Abstract
Objectives
To assess the clinical characteristics and course of patients with mild cognitive impairment (MCI) and mild Alzheimer disease (AD) treated with cholinesterase inhibitors (ChEIs) and memantine hydrochloride.
Design
Cohort study.
Setting
The 59 recruiting sites for the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Participants
Outpatients with MCI and AD in ADNI.
Main Outcome Measures
The AD Assessment Scale–cognitive subscale (ADAS-cog), Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR) scale, and Functional Activities Questionnaire (FAQ).
Results
A total of 177 (44.0%) of 402 MCI patients and 159 (84.6%) of 188 mild-AD patients were treated with ChEIs and 11.4% of MCI patients and 45.7% of AD patients with memantine at entry. Mild-cognitive-impairment patients who received ChEIs with or without memantine were more impaired, showed greater decline in scores, and progressed to dementia sooner than patients who did not receive ChEIs. Alzheimer-disease patients who received ChEIs and memantine took them longer, were more functionally impaired, and showed greater decline on the MMSE and CDR (but not on the ADAS-cog or FAQ) than those who received ChEIs only.
Conclusions
Academic physicians frequently prescribe ChEIs and memantine earlier than indicated in the US Food and Drug Administration–approved labeling to patients who are relatively more severely impaired or who are rapidly progressing toward cognitive impairment. The use of these medications in ADNI is associated with clinical decline and may affect the interpretation of clinical trial outcomes.
Study Registration
clinicalTrials.gov Identifier: NCT00106899
Many patients with mild cognitive impairment (MCI) and mild Alzheimer disease(AD) participating in the National Institutes of Health (NIH) Alzheimer’s Disease Neuroimaging Initiative (ADNI)1 are receiving cholinesterase inhibitors (ChEIs) and memantine hydrochloride. The prescription of the former for MCI and the latter for mild AD is not approved by the US Food and Drug Administration (FDA). Rather, ChEIs are indicated for AD2 and memantine for moderate to severe AD (defined as AD with Mini-Mental State Examination [MMSE] scores below 15), per FDA-approved labeling.3
Clinical trial results do not show efficacy for ChEIs in MCI4–9 or for memantine in mild to moderate AD.10–14 In 1 placebo-controlled MCI trial,4 however, donepezil hydrochloride was associated with small effects on secondary outcomes, including memory and language subscales, as well as a clinical dementia rating(CDR)at12 to 18 months and an MMSE score at 24 months of treatment that were not maintained.
We compared MCI and AD patients enrolled in ADNI who were receiving ChEIs and memantine with those who were not receiving those medications on clinical differences at study entry and outcomes over 2 years to assess the medications’ potential for efficacy or for affecting clinical outcomes.
METHODS
STUDY OVERVIEW AND PARTICIPANTS
The ADNI is a natural-history, nontreatment, observational study aimed at setting standards for brain imaging and chemical bio-markers for diagnosis and treatment trials.1 Most of the 59 recruiting sites are academic, from which 188 participants with mild AD (ie, who had MMSE scores from 21 through 26), 402 with MCI (ie, who had MMSE scores from 24 through 30), and 229 with no cognitive impairment were enrolled and followed up with regular clinical, imaging, and biomarker assessments.1 Inclusion criteria are detailed elsewhere; participants are allowed to continue their use of marketed antidementia medications if they had been taking stable doses for at least 4 weeks prior to entry.1
CLINICAL OUTCOMES
The main clinical outcomes in ADNI are the AD Assessment Scale–cognitive subscale (ADAS-cog),15,16 CDR,17 MMSE,18 and Functional Activities Questionnaire (FAQ).19 Assessments were per-formedat6-month intervals during the first 2 years(except month 18 for AD patients). The ADAS-cog15,16 is a structured scale used to evaluate memory, reasoning, language, orientation, praxis, language, and word-finding difficulty and is scored from 0 to 70, with higher scores indicating worse performance. The CDR17 is used to rate 5 levels of impairment (0 [not impaired], 0.5, 1, 2, and 3 [severely impaired] in each of 6 categories: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. The CDR sum of boxes (CDR-SB) score is used as a measure of severity and outcome, ranging from 0 to 18.
The MMSE18 is used to evaluate orientation, registration, attention, concentration, recall, language, and visual construction. Scores are the number of correct items, with a range from 0 through 30. The FAQ19 relies on an interview with a study partner to rate a participant’s ability to perform 10 complex activities of daily living (eg, manage finances, shop, prepare a meal, and travel). Each activity is rated on 3 levels (0=does without difficulty, 1=needs frequent advice or assistance, and 2=some-one else has taken over the activity); scores range from 0 to 20.
STATISTICAL ANALYSIS
We tested for associations between diagnostic groups (MCI vs AD) at study entry on clinical characteristics and medication use (including dose and previous duration of use) and between treatment groups within diagnoses using the Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables. (We excluded the few MCI patients who received only memantine and the few AD patients who received no medication or memantine only.)
We used linear mixed-effects models to assess the rate of change for each of the 4 clinical outcomes over 24-month follow-up periods between treatment groups. Time was modeled continuously and calculated from participant visit dates. Prior drug exposure was estimated using concurrent medication start and stop dates. Diagnostics for model fit were done by visual inspection of residuals. Imbalances of age, sex, educational level, apolipoprotein E (APOE) ε4 carrier status, and family history were assessed among treatment groups. Covariates were included if they were associated with the outcome (α=.15) and treatment group (α=.10). Estimates were adjusted for age regardless of observed association.
We assessed time to progression from MCI to dementia, defined as change in CDR score from 0.5 through 1.0, using Weibull regression, an interval-censored parametric survival model, because the time could only be known to have occurred between 6-month visits and not on an exact date. Ratios of mean time to progression derived from the survival analysis were used to compare the risk for progression in the medication-treated groups with that in the nontreated group. Covariates were included using the same criteria as the mixed models.
We compared by group the proportions discontinuing their medications during follow-up, the reasons for discontinuation, and serious adverse events (by FDA definition), including deaths. We then assessed the numbers of patients who started these medications after study entry. Data were downloaded from the ADNI database (http://adni.loni.ucla.edu/) on May 7, 2009. Statistical analyses were performed on all participants with available data using R software, version 2.9.2 (http://www.r-project.org).
RESULTS
MCI COMPARED WITH AD PATIENTS AT STUDY ENTRY
Most of the MCI and AD patients were male (Table 1). One-half of MCI and two-thirds of AD patients were APOE ε4 carriers. Mild-cognitive-impairment patients showed less impairment on clinical ratings scales. Among MCI patients, 177 (44.0%) received ChEIs; 46 (11.4%), memantine; and 215 (53.5%), neither. Among AD patients, 159 (84.6%) received ChEIs; 86 (45.7%), memantine; and 16 (8.5%), neither. Median duration of prior ChEI use was 0.97 years for MCI and 1.42 years for AD patients (P=.02), and duration of prior treatment with memantine was 0.88 and 0.94 years (P=.38), respectively.
Table 1.
Characteristics and Medication Use for All MCI and AD Patients at Study Entrya
| Characteristic | MCI (n=402) | AD (n=188) | P Valueb |
|---|---|---|---|
| Age (SD), y | 74.8 (7.42) | 75.3 (7.56) | .47 |
| Female sex | 143 (35.6) | 89 (47.3) | .007 |
| Educational level (SD), y | 15.7 (3.04) | 14.7 (3.14) | <.001 |
| Family history of AD or dementiac | 170 (49.6) | 78 (50.3) | .92 |
| APOE ε4 genotype carriers, 1 or 2 alleles | 215 (53.5) | 124 (66.0) | .004 |
| GDS score (SD)d | 1.58 (1.37) | 1.67 (1.42) | .50 |
| MMSE score (SD)d | 27.0 (1.78) | 23.3 (2.04) | <.001 |
| ADAS-cog, errors, mean (SD) | 11.54 (4.43) | 18.72 (6.33) | <.001 |
| CDR-SB score (SD) | 1.60 (0.88) | 4.36 (1.61) | <.001 |
| FAQ score (SD) | 3.88 (4.48) | 13.14 (6.84) | <.001 |
| ChEI use | 177 (44.0) | 159 (84.6) | <.001 |
| ChEI type | .22 | ||
| Donepezil hydrochloride | 150 (84.7) | 123 (77.4) | . . . |
| Galantamine | 18 (10.2) | 25 (15.7) | . . . |
| Rivastigmine | 9 (5.1) | 11 (6.9) | . . . |
| Prior exposure, median (IQR), y | 0.97 (0.41–2.14) | 1.42 (0.57–3.01) | .02 |
| Memantine hydrochloride use | 46 (11.4) | 86 (45.7) | <.001 |
| Prior exposure, median (IQR), y | 0.88 (0.30–1.42) | 0.94 (0.32–1.93) | .38 |
| ChEI and memantine prescription | 36 (9.0) | 73 (38.8) | <.001 |
| Neither ChEI nor memantine | 215 (53.5) | 16 (8.5) | <.001 |
Abbreviations: AD, Alzheimer disease; ADAS-cog, AD Assessment Scale–cognitive subscale; APOE, apolipoprotein E; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ChEI, cholinesterase inhibitor; ellipses, not applicable; FAQ, Functional Activities Questionnaire; GDS, Geriatric Depression Scale; IQR, interquartile range; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Data are given as number (percentage) unless otherwise indicated.
P values based on Fisher exact tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Family history of AD or dementia in first-degree relatives was missing for 59 MCI and 33 AD patients.
Inclusion criteria required GDS scores of less than 6 and MMSE scores from 24 to 30 for MCI and from 21 through 26 for mild-AD patients.
MCI RESULTS: ChEIs AND MEMANTINE VS NO TREATMENT
Patient Characteristics
There were virtually no differences in age, sex, and educational level between MCI patients who received ChEIs only or ChEIs and memantine and patients who received none (Table 2). A total of 93.9% were classified as having MCI due to Alzheimer disease. Carriers of the genotype APOE ε4 were more prevalent in the treated groups. The 2 medication-treated groups performed worse on the ADAS-cog, CDR, and FAQ than the no-treatment group, with the group that received a ChEI and memantine performing worse than the ChEI-only group. Median prior treatment with ChEIs was 0.90 years for patients receiving ChEIs only and 1.54 years for those receiving both types of medication; median treatment with memantine was 0.80 years prior to study entry.
Table 2.
Characteristics of MCI Patients by Treatment Groups at Study Entrya
| Characteristic | None (n=215) | ChEI (n=141) | ChEI and Memantine Hydrochloride (n=36) | P Valueb |
|---|---|---|---|---|
| Age (SD), y | 75.4 (7.60) | 74.2 (7.06) | 74.0 (8.23) | .30 |
| Female sex | 80 (37.2) | 52 (36.9) | 10 (27.8) | .58 |
| Educational level (SD), y | 15.5 (3.15) | 15.7 (2.90) | 16.7 (2.95) | .08 |
| MCI due to AD | 205 (95.3) | 132 (93.6) | 32 (88.9) | .61 |
| APOE ε4 genotype carrier on 1 or 2 alleles | 99 (46.0) | 86 (61.0) | 25 (69.4) | <.01 |
| Family history of AD or dementiac | 85 (47.0) | 63 (51.2) | 18 (62.1) | .29 |
| MMSE score (SD)d | 27.1 (1.80) | 27.1 (1.75) | 26.4 (1.70) | .10 |
| ADAS-cog, errors (SD) | 10.7 (4.12) | 12.2 (4.55) | 13.7 (4.54) | <.001 |
| CDR-SB score (SD) | 1.45 (0.77) | 1.74 (0.93) | 1.97 (1.08) | <.001 |
| FAQ score (SD) | 2.88 (3.54) | 4.60 (4.93) | 6.43 (5.58) | <.001 |
| Prior exposure, median (IQR), y | ||||
| ChEI | 0.90 (0.31–2.09) | 1.54 (0.78–2.75) | .02 | |
| Memantine | . . . | 0.80 (0.33–1.35) | . . . | |
Abbreviations: AD, Alzheimer disease; ADAS-cog, AD Assessment Scale–cognitive subscale; APOE, apolipoprotein E; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ChEI, cholinesterase inhibitor; ellipses, not applicable; FAQ, Functional Activities Questionnaire; IQR, interquartile range; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Data are given as number (percentage) unless otherwise indicated. Ten MCI patients who received only memantine were excluded.
P values based on Fisher exact and Kruskal-Wallis tests.
AD or dementia in first-degree relatives was missing for 59 MCI patients.
Inclusion criteria for MCI patients required MMSE scores from 24 through 30.
Of the 150 donepezil-treated patients, 116 (77.3%) were taking 10 mg/d or higher, 33 (22.0%) were taking 5 mg/d, and 1 (0.7%) was taking 2.5 mg/d. Of the 18 galantamine-treated patients, 14 (77.8%) were taking 16.0 to 24.0 mg/d and 4 (22.2%) were taking 8.0 to 12.0 mg/d. Of the 9 rivastigmine-treated patients, 8 (88.9%) were taking 6.0 to 12.0 mg/d and 1 (11.1%) was taking 3.0 mg/d. Of the 36 memantine-treated patients (not including 10 who were taking memantine only), 30 (83.3%) were taking 20.0 mg/d and 6 (16.7%) were taking 10.0 mg/d.
Change in Rating Scales Scores
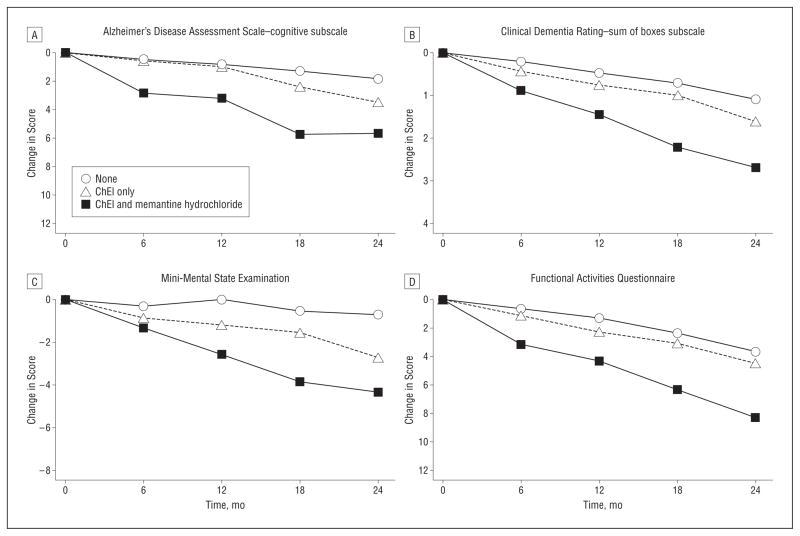
Mild-cognitive-impairment patients treated with ChEIs only or with ChEI and memantine showed decline on the ADAS-cog, MMSE, CDR-SB, and FAQ to a greater extent than patients not receiving those medications (Table 3, Figure 1). The mean differences generally increased from month 6 to month 24. The magnitude of decline was more than 2-fold greater in patients treated with both types of medication than in those treated with ChEIs only on the observed change scores at the 6-, 12-, 18-, and 24-month follow-ups and the model-based change per year (except the ADAS-cog). For example, decline on the MMSE was 0.87 point greater per year in patients treated with ChEIs only and 1.89 points greater per year in those treated with both types of medication compared with patients not treated with either type.
Table 3.
Observed Change From Baseline and Modeled Difference in Annual Rate of Decline on Clinical Outcomes by Treatment for MCI Patients (See Figure 1)a
| Assessment Instrument and Treatment Group | Observed Change From Study Entry, mo
|
Increase in Rate of Declineb |
||||
|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | Compared With None (Points/y) | P Value for Model | |
| ADAS-cog | ||||||
| None | 0.49 | 0.84 | 1.32 | 1.83 | ||
| ChEI only | 0.59 | 1.00 | 2.41 | 3.46 | 0.78 | .03 |
| ChEI and memantine hydrochloride | 2.83 | 3.23 | 5.74 | 5.64 | 0.86 | .14 |
| MMSE | ||||||
| None | −0.32 | 0.01 | −0.54 | −0.72 | ||
| ChEI only | −0.87 | −1.20 | −1.55 | −2.72 | −0.87 | <.001 |
| ChEI and memantine | −1.33 | −2.56 | −3.86 | −4.36 | −1.89 | <.001 |
| CDR–SB | ||||||
| None | 0.22 | 0.48 | 0.73 | 1.10 | ||
| ChEI only | 0.45 | 0.77 | 1.00 | 1.62 | 0.26 | <.001 |
| ChEI and memantine | 0.89 | 1.45 | 2.21 | 2.69 | 0.72 | <.001 |
| FAQ | ||||||
| None | 0.64 | 1.28 | 2.36 | 3.64 | ||
| ChEI only | 1.12 | 2.27 | 3.07 | 4.46 | 0.63 | .007 |
| ChEI and memantine | 3.16 | 4.29 | 6.36 | 8.28 | 2.29 | <.001 |
Abbreviations: ADAS-cog, AD Assessment Scale–cognitive subscale; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ChEI, cholinesterase inhibitor; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Sample sizes for the 3 treatment groups were from 215 to 147 for no treatment, 141 to 92 for ChEI only, and 36 to 25 for ChEI and memantine. Overall sample sizes for the models were 375 to 392 patients.
Model-based estimates compared with the no-treatment group. Estimates were adjusted for age, educational level, APOE genotype, and baseline CDR-SB or ADAS-cog score.
Figure 1.
Observed change on clinical outcomes by treatment for patients with mild cognitive impairment. See Table 3 for values. ChEI indicates cholinesterase inhibitor.
Progression to AD
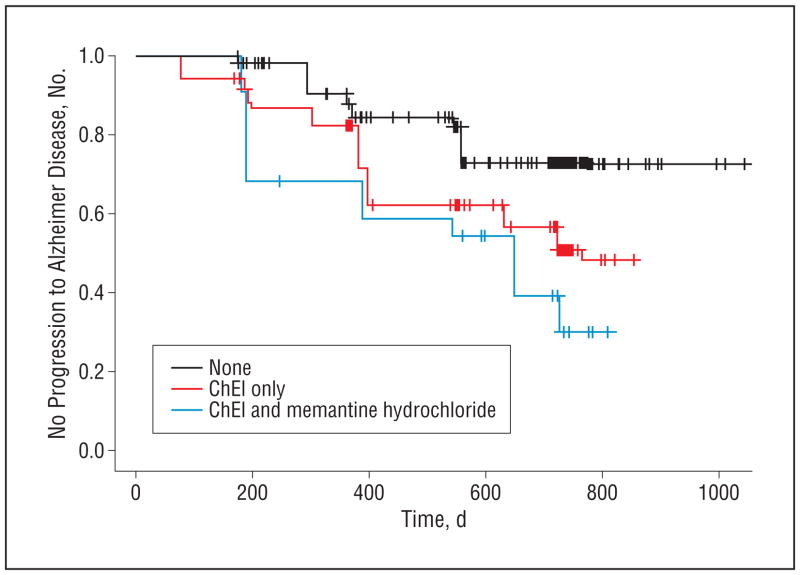
One hundred twenty-eight MCI patients progressed to having dementia, including 48 (22.3%), 60 (42.6%), and 20 (55.6%) in the nontreated, ChEI-only–treated, and ChEI and memantine–treated groups, respectively. The mean time to dementia for ChEI-only–treated patients was reduced by 29.8% (P =.005) and for ChEI and memantine–treated patients by 41.8% (P=.003) compared with the no-treatment group, and the risk for progression was higher for patients taking medications (Figure 2). Estimates were adjusted for age, APOE ε4 genotype carrier status, educational level, and baseline ADAS-cog score.
Figure 2.
Kaplan-Meier estimates of the rate of progression from mild cognitive impairment to Alzheimer disease, showing the observed estimates for no progression to Alzheimer disease for the 3 treatment groups during follow-up (P=.003). ChEI indicates cholinesterase inhibitor.
Duration of Prior Drug Exposure
Duration of exposure to ChEI treatment prior to study entry was not associated with change on the ADAS-cog compared with no treatment (P=.57). However, every year of ChEI exposure prior to study entry was associated with a rate of decline of 0.13 point per year slower on the CDR-SB (P<.001), 0.12 point per year slower on the MMSE (P=.04), and 0.41 point per year slower on the FAQ (P<.001) compared with no treatment. For example, during a 2-year period, a participant taking a ChEI for 2.5 years at baseline would be expected to show a decline of 0.24 point less on the MMSE than a participant with only 0.5 years of exposure.
Discontinuations, Adverse Events, and Medication Discontinuation and Initiation
Thirty-seven (9.2%) of 402 MCI patients discontinued treatment during follow-up. The main reasons were withdrawal of consent (n=23), loss to follow-up (n=4), and protocol noncompliance (n=2). Virtually no differences were found among treatment groups. Serious adverse events were reported in 59 (27.4%), 30 (21.3%), and 12 (33.3%) (including deaths in 5, 3, and 2 patients) no-treatment, ChEI-only–, and ChEI and meman-tine–treated patients, respectively. (There was 1 serious adverse event and 0 deaths in the 10 patients treated with memantine only.)
Sixteen (9.0%) of 177 MCI patients taking ChEIs discontinued treatment during follow-up, and 45 (20.9%) of 215 not taking ChEIs started to do so. Five (13.9%) of 36 patients taking memantine at baseline discontinued doing so. Of 356 not taking memantine at baseline, 45 (12.6%) started doing so, with 29 (8.1%) taking it in addition to their ChEI.
AD RESULTS: ChEIs AND MEMANTINE VS ChEI TREATMENT
Patient Characteristics
There were virtually no differences in age, sex, educational level, or family history of AD or dementia between mild-AD patients receiving ChEIs only and those receiving ChEIs with memantine (Table 4). Carriers of APOE ε4 were somewhat more prevalent in the ChEI-only group than in the group treated with both types of medication (74.4% vs 58.9%). At entry, the group receiving both types of medication performed worse on the CDR and FAQ but not the ADAS-cog (P=.11) or MMSE compared with the group receiving only a ChEI. Median duration of prior use of ChEIs was 2.20 years for patients receiving both types of medication and 0.97 years for those receiving only ChEIs. The median duration for prior memantine treatment was 1.03 years.
Table 4.
Characteristics of AD Patients Taking Antidementia Medications at Study Entrya
| Characteristic | ChEI Only (n=86) | ChEI and Memantine Hydrochloride (n=73) | P Valueb |
|---|---|---|---|
| Age (SD), y | 76.0 (6.69) | 74.0 (8.63) | .21 |
| Female sex, No. (%) | 38 (44.2) | 31 (42.5) | .87 |
| Educational level (SD), y | 14.8 (3.05) | 15.1 (2.85) | .43 |
| APOE ε4 genotype carrier on 1 or 2 alleles, No. (%) | 64 (74.4) | 43 (58.9) | .04 |
| Family history of AD or dementia, No. (%)c | 35 (50) | 34 (54) | .73 |
| MMSE score (SD)d | 23.4 (2.02) | 23.1 (2.05) | .35 |
| ADAS-cog, errors (SD) | 18.1 (5.87) | 19.7 (6.64) | .11 |
| CDR-SB score (SD) | 4.15 (1.47) | 4.82 (1.64) | .001 |
| FAQ score (SD) | 11.7 (6.40) | 15.8 (7.05) | <.001 |
| ChEI exposure, median (IQR), y | 0.97 (0.33–2.15) | 2.20 (1.00–3.66) | <.001 |
| Memantine exposure, median (IQR), y | . . . | 1.03 (0.38–1.97) | . . . |
Abbreviations: AD, Alzheimer disease; ADAS-cog, AD Assessment Scale–cognitive subscale; APOE, apolipoprotein E; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ChEI, cholinesterase inhibitor; ellipses, not applicable; FAQ, Functional Activities Questionnaire; IQR, interquartile range; MMSE, Mini-Mental State Examination.
Sixteen (8.5%) patients who received no medication and 13 (7.0%) who received memantine only were excluded.
P values based on Fisher exact and Kruskal-Wallis tests.
Family history of AD or dementia in 1st-degree relatives was missing for 26 AD patients.
Inclusion criteria for AD patients required MMSE scores from 21 through 26.
Overall, 108 (87.8%) of donepezil-treated patients were taking 10 mg/d or greater and 15 (12.2%) were taking 5 mg/d; 23 (92.0%) of galantamine-treated patients were taking 16 to 24 mg/d and 2 (8.0%), 8 mg/d; 10 (90.9%) of rivastigmine-treated patients took 6 to 12 mg/d, and 1 (9.1%) took 3 mg/d. For memantine, 63 (86.3%) were taking 20 mg/d, 8 (11.0%) were taking 10 mg/d, 1 (1.4%) was taking 15 mg/d, and 1 (1.4%) was taking 40 mg/d.
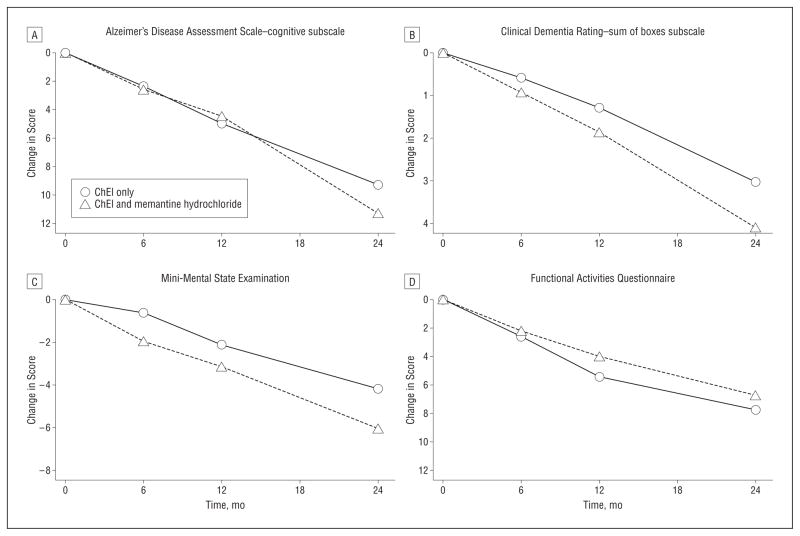
Change in Rating Scales
Alzheimer-disease patients treated with ChEIs and memantine showed greater clinical decline than patients treated with only ChEIs on the MMSE and CDR-SB scales, by 0.93 and 0.50 points per year, respectively (Table 5, PFigure 3). There were no significant differences between the groups on the ADAS-cog or the FAQ based on the modeled differences, although at 24 months, patients taking both types of medication had a worse score, by 2 ADAS-cog points, on observed change. Duration of exposure prior to entry was not associated with change on the ADAS-cog ( = .60), MMSE (P = .05), CDR-SB (P = .27), or FAQ (P=.76) among AD patients treated only with a ChEI vs treatment with both types of medication.
Table 5.
Observed Change From Baseline and Modeled Difference in Annual Rate of Decline on Clinical Outcomes by Treatment for AD Patients (See Figure 3)a
| Assessment Instrument and Treatment Group | Observed Change From Study Entry, mo
|
Increase in Rate of Declineb |
||||
|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | Compared With ChEI Only (Points/y) | P Value for Model | |
| ADAS-cog | ||||||
| ChEI only | 2.35 | 4.96 | . . . | 9.25 | ||
| ChEI and memantine hydrochloride | 2.56 | 4.44 | . . . | 11.26 | −0.12 | .87 |
| MMSE | ||||||
| ChEI only | −0.64 | −2.15 | . . . | −4.19 | ||
| ChEI and memantine | −1.96 | −3.15 | . . . | −6.04 | −0.93 | .005 |
| CDR-SB | ||||||
| ChEI only | 0.59 | 1.30 | . . . | 3.02 | ||
| ChEI and memantine | 0.93 | 1.86 | . . . | 4.09 | 0.50 | .007 |
| FAQ | ||||||
| ChEI only | 2.60 | 5.43 | . . . | 7.79 | ||
| ChEI and memantine | 2.22 | 4.02 | . . . | 6.74 | −0.55 | .21 |
Abbreviations: AD, Alzheimer Disease; ADAS-cog, AD Assessment Scale–cognitive subscale; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ChEI, cholinesterase inhibitor; ellipses, not applicable; FAQ, Functional Activities Questionnaire; MMSE, Mini-Mental State Examination.
Sample sizes for the 2 treatment groups were from 86 through 54 for ChEI only and between 73 and 49 for ChEI and memantine. Overall, sample sizes for the models were 158 through 149.
Model-based estimates comparing treatment with ChEI and memantine to ChEI only. Estimates were adjusted for age, educational level, APOE genotype, and baseline CDR-SB or ADAS-cog score.
Figure 3.
Observed change on clinical outcomes by treatment for mild–Alzheimer Disease patients. See Table 5 for values. ChEI indicates cholinesterase inhibitor.
Discontinuations, Adverse Events, and Medication Discontinuation and Initiation
Sixteen (8.5%) of 188 AD patients discontinued medications during follow-up. The main reasons were withdrawal of consent (n=10) and protocol noncompliance(n=3), and there were no significant differences between the groups. Serious adverse events were reported in 18 (20.9%) and 22 (30.1%) (P=.02), including deaths in 1 ChEI-only–treated and 2 ChEI and memantine–treated patients, respectively.
Of 159 AD patients taking ChEIs at entry, 25 (15.7%) discontinued taking them during follow-up. Fourteen (19.2%) of 73 patients taking memantine discontinued doing so, and 30 (34.9%) of 86 patients not taking memantine at entry started doing so.
COMMENT
Rates of ChEI (84.6%) and memantine treatment (45.7%) for mild-AD patients among the mostly academic ADNI centers are similar to rates found in recent mild to moderate AD clinical trials conducted from 2003 through 2009, wherein mean ChEI treatment increased from 52.9% to nearly 100% and memantine treatment from 13.5% to 63.4%.20 Rates were comparable to a recent tarenflurbil trial in mild AD, in which ChEI treatment was 75.1% and memantine treatment was 48.1%.21 However, rates of treatment with those medications were higher than for similar mild-AD patients registered in the NIH National Alzheimer’s Coordinating Center (NACC) during 2009, of whom 152 (58.5%) of 260 received ChEIs and 65 (25.0%) received memantine (Walter Kukull, PhD; oral communication; January 13, 2010).
Cholinesterase inhibitor treatment of 44.0% for MCI patients in ADNI was nearly twice that of MCI patients in the NACC and the California Alzheimer’s Disease Centers.22 Specifically, 23.9% of 351 MCI patients in the NIH centers and 25.1% of 578 MCI patients in the California centers received these medications. Treatment with memantine (at 11.1% and 10.9%, respectively) was similar to that in ADNI (11.4%).
The MCI patients who received ChEIs had, on average, a more severe decline in scores than those who did not, and their scores deteriorated more rapidly. They were very similar to AD patients, as evidenced by lower performance scores; APOE ε4 carrier rates of 61.0% to 69.5% similar to AD patients enrolled in clinical trials20; and greater rate of worsening of clinical ratings compared with MCI participants from recent trials.4,8,9 Retrospectively, the study physicians probably considered the patients to have technically fulfilled the MCI criteria used in ADNI while also having AD, as further evidenced by their characterizing 95% of the MCI diagnoses as MCI due to AD. Under these circumstances, use of ChEIs could be expected to be consistent with the treatment of early AD.
Although unlikely, other hypotheses merit consideration, including the possibility that treatment with ChEIs in MCI during 1 to 1.5 years is associated with worse outcomes compared with no treatment. Notably, the approximately 1.5-point ADAS-cog and 0.5- to 1.5-point CDR-SB differences between the treatment and no-treatment groups during 1 to 2 years have the same magnitude as the effect of ChEIs in placebo-controlled AD trials23 and as the effect sizes expected for experimental drugs in current clinical trials20,21 but in the opposite (counter therapeutic) direction. This observation is also consistent with the observation herein that the mild-AD patients who received both ChEIs and memantine and received the ChEI for longer than 2 years prior to entering ADNI had greater dementia severity and a worse disease course compared with those who received ChEIs only and had been treated less than half as long. It should be emphasized, however, that none of the placebo-controlled MCI trials of ChEIs suggest cognitive or behavioral toxicity of the medications during the 2- to 4-year trial periods.4–6
Although a relatively longer duration of treatment with ChEIs in MCI prior to entering ADNI was associated with less decline compared with no treatment in 3 of 4 outcomes, the effects, such as 0.12 MMSE point per year, were slight. This finding may represent the combination of a group taking medications who had not shown clinical decline and whose medication regimens are maintained on a long-term basis and another group who had recently started taking medications because their symptoms were more severe or their scores were showing more rapid decline.
Evidence for the efficacy of memantine in mild AD is lacking despite its widespread use.13 The 3 placebo-controlled trials for mild to moderate AD included few mild-AD patients (ie, they allowed only patients with MMSE scores ≤22 in 2 trials and ≤24 in 1) and were not statistically significant overall.10–13 As with MCI patients, one consideration is that the mild-AD patients who have worsening scores on the assessment measures may have had memantine added to their ChEI regimen with hope of added benefit. In other words, physicians in a predicament may choose to treat early with memantine rather than delay treatment until patients’ conditions deteriorate into the severity range for which the medication has been demonstrated effective.3
Important limitations to making inferences from these analyses include that ADNI is not a treatment study and not a clinical trial and that medication was not assigned randomly or in a double-blind manner to minimize biased outcomes. As with all observational studies, known and unknown potential biases cannot be fully corrected for by multivariable analysis. As we have discussed herein, physicians at these academic centers could have made treatment decisions based on biomarkers including APOE ε4 genotype carrier status, clinical severity, neuropsychological test performance, and clinical course.
The results raise issues regarding MCI diagnoses and in particular whether the diagnosis of MCI due to Alzheimer disease, as used by research physicians in ADNI, is actually early or prodromal AD before or early in the dementia syndrome.24 Moreover, there are substantial implications for health policy and clinical trial planning and interpretations because MCI and mild-AD patients receiving marketed antidementia medications may have different responses to experimental drugs and different, counterintuitive clinical courses compared with those not receiving medications. It does not necessarily follow, however, that because medication-treated patients show worsening on assessments to a greater extent than non-treated patients, they would be more likely to respond to an experimental drug or that a therapeutic effect to such treatment may be detected more readily. Rather, the opposite could be true. Much more investigation needs to be given to the long-term effects of marketed antidementia medications; the imaging and biomarker studies in ADNI may provide additional information.
Acknowledgments
Financial Disclosure: Dr Schneider is an editor for the Cochrane Dementia and Cognitive Improvement Group, which oversees systematic reviews of medications for cognitive impairment and dementia; has received a grant from the Alzheimer’s Association for a registry for dementia and cognitive impairment trials and grant or research support from AstraZeneca Pharmaceuticals, Baxter International Inc, Elan Pharmaceuticals, Forest Laboratories Inc, Johnson & Johnson Consumer Companies Inc, Eli Lilly and Company, Myriad Pharmaceuticals Inc, Novartis Pharmaceuticals Corporation, Pfizer Incorporated, Takeda Pharmaceutical Company Ltd, and Wyeth Pharmaceuticals; has served as a consultant for or received consulting fees from Abbott Laboratories, AC Immune SA, Allergan Inc, Allon Therapeutics, Alzheimer’s Drug Discovery Foundation, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Elan Pharmaceuticals, Eli Lilly and Company, ExonHit Therapeutics, Forest Laboratories Inc, GlaxoSmithKline plc, Ipsen Group, Johnson & Johnson Consumer Companies Inc, H. Lundbeck A/S, Myriad Pharmaceuticals Inc, MedAvante Inc, Medivation Inc, Merck & Co Inc, Merz Pharmaceuticals GmbH, Novartis Pharmaceuticals Corporation, F. Hoffman–La Roche Ltd, Sanofi-aventis LLC, Servier Laboratories, Schering-Plough Corporation, Schwabe Pharmaceuticals, Teva Pharmaceutical Industries Ltd, Toyama Pharmaceutical Co Ltd, Transition Therapeutics Inc, Voyager Pharmaceuticals Corp, and Wyeth Pharmaceuticals. Dr Weiner serves on scientific advisory boards for Aegis Therapies, Allergan Inc, Araclon Biotech, Avid Radiopharmaceuticals Inc, the Banner Pharmacaps Inc Alzheimer’s Institute Alzheimer’s Prevention Initiative; Bayer Schering Pharma AG, Biogen Idec, Bristol-Myers Squibb, CoMentis Inc, the Elan Corporation plc/Wyeth Pharmaceuticals Alzheimer’s Immunotherapy Program, Eli Lilly and Company, Eisai Inc, Forest Laboratories Inc, Genentech Inc, Institut Català de Neurociencias Aplicades, Neurochem Inc, Lippincott Williams & Wilkins, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals Corporation, Pfizer Inc, and the United States Department of Veterans Affairs Central Office Gulf War Veterans’ Illnesses Advisory Committee; has received funding for traval from l’Alliance Médicale et Scientifique, Alzheimer’s Association, Alzheimer’s Disease Neuroimaging Initiative, AstraZeneca Pharmaceuticals, Avid Pharmaceuticals Inc, the Banner Pharmacaps Inc Alzheimer’s Institute, Bayer Health-Care, Elan Corporation plc/Wyeth Pharmaceuticals Alzheimer’s Immunotherapy Program North American Advisory Board, Beijing Institute of Geriatrics, Centre Hospitalier Régional Universitaire—Hôpital Roger Salengro, Clinical Trials on Alzheimer’s Disease, Colloquium Paris, Eli Lilly and Company, Forest Laboratories Inc, Foundation for the National Institutes of Health, GE Healthcare Japan, Geneva University Hospitals, In-nogenetics NV, Institut Català de Neurociencias Aplicades, Ipsen, The Johns Hopkins University, Korean Neurological Association, The Michael J. Fox Foundation for Parkinson’s Research, Montreal Neurological Institute and Hospital at McGill University, Mount Sinai Medical Center, National Multiple Sclerosis Society, Neuroscience Early Stage Research Training, NeuroVigil Inc, New York University, Pfizer Inc Global Research and Development, Research Association for Biotechnology, Rotman Research Institute of Baycrest, Siemens AG, Society of Photo-Optical Instrumentation Engineers, Tel-Aviv University Medical School, Telemedicine & Advanced Technology Research Center, University of Pittsburgh, Wien Center for Alzheimer’s Disease and Memory Disorders, University of Califonia Davis, United States Social Security Administration, Wenner-Grem Foundation, Washington University in St Louis, United States Department of Veterans Affairs Central Office, University of New Mexico School of Medicine, and University of Paris and from Kenes International and Nestlé SA to attend conferences not funded by industry; serves on the editorial board of Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association; has received honoraria from American Academy of Neurology, BOLT International, GE Healthcare Japan, Institut Català de Neurociencias Aplicades, Ipsen, The Johns Hopkins University, NeuroVigil Inc, the Rotman Research Institute, and the Research Institute for Biotechnology; serves as a consultant for Araclon Biotech, AstraZeneca Pharmaceuticals, Bayer Healthcare, Bayer Schering Pharma AG, Biogen Idec, Co-Mentis Inc, Daiichi Sankyo Co Ltd, Elan Corporation plc, Eli Lilly and Company, ExonHit Therapeutics SA, Forest Laboratories Inc, Medivation Inc/Pfizer Inc, Ipsen, Neurochem International Limited (now Bellus Health International Limited), Novartis Pharmaceuticals Corporation, Pfizer Inc Global Research and Development, Servier Laboratories, Synarc Inc, and TauRx Therapeutics LTD; receives research support from Merck & Co Inc, Avid Radiopharmaceuticals Inc, the National Institutes of Health (as a private investigator: U01AG024904, P41 RR023953, and R01AG10897; and as a coinvestigator: P01AG19724, P50AG23501, R24 RR021992, R01 NS031966, and P01AG012435), the United States Department of Defense (as a private investigator: DAMD17-01-0764), and the Veterans Administration (as a core private investigator: MIRECC VISN 21); and holds stock in Synarc Inc and Elan Corporation plc.
Additional Contributions: The Foundation for the National Institutes of Health (http://www.fnih.org) coordinates the private sector participation of the $60 million ADNI public/private partnership begun by the National Institute on Aging and supported by the National Institutes of Health. To date, more than $27 million has been provided to the FNIH by Abbott Laboratotries, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation plc, Genentech Inc, GE HealthCare, GlaxoSmithKline plc, In-nogenetics, Johnson & Johnson Consumer Companies Inc, Eli Lilly and Company, Merck & Co Inc, Novartis AG, Pfizer Inc, F. Hoffmann–La Roche Ltd, Schering-Plough Corporation, Synarc Inc, and Wyeth Pharmaceuticals, as well as nonprofit partners the Alzheimer’s Association and the Alzheimer’s Drug Discovery Foundation. This research was also supported by National Institutes of Health grant P50AG05142 in conjunction with the University of Southern California Alzheimer’s Disease Research Center.
Footnotes
Additional Information: The Alzheimer’s Disease Neuro-imaging Initiative: Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuro-imaging Initiative (ADNI, NIA U01 AG024904) database (www.loni.ucla.edu/ADNI). As such, the investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete list of investigators in ADNI is listed at: http://adni.loni.ucla.edu/about/who-we-are/principal-investigators/. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, positron emission tomography, other biological markers, and clinical and neuropsycho-logical assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as to lessen the time and cost of clinical trials.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Schneider, Insel, and Weiner. Acquisition of data: Schneider and Weiner. Analysis and interpretation of data: Schneider, Insel, and Weiner. Drafting of the manuscript: Schneider and Insel. Critical revision of the manuscript for important intellectual content: Schneider and Weiner. Statistical analysis: Schneider and Insel. Obtained funding: Weiner. Administrative, technical, and material support: Schneider and Weiner. Study supervision: Schneider.
References
- 1.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aricept prescribing information. Aricept; [Accessed November 2, 2009]. Web site. http://aricept.com/images/AriceptComboFullPLNovember02006.pdf. [Google Scholar]
- 3.Memantine hydrochloride prescribing information. Forest Laboratories Inc; [Accessed November 2, 2009]. Web site. http://www.frx.com/pi/namenda_pi.pdf. [Google Scholar]
- 4.Petersen RC, Thomas RG, Grundman M, et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 5.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 6.Winblad B, Gauthier S, Scinto L, et al. GAL-INT-11/18 Study Group. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 7.Salloway S, Ferris S, Kluger A, et al. Donepezil 401 Study Group. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 8.Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 9.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peskind ER, Potkin SG, Pomara N, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14(8):704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 11.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 12.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2007;11(4):471–479. doi: 10.3233/jad-2007-11409. [DOI] [PubMed] [Google Scholar]
- 13.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 14.Winblad B, Jones RW, Wirth Y, Stöffler A, Möbius HJ. Memantine in moderate to severe Alzheimer’s disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord. 2007;24(1):20–27. doi: 10.1159/000102568. [DOI] [PubMed] [Google Scholar]
- 15.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 20.Schneider LS, Sano M. Current Alzheimer’s disease clinical trials: methods and placebo outcomes. Alzheimers Dement. 2009;5(5):388–397. doi: 10.1016/j.jalz.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green RC, Schneider LS, Amato DA, et al. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstein AM, Barton C, Ross L, Kramer JH, Yaffe K. Treatment practices of mild cognitive impairment in California Alzheimer’s Disease Centers. J Am Geriatr Soc. 2009;57(4):686–690. doi: 10.1111/j.1532-5415.2009.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]