituted in vitro from collagen I fibrils and recombinant collagen VII or fragments thereof. Fig. 5 schematically represents the structure and domain organizations of collagen VII and of its truncated forms designed to contain or lack putative binding sites for collagen fibrils. The corresponding recombinant proteins carrying oligo-His tags were expressed in eukaryotic HEK293 cells and were purified by affinity chromatography on nickel-Sepharose (25). Banded fibrils were reconstituted in vitro from purified collagen I and were adsorbed to carbon-coated EM grids. After blocking of unspecific binding sites, the fibrils were reacted with soluble recombinant collagen VII, mini-collagen VII, or the short form of mini-collagen VII (Fig. 5). Binding of the soluble ligands to the banded fibrils was assessed by immunogold EM with an antibody to the NC-2 domain of collagen VII (Fig. 6). Gold particles representing collagen VII and its truncated forms were counted in randomly selected electron micrographs, which were observed either on collagen I fibrils or within a perimeter of 200 nm, i.e. a distance corresponding to the approximate hydrodynamic size of collagen VII. As shown in Fig. 7, collagen VII or truncated mini-collagen VII bound to reconstituted collagen I fibrils in a dose-dependent manner. In contrast, the short form of mini-collagen VII, which lacks the helix-proximal von Willebrand A motif within the amino-terminal NC-1 domain, failed to bind to reconstituted collagen I fibrils. These results indicated that the von Willebrand factor A motif was essential for the interaction between anchoring fibrils and collagen I-containing fibrils.
FIGURE 6.
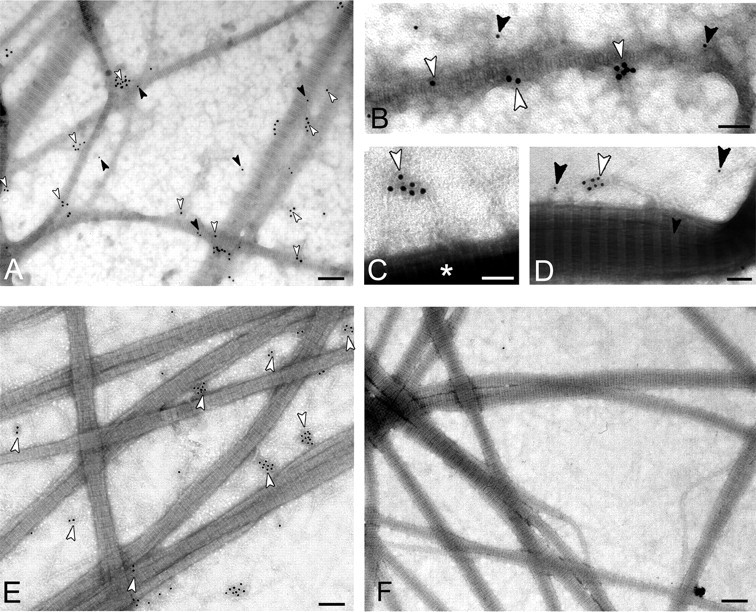
Procollagen VII and mini-collagen VII but not mini-collagen VII short-bind to reconstituted collagen I fibrils. In vitro fibrillogenesis was performed with solutions of purified collagen I. Newly formed fibrils were reacted with variants of recombinant collagen VII, and immunogold labeling was performed analogously to the experiments shown in Fig. 3. Collagen I fibrils reacted with full-length procollagen VII (A, overview; B–D, gallery of filamentous structures in detail). Black and white arrowheads, double labeling with antibodies against the NC-1(VII) domain (12-nm gold particles, black arrowheads) and the carboxyl terminus of collagen VII (18-nm gold particles, white arrowheads). Reconstituted collagen I fibrils were exposed to mini-collagen VII (E) and mini-collagen VII short form (F). If present, these proteins were identified with antibodies to the carboxyl terminus (white arrowheads). * highlights collagen I fibrils. Note the absence of gold labeling in panel F. Bars: 200 nm in A, E, and F; 100 nm in B, C, and D.
FIGURE 7.
Procollagen VII and mini-collagen VII but not mini-collagen VII short-bind to reconstituted collagen I fibrils. The graphs show the quantification of the binding of procollagen VII and procollagen VII fragments constructs to reconstituted collagen I fibrils. Procollagen VII (A) and the procollagen VII fragments (B) were incubated at different concentrations with collagen I fibrils and labeled with antibodies to the carboxyl terminus of collagen VII (NC-2-10). Binding was quantified by counting gold particles per μm2 of collagen I fibril in 10 arbitrarily chosen EM fields. Control: collagen VII ligand omitted.
To pinpoint further interaction sites in collagen I and in anchoring fibrils, tag transfer cross-link experiments (28) were performed. A biotin-tagged, multifunctional, and reducible cross-link reagent was bound to collagen VII, mini-collagen VII, or mini-collagen VII short form. Thereafter, the tagged collagen VII proteins were allowed to react with collagen I fibrils reconstituted in vitro, and chemical cross-linking of collagen I to collagen VII was induced by photoactivation of the second cross-link function. The disulfide bonds within the cross-link reagent were then cleaved by reduction with β-mercaptoethanol, which leads to a transfer of the biotin tag from collagen VII to collagen I molecules within the fibrils. As shown in Fig. 8, collagen I was biotin-tagged only if the interaction partner was full-length or mini collagen VII but not the short form of mini collagen VII. This corroborated the binding specificity of the interaction between anchoring and banded collagen I fibrils and assigned the binding epitope in collagen VII to the von Willebrand A domain within the amino-terminal NC-1 domain, which lies adjacently to the amino-terminal end of the large triple helical domain.
FIGURE 8.
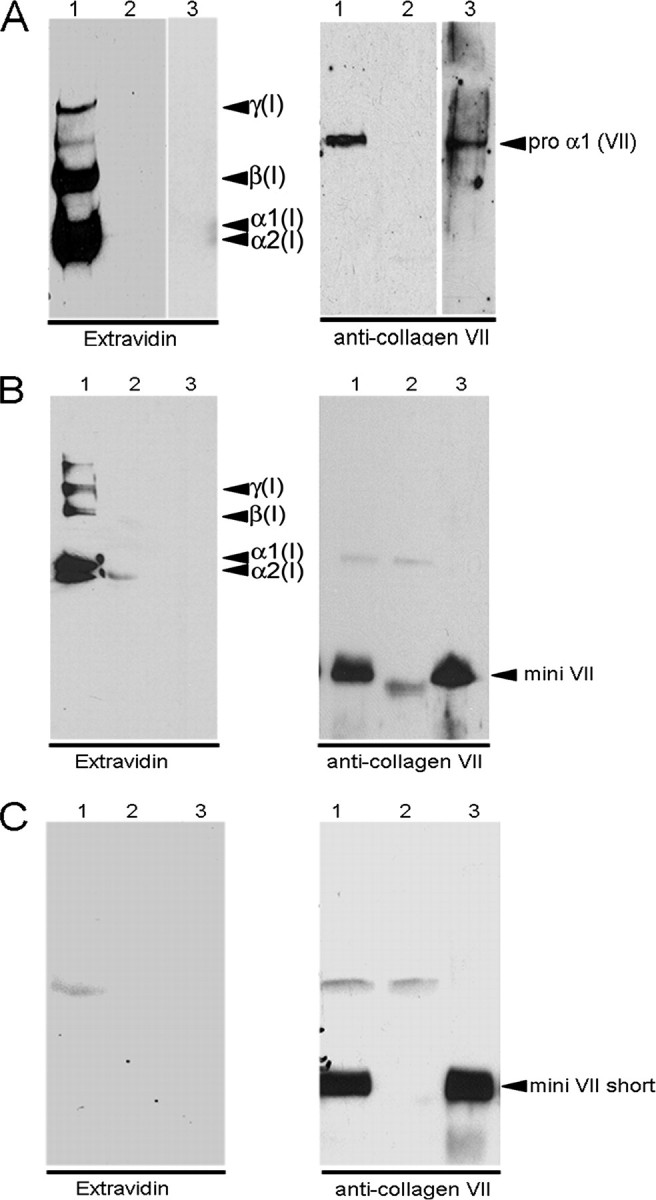
Procollagen VII and mini-collagen VII but not mini-collagen VII short are covalently cross-linked to collagen I fibrils in vitro. Full-length procollagen VII (A), mini-collagen VII (B), and mini-collagen VII short form (C) were coupled to Sulfo-SBED and incubated with reconstituted collagen I fibrils. After the irradiation with UV light, the reaction products were reduced, submitted to SDS-PAGE, and electrotransferred to nitrocellulose membrane (lanes 1). For controls, collagen VII ligands (lanes 2) or collagen I fibrils (lanes 3) were omitted from reaction mixtures. The biotin transfer was detected by ExtrAvidin coupled to horseradish peroxidase. Note: Biotin was transferred to collagen I only in complete reaction mixtures (lanes 1) and only from procollagen VII (A) and mini-collagen VII (B). For further controls (right panels), immunoblotting was conducted with antibodies to procollagen VII.
DISCUSSION
In this study, novel insights were gained on mechanisms of tissue stabilization within the DEJZ that operate at the level of aggregated suprastructures rather than separate macromolecules. Fragments of banded dermal collagen fibrils were generated by mechanical disruption and were separated from other suprastructural fragments by a purification technique employing immunomagnetic beads. Experimental conditions were developed, which allowed for the maintenance of both macromolecular organizations and interactions at the level of integrated suprastructures. The purified banded fibrils still were associated with structures closely resembling anchoring fibrils. This finding was surprising since earlier reports indicated that anchoring fibrils interacted with banded dermal collagen fibrils solely by unspecific mechanical entanglement (5). Further observations indicated that strong interactions did occur between collagen VII in anchoring fibrils and collagen IV or laminin 332 present in the dermo-epidermal basement membrane (14, 15) but not between collagen VII and the major component of banded fibrils of the papillary dermis, i.e. collagen I (3, 14). However, others reported direct but weak interactions between collagens I and VII (13). These ambiguities may not easily be resolved and may be related to the fact that, in the previous studies, interactions were investigated between individual macromolecules rather than organized aggregates thereof. It has been shown previously that affinities of ligand binding to matrix macromolecules are strongly affected by their stage of aggregation. For example, the anchorless adhesin Eap from Staphylococcus aureus selectively recognizes extracellular matrix aggregates but binds promiscuously to monomeric matrix macromolecules (29). Furthermore, the importance of suprastructural organization is highlighted by differential binding of integrins to extracellular matrix ligands in the aggregated and monomolecular states, respectively (30).
The strong interactions observed here between anchoring fibrils and banded collagen fibrils are further stabilized in situ. It is tempting to speculate that covalent cross-linking between collagens I and VII underlies the unusual stability of the complexes of the two proteins. However, very stable non-covalent interactions withstanding prolonged treatment with SDS at high temperatures are not uncommon. This problem is not easily resolved for several reasons. Firstly, collagen VII, although functionally crucial for tissue integrity, constitutes only about 0.001% of total collagens in skin (31). Furthermore, the nondissociable species represents a small fraction of the total amount of collagen VII. Moreover, there are several candidate varieties of covalent cross-links with diverse chemistry. A group of cross-links may result from several types of condensation reactions of enzymatically introduced lysyl or hydroxylysyl aldehyde residues as typically found in collagen molecules. A likely alternative, however, are isopeptide bonds introduced by the action of transglutaminases because collagen VII is a proven substrate for tissue transglutaminases (32). Disulfide bond formation is another option but less likely because the cross-links resist treatment with sulfhydryl reagents. For these reasons, the identification of putative covalent cross-links involving collagen VII in human skin requires the availability of new methods of structural protein analysis with appropriate sensitivity.
Reconstitution experiments demonstrated that full-length and truncated collagen VII molecules still containing the von Willebrand factor A-like domain adjacent to the major triple helix directly interacted with fibrillar collagen I. This interaction was corroborated by chemical cross-linking resulting in a specific transfer of the biotin label within the cross-linking reagent from collagen VII to collagen I and vice versa.
In conclusion, we have identified here an additional molecular mechanisms of tissue stabilization within the DEJZ, which is evident only at the suprastructural level. We have found a very strong bonding between anchoring fibrils and banded fibrils, as schematically represented in Fig. 9. Such interactions are bound to be essential also for the tissue integrity and homeostasis in skin and in the pathogenetic mechanisms of hereditary skin blistering diseases such as dystrophic epidermolysis bullosa.
FIGURE 9.
Schematic representation of the organization of the anchoring complex within the DEJZ. The novel, covalently stabilized interaction between collagen I-containing fibrils and anchoring fibrils is highlighted in black, whereas interactions previously described are kept in gray. Inset: molecular organization of anchoring fibrils; antiparallel dimers of collagen VII molecules subsequently form lateral aggregates.
Acknowledgments
We thank Margret Bahl for excellent technical help.
This work was supported by the Deutsche Forschungsgemeinschaft SFB 492 Grants A2 (to P. B.), KO 2247/3-1 (to M. K.), and Br 1475/8-2 (to L. B.-T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: DEJZ, dermo-epidermal junction zone; NC-1(VII), amino-terminal, non-collagen-like domain 1 of collagen VII; NC-2(VII), carboxyl-terminal, non-collagen-like domain 2 of collagen VII; PBS, phosphate-buffered saline; TBS, Tris-buffered saline; EM, electron microscopy; HPLC, high pressure liquid chromatography.
References
- 1.Ellison, J., and Garrod, D. R. (1984) J. Cell Sci. 72 163–172 [DOI] [PubMed] [Google Scholar]
- 2.Palade, G. E., and Farquhar, M. G. (1965) J. Cell Biol. 27 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgeson, R. E., Lunstrum, G. P., Rokosova, B., Rimberg, C. S., Rosenbaum, L. M., and Keene, D. R. (1990) Ann. N. Y. Acad. Sci. 580 32–43 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu, H., Ishiko, A., Masunaga, T., Kurihara, Y., Sato, M., Bruckner-Tuderman, L., and Nishikawa, T. (1997) Lab. Investig. 76 753–763 [PubMed] [Google Scholar]
- 5.Keene, D. R., Sakai, L. Y., Lunstrum, G. P., Morris, N. P., and Burgeson, R. E. (1987) J. Cell Biol. 104 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clermont, Y., and Hermo, L. (1988) Anat. Rec. 221 482–493 [DOI] [PubMed] [Google Scholar]
- 7.Sakai, L. Y., Keene, D. R., Morris, N. P., and Burgeson, R. E. (1986) J. Cell Biol. 103 1577–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkel, W., and Glanville, R. W. (1982) Eur. J. Biochem. 122 205–213 [DOI] [PubMed] [Google Scholar]
- 9.Keene, D. R., Lunstrum, G. P., Morris, N. P., Stoddard, D. W., and Burgeson, R. E. (1991) J. Cell Biol. 113 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henkel, W., and Dreisewerd, K. (2007) J. Proteome Res. 6 4269–4289 [DOI] [PubMed] [Google Scholar]
- 11.Aumailley, M., Has, C., Tunggal, L., and Bruckner-Tuderman, L. (2006) Expert Rev. Mol. Med. 8 1–21 [DOI] [PubMed] [Google Scholar]
- 12.Bruckner-Tuderman, L., Mitsuhashi, Y., Schnyder, U. W., and Bruckner, P. (1989) J. Investig. Dermatol. 93 3–9 [DOI] [PubMed] [Google Scholar]
- 13.Chen, M., Marinkovich, M. P., Veis, A., Cai, X. Y., Rao, C. N., O'Toole, E. A., and Woodley, D. T. (1997) J. Biol. Chem. 272 14516–14522 [DOI] [PubMed] [Google Scholar]
- 14.Brittingham, R., Uitto, J., and Fertala, A. (2006) Biochem. Biophys. Res. Commun. 343 692–699 [DOI] [PubMed] [Google Scholar]
- 15.Rousselle, P., Keene, D. R., Ruggiero, F., Champliaud, M. F., van der Rest, M., and Burgeson, R. E. (1997) J. Cell Biol. 138 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heagerty, A. H., Kennedy, A. R., Leigh, I. M., Purkis, P., and Eady, R. A. (1986) Br. J. Dermatol. 115 125–131 [DOI] [PubMed] [Google Scholar]
- 17.Bruckner-Tuderman, L., Nilssen, O., Zimmermann, D. R., Dours-Zimmermann, M. T., Kalinke, D. U., Gedde-Dahl, T., and Winberg, J. O. (1995) J. Cell Biol. 131 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassner, A., Hansen, U., Miosge, N., Reinhardt, D. P., Aigner, T., Bruckner-Tuderman, L., Bruckner, P., and Grässel, S. (2003) Matrix Biol. 22 131–143 [DOI] [PubMed] [Google Scholar]
- 19.Christiano, A. M., Greenspan, D. S., Lee, S., and Uitto, J. (1994) J. Biol. Chem. 269 20256–20262 [PubMed] [Google Scholar]
- 20.Bentz, H., Morris, N. P., Murray, L. W., Sakai, L. Y., Hollister, D. W., and Burgeson, R. E. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 3168–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunstrum, G. P., Sakai, L. Y., Keene, D. R., Morris, N. P., and Burgeson, R. E. (1986) J. Biol. Chem. 261 9042–9048 [PubMed] [Google Scholar]
- 22.Bächinger, H. P., Morris, N. P., Lunstrum, G. P., Keene, D. R., Rosenbaum, L. M., Compton, L. A., and Burgeson, R. E. (1990) J. Biol. Chem. 265 10095–10101 [PubMed] [Google Scholar]
- 23.Gayraud, B., Höpfner, B., Jassim, A., Aumailley, M., and Bruckner-Tuderman, L. (1997) J. Biol. Chem. 272 9531–9538 [DOI] [PubMed] [Google Scholar]
- 24.Bruckner-Tuderman, L., Höpfner, B., and Hammami-Hauasli, N. (1999) Matrix Biol. 18 43–54 [DOI] [PubMed] [Google Scholar]
- 25.Rattenholl, A., Pappano, W. N., Koch, M., Keene, D. R., Kadler, K. E., Sasaki, T., Timpl, R., Burgeson, R. E., Greenspan, D. S., and Bruckner-Tuderman, L. (2002) J. Biol. Chem. 277 26372–26378 [DOI] [PubMed] [Google Scholar]
- 26.Hansen, U., and Bruckner, P. (2003) J. Biol. Chem. 278 37352–37359 [DOI] [PubMed] [Google Scholar]
- 27.Blaschke, U. K., Eikenberry, E. F., Hulmes, D. J. S., Galla, H. J., and Bruckner, P. (2000) J. Biol. Chem. 275 10370–10378 [DOI] [PubMed] [Google Scholar]
- 28.Geselowitz, D. A., and Neumann, R. D. (1995) Bioconjugate Chem. 6 502–506 [DOI] [PubMed] [Google Scholar]
- 29.Hansen, U., Hussain, M., Villone, D., Herrmann, M., Robenek, H., Peters, G., Sinha, B., and Bruckner, P. (2006) Matrix Biol. 25 252–260 [DOI] [PubMed] [Google Scholar]
- 30.Jokinen, J., Dadu, E., Nykvist, P., Käpylä, J., White, D. J., Ivaska, J., Vehviläinen, P., Reunanen, H., Larjava, H., Häkkinen, L., and Heino, J. (2004) J. Biol. Chem. 279 31956–31963 [DOI] [PubMed] [Google Scholar]
- 31.Bruckner-Tuderman, L., Schnyder, U. W., Winterhalter, K. H., and Bruckner, P. (1987) Eur. J. Biochem. 165 607–611 [DOI] [PubMed] [Google Scholar]
- 32.Raghunath, M., Höpfner, B., Aeschlimann, D., Lüthi, U., Meuli, M., Altermatt, S., Gobet, R., Bruckner-Tuderman, L., and Steinmann, B. (1996) J. Clin. Investig. 98 1174–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]