Abstract
Memories are enhanced during sleep through memory consolidation processes. A recent study reported that sleep increases competitive forgetting in the absence of sleep-dependent consolidation of the target memory (Racsmany et al. in Psychol Sci 21:80–85, 2010). Here, using a modified retrieval-induced forgetting task, we examined whether sleep-dependent modulation of forgetting occurs concurrently with the consolidation of related target memories. Participants encoded a word-pair list and then practiced retrieving a portion of these pairs. Following a break with sleep or wake, recall of all pairs was tested. As expected, recall for practiced pairs was greater following sleep relative to wake. Contrary to Racsmany et al. (Psychol Sci 21:80–85, 2010), competitive forgetting decreased following sleep. Moreover, recall for practiced pairs correlated with slow wave sleep (SWS) while forgetting of competing targets correlated negatively with REM, suggesting a novel function of these sequential brain states on memory processing.
Keywords: Sleep, Memory, Consolidation, Forgetting, Slow wave sleep, REM
Introduction
Recent research has emphasized the benefit of sleep on memory through consolidation processes (e.g., Walker 2005). However, memory enhancement may also come through suppression or forgetting of competing memories—increasing the relative activation of a target over an interloper at retrieval (Jones 1989).
Crick and Mitchison (1983) hypothesized that sleep increases forgetting of conflicting memories by triggering processes which prune against their neural representations during REM. Likewise, the Synaptic Homeostasis Hypothesis (Tononi and Cirelli 2006) suggests that global synaptic downscaling occurs during sleep which would result in forgetting of the weakest memories from the day. However, neural simulations support the converse: Norman and colleagues (Norman et al. 2005, 2007) have illustrated the potential for REM to reduce forgetting of competing memories by repairing earlier suppression.
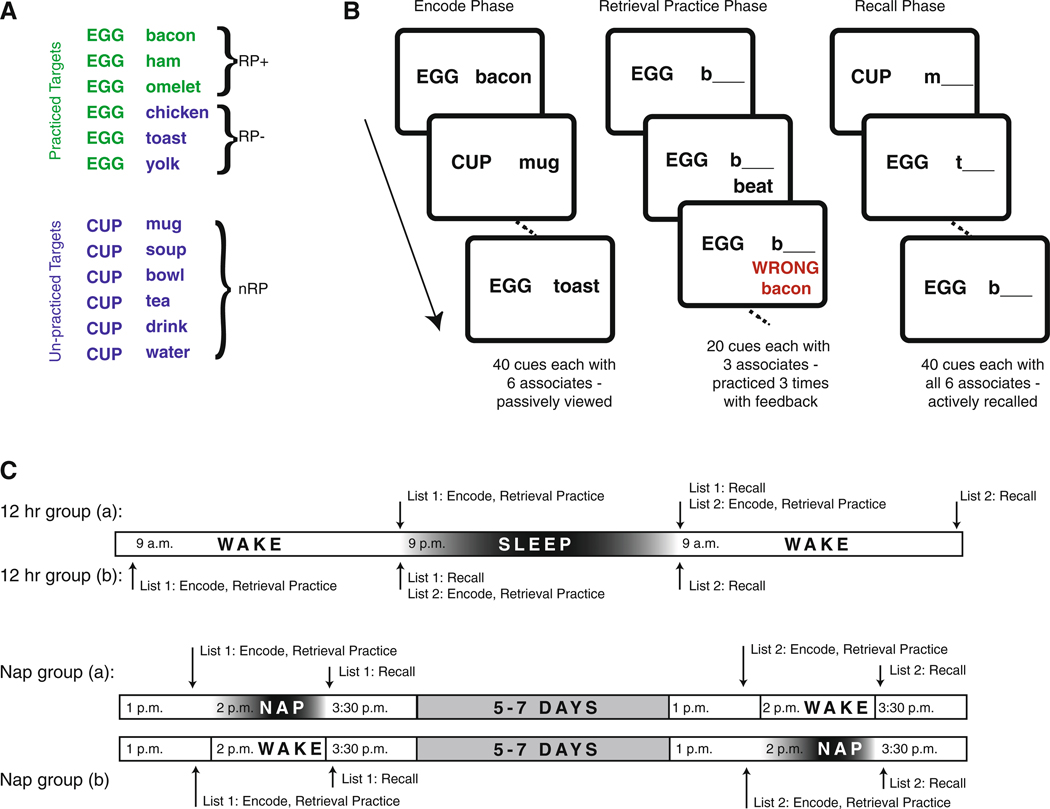
In the present study, we employed a variant of the retrieval-induced forgetting task (Anderson et al. 1994) to assess learning and competitive forgetting over sleep and wake. In this task, participants are presented with word pairs to encode. These pairs are composed of cues, each of which is paired with six targets. Participants practice retrieving half of the targets associated with half of the cues (RP+; Fig. 1a). The remaining targets associated with these practiced cues (RP−) and the remaining cue-target pairs (nRP) are unpracticed. Competing targets (RP−) are suppressed during retrieval practice (Kuhl et al. 2007) resulting in worse recall of the RP− pairs relative to the baseline pairs. The difference in recall for these pair types (i.e., nRP minus RP−) is used as the measure of competitive forgetting (Kuhl et al. 2007).
Fig. 1.
The retrieval-induced forgetting task. a Sample stimuli used in the Retrieval-Induced Forgetting Task. b The three phases of the task. All word pairs are initially encoded in the Encode Phase. A subset of these pairs is practiced in the Retrieval Practice Phase. All pairs are then recalled in the Recall Phase. c Participants first performed the Encode and Retrieval Practice Phases. An interval, containing either sleep (12-h containing overnight sleep or 90 min containing a nap) or an equivalent interval spent awake, preceded the Recall Phase. Participants performed the task twice, with either sleep or wake in the intervening interval and the order of sleep and wake delays was counterbalanced across participants
Recently, Racsmany et al. (2010) assessed competitive forgetting following a 12-h break which either contained sleep (8 p.m. to 8 a.m.) or wake (8 a.m. to 8 p.m.). They report that competitive forgetting only occurs over sleep. No forgetting was found over a 1 or 12-h interval awake, suggesting that sleep mediates memory suppression. Interestingly, however, suppression over the sleep interval was not paralleled by enhanced memory for the practiced pairs (RP+), possibly due to the small sample of RP+ pairs and no feedback during the training phase. As such, the question of whether sleep can modulate competitive memories in conjunction with enhancement of a target memory, remains unanswered.
We employed a modified RIF task (Kuhl et al. 2007) that pilot data suggested would be more likely to elicit sleep-dependent consolidation of practiced pairs (RP+). Compared to Racsmany et al. our task used more word pairs and, uniquely, we provided feedback during the retrieval practice phase. Performance was assessed following a 12-h interval containing sleep and a 12-h interval spent awake or after a mid-day nap and wake interval. Polysomnography (PSG) recorded in the overnight interval for a subset of participants allowed for direct measure of the role of physiological sleep state on the modulation of competing memories.
Experimental procedure
Participants
All participants were young adults with no known neurological disorders, were free of medication known to interfere with sleep, habitually slept 5 h or more at night, and were native English speakers. Procedures were approved by the University of Massachusetts, Amherst and University of California, Berkeley Institutional Review Boards (IRB). Following an explanation of the experimental procedures, informed consent was obtained. Participants were debriefed after their participation.
The 12-h group was composed of 52 individuals (20 males, 32 females; mean age = 20.8 years) who were recruited from University of Massachusetts psychology courses in exchange for course credit or responded to flyers on campus and received payment for their time. Data from three participants were excluded given that performance for the RP+ pairs was at ceiling (>98%) prior to the break and data from three participants were lost due to a technical failure. Of the remaining 46 participants, 23 participants performed the first session in the evening (sleep interval preceded wake interval; sleep–wake subgroup), and 23 participants performed the first session in the morning (wake–sleep subgroup).
PSG was recorded on 20 of these participants—ten from the wake–sleep subgroup and ten from the sleep–wake subgroup. However, four participants were excluded from the PSG analysis due to insufficient PSG data, and one participant was excluded for sleep stage distributions significantly outside normative data (Danker-Hopfe et al. 2005). As such, PSG results are based on 15 participants, six in the wake–sleep subgroup and nine in the sleep–wake subgroup.
The nap group was composed of 24 individuals (12 males, 12 females; mean age = 20.2 years) recruited from the University of California, Berkeley and the University of Massachusetts, Amherst, student populations in exchange for pay. We screened against participants who deemed themselves unlikely to nap if given the opportunity. Nonetheless, data were excluded from one participant who was unable to nap. Three additional participants were excluded given that performance for the RP + pairs was at ceiling prior to the break, leaving 20 participants, 10 participants in the nap–wake subgroup and 10 participants in the wake–nap subgroup.
Retrieval-induced forgetting task
Stimuli were 480 word-pairs that were divided into two lists. Each list was composed of 40 cues (e.g., WINDOW), and each cue was paired with six semantically-related target words. All six targets for a given cue started with a unique first letter (e.g., WINDOW-pane, WINDOW-door, WINDOW-curtain, WINDOW-shade, WINDOW-glass, WINDOW-mirror). Each cue-target pair had moderate associative strength (http://web.usf.edu/FreeAssociation/; mean = 0.055, sd = 0.003), frequency (mean = 70.8, sd = 5.1), and concreteness (mean = 5.2, sd = 0.06). Strength was equated across lists and across pair-types (RP+/RP− and nRP). Half of these pairs were used in an earlier study (Kuhl et al. 2007) and half of these pairs were assigned to list 1 and half to list 2.
The experiment consisted of three phases: encoding, retrieval practice, and delayed recall (Fig. 1b). During the encode phase, participants passively viewed 240 word pairs (40 cues each having 6 targets), presented in the center of a computer monitor, and were instructed to remember them. Cues appeared in uppercase letters on the left and the targets appeared on the right in lowercase letters. Each word pair was shown for 3 s.
Following the encode phase, participants performed the retrieval practice phase in which a subset of pairs (RP+) were practiced. These pairs were composed of half of the cues from the encode phase (20 cues) and only three of the respective targets. Participants were presented with the cue and the first letter of a target, and were asked to type the correct response. They were given feedback after each response: a correct response was followed with “CORRECT!” flashed on screen for 1 s and each incorrect response was followed by the presentation of “WRONG!” as well as the correct target for 1 s. Participants practiced retrieving all items three times consecutively, regardless of accuracy, with each presentation of the list in a pseudorandom order.
The recall phase was similar to the practice retrieval phase with the exceptions that all cue-target pairs were presented, pairs were tested only once, and no feedback was given.
Response files were scanned for typographical errors by two experimenters, working independently. Words that were clearly misspelled (e.g., omlet for omelet) or did not match in plurality (e.g., sheet for sheets) were corrected and further analyses were based on these corrected responses.
Procedure
We used a repeated measures design in which participants were tested on both word lists, once following an interval containing sleep and once following an interval containing wake. The order of wake and sleep conditions was counterbalanced. Participants in the 12-h group encoded and practiced retrieving the first list either in the morning (7–10 a.m.; wake–sleep subgroup) or the evening (7–10 p.m.; sleep–wake subgroup). Recall was assessed in a second session 12 h later. Following recall, participants encoded and practiced retrieving the second list. Recall of this list was assessed 12 h later. The order of the two lists of word pairs was counterbalanced across participants (Fig. 1c). Following the overnight interval, participants completed a modified Pittsburgh Sleep Diary (Monk et al. 1994) which provided a subjective measure of total sleep time.
Participants in the nap group encoded and practiced retrieving the first list mid-day (1–3 p.m.). Afterwards, participants were given the opportunity to nap for 90 min. Following the break, recall was assessed and participants completed a modified sleep diary that provided a subjective measure of total nap time. Participants were informally monitored for compliance during the nap interval. We again used a repeated measures design in which the order of wake and sleep conditions was counterbalanced. Participants came to the lab either 5–7 days prior-to (wake–nap subgroup) or 5–7 days following (nap–wake subgroup) the nap session for the same procedure except the offline interval was spent watching a nature video (“Planet Earth”).
Polysomnography (PSG)
PSG was recorded for 20 participants in the 12-h group with the Aura PSG mobile system (Grass Technologies, Astro-Med Inc., RI). In the home of the participant, 1 h or more prior to the participant’s estimated bedtime, the experimenter applied the PSG montage. The montage included EOG (ROC and LOC), two chin EMG, and five cortical EEG leads (O1, O2, C3, C4, Cz) with all electrodes referenced to one separate electrode. Data were obtained and analyzed according to the specifications provided in the revised AASM manual (AASM 2007).
Results
Based on the sleep surveys, participants in the 12-h group without PSG slept for an average of 6.8 h (sd = 1.3 h) in the overnight intervals. Subjective estimates of total sleep time did not differ by session order (wake–sleep vs. sleep–wake subgroup; F(1,22) = 1.4, p = 0.25). The average total sleep time for the PSG-recorded interval was 6.4 h (sd = 0.7 h; based on subjective reports: mean = 6.5 h; sd = 0.6 h). Total sleep time for this group also did not differ for the wake–sleep and sleep–wake subgroups (F(1,13) = 0.06, p = 0.82).
The average subjective estimate of nap length was 58.2 min (sd = 16.4 min). Nap length estimate did not differ based on session order (nap–wake vs. wake–nap subgroup; F(1,18) = 0.99, p = 0.33). The majority of participants (n = 16) appeared to be sleeping when the experimenter entered at the end of the 90-min interval, which further verifies nap compliance.
There were no significant differences in initial encoding efficiency based on the time at which encoding took place (morning vs. evening for the 12-h group and pre-wake vs. pre-nap session for the nap group). First, passive encoding, as estimated by accuracy for the first 60 pairs of the practice retrieval phase, did not differ across time of encoding (wake–sleep vs. sleep–wake subgroup: F(1,84) = 0.03, p = 0.99; wake–nap vs. nap–wake: F(1,32) = 0, p = 0.99) nor across word lists (12-h F(1,84) = 0.22, p = 0.64; nap F(1,32) = 0.06, p = 0.82). Second, retrieval practice was equally successful with no difference in accuracy at the end of the practice retrieval phase across time of encoding (12-h F(1,84) = 0.04, p = 0.83; nap F(1,32) = 0, p = 0.98) or lists (12-h F(1,84) = 1.3, p = 0.26; nap F(1,32) = 0.55, p = 0.46). As order effects were consistently non-significant, all comparisons are within-subject and collapsed across wake–sleep and sleep–wake subgroups and across nap–wake and wake–nap subgroups.
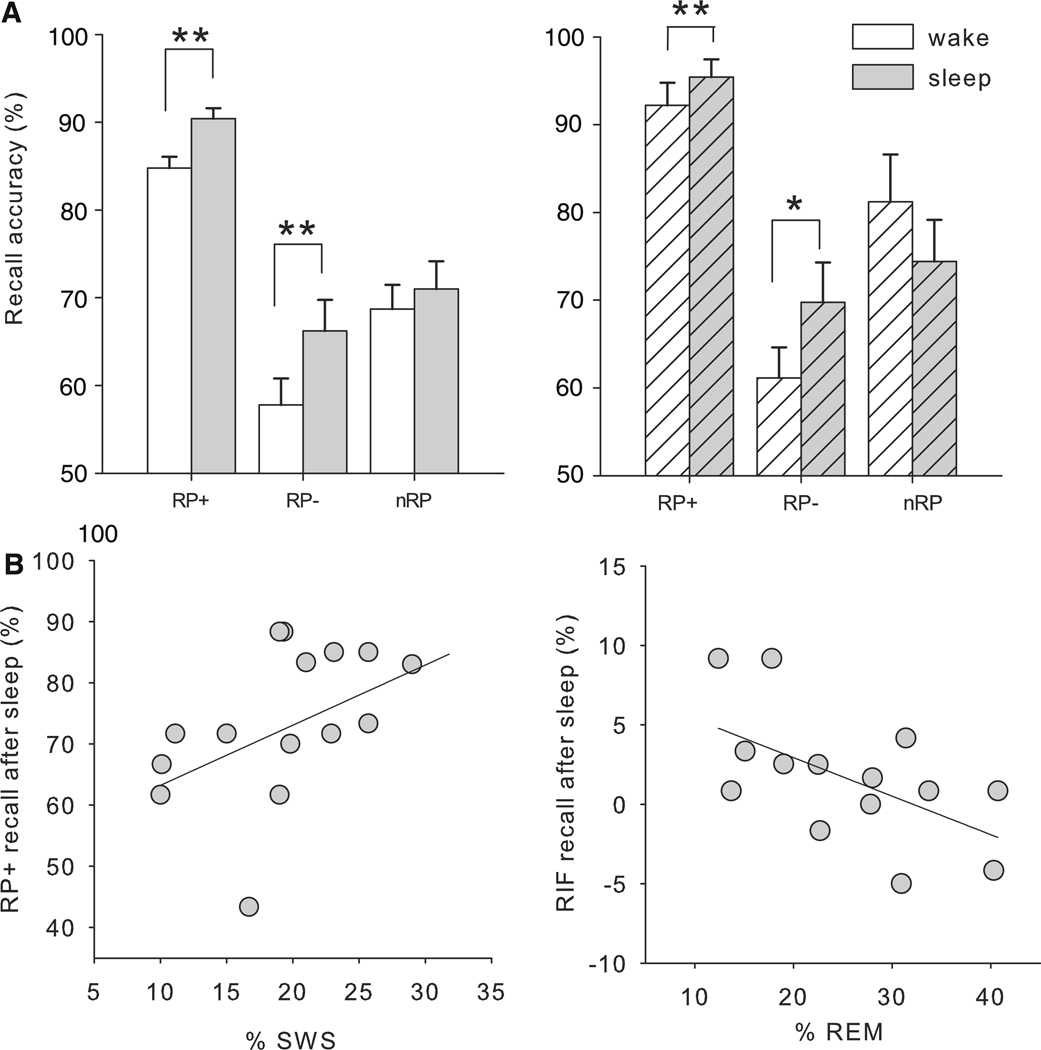
Consistent with previous work on the role of sleep in the consolidation of memory for associated word pairs (e.g., Plihal and Born 1997, 1999; Tucker et al. 2006), recall of the practiced pairs (RP+) was greater following a 12-h interval containing sleep relative to a 12-h interval spent fully awake (12-h F(1,88) = 9.2, p = 0.003). Likewise, RP+ recall was greater following a 90-min mid-day interval containing sleep relative to a 90-min interval spent awake (F(1,36) = 3.6, p = 0.058; Fig. 2a).
Fig. 2.
a Recall accuracy for all pair types (open bars 12 h grp; hatched bars nap grp). b Relationship between sleep and the overnight change in memory and forgetting. On left the percent of the night spent in SWS significantly correlated with the recall of the practiced pairs. On right the percent of the night spent in REM had a significant negative correlation with competitive forgetting after sleep
Overall, we were successful in inducing competitive forgetting: Performance for RP− pairs was diminished beyond that of nRP pairs across 12-h (F(1,178) = 5.1, p = 0.02) and 90-min (F(1,72) = 5.2, p = 0.03) intervals. To examine whether sleep modulates competitive forgetting, we compared forgetting, the difference in recall for RP− and nRP pairs, following intervals with sleep and wake. Competitive forgetting was significantly greater following the wake interval relative to the sleep interval (12-h F(1,88) = 4.6, p = 0.04; nap F(1,36) = 6.5, p = 0.01). Importantly, this reduction was driven by a change in RP− recall: While there was no difference in recall of nRP pairs following sleep and wake (12-h F(1,88) = 0.35, p = 0.56; nap F(1,36) = 0.50, p = 0.48), recall for the RP− pairs was greater following an interval with sleep relative to the interval spent awake in the 12-h group (F(1,88) = 5.7, p = 0.02). This was also a statistical trend in the nap group (F(1,36) = 2.9, p = 0.09; Fig. 2a).
These behavioral results suggest a relationship between brain state and the process of competitive forgetting. However, sleep may decrease competitive forgetting through an active process of memory consolidation or repair, or, conversely, wake may increase competitive forgetting through specific interference effects on the RP-pairs. In the latter case, sleep has no active role in the forgetting process. To distinguish between these two alternatives, we turned to sleep physiology. Competitive forgetting (RP− performance relative to nRP) following sleep was correlated with REM: participants with a greater percent of the night spent in REM had reduced competitive forgetting (r = −0.54, p = 0.04). Percent of the night spent in SWS did not correlate with competitive forgetting (r = −0.24, p = 0.38), nor did measures of fourth quarter REM (r = −0.22, p = 0.43), a factor which has been considered in previous studies (see Karni et al. 1994; Stickgold et al. 2000; Walker et al. 2002).
This result suggests an active role of sleep, particularly REM sleep in reducing forgetting. This reduction may come through consolidation processes acting on the competing pairs, the same mechanism that enhances memory for practiced pairs. Alternatively, modulation of forgetting may result from a separate process acting on the competing pairs, for example, a process as proposed by Norman and colleagues (Norman et al. 2005, 2007).which repairs or separately consolidates suppressed memories. Overall recall following sleep, performance collapsed across RP+, RP− and nRP pairs, did not correlate with REM (r = 0.16, p = 0.57) or SWS (r = 0.26, p = 0.35). Rather, recall of practiced pairs (RP+) correlated with the percent of the night spent in SWS. Specifically, as suggested by previous reports (Plihal and Born 1997, 1999; Tucker et al. 2006) the benefit of sleep on RP+ pairs was greatest for those individuals who spent the most time in SWS (r = 0.49, p = 0.03; Fig. 2b). Performance on these pairs did not significantly correlate with the percent of the night spent in REM (r = 0.16, p = 0.57) nor fourth quarter REM (r = 0.43, p = 0.11).
Discussion
These results suggest that the enhancement of memory during sleep is not mediated by forgetting of competing memories. To the contrary, retrieval of competing memories was greater following sleep relative to wake in this modified retrieval induced forgetting task.
It may be argued that improved retrieval of the RP− pairs following sleep was due to decreased interference over this interval or circadian influences on attention or arousal. These accounts can be discounted in two ways. First, RP− pair retrieval was also greater following a mid-day nap relative to an equivalent interval spent awake. In this case, wake activities were minimized and controlled and circadian differences were eliminated. However, only subjective reports of sleep were available for this group, and we cannot exclude the possibility that participants practiced the word-pairs further while awaiting sleep onset. As such, physiological evidence from the 12-h group provides a second test of these alternative accounts, also suggesting a direct role of sleep in modulating memory for RP− pairs. If circadian influences or daytime interference caused the differential performance on RP− pairs over the sleep and wake intervals, a correlation between REM and performance following sleep would be unlikely.
When learning occurs, information is communicated from the cortex to the hippocampus. This pattern is reversed during SWS when activation spreads from hippocampus back to the cortex. REM, which typically follows bouts of SWS, resembles wake in that information again flows from cortical areas to the hippocampus. This sequence of brain states has been hypothesized to perform a sequence of processing steps on memory (Stickgold 1998). Taken in conjunction with the present physiological results, we speculate that the RP+ pairs represent a strong memory and, as such, received preferential processing during SWS. During subsequent REM, the memory for RP− pairs was repaired (Norman et al. 2005, 2007), perhaps through reactivation.
Importantly, our results present a striking contrast to that of Racsmany et al. (2010). In that study, competitive forgetting was observed following a 12-h interval with sleep, yet no such suppression was found after 1- or 12-h spent awake. While there are many similarities between our study and that of Racsmany and colleagues (particularly their Experiment 2), we consider three key differences that may underlie these conflicting results. First, we chose to use a modified version of the retrieval-induced forgetting task in which feedback was provided during the retrieval practice phase. While the design of a pilot study mimicked that of Kuhl et al. (2007), we added feedback given that we were not eliciting a sleep benefit on practiced pairs (RP+), a result which would stand in stark contrast to many other studies. To our knowledge, only one other RIF study provided feedback during the retrieval practice phase (Hogge et al. 2008) but forgetting under conditions with and without feedback has not been compared or directly addressed. Clearly, however, RP+ performance is greater when feedback is provided and, in parallel, the amount of suppression likely differed for our participants and those of Racsmany’s study. Notably, unlike in Racsmany’s study, we observed significant retrieval-induced forgetting following both intervals, but the amount of forgetting was decreased when the interval contained sleep.
A second difference between the present task and that of Racsmany is the number of stimuli encoded and retrieved. Given that our task used four times the number of word pairs, this too is reason to assume that the amount of suppression differed greatly across these studies. However, given the experimental differences, we cannot quantify the difference in suppression. We also cannot directly contrast the stimuli and the relative associative strengths of the stimuli for these studies, given that the stimuli of Racsmany and colleagues are in Hungarian.
Third, while we chose to use a three-session, within-subject design, Racsmany et al. (2010) used a two-session, between-groups design in which encoding and practice retrieval were performed in the first session and recall was tested 12-h later following either an interval with wake (e.g., 8 a.m.–8 p.m.) or sleep (e.g., 8 p.m.–8 a.m.). As such, it is unclear as to whether increased forgetting over sleep that they observed was driven by changes in RP− pairs or was due to a change in nRP recall. The results suggest the latter, implying that sleep did not increase suppression of the competing memories (RP), but rather that there was a change in “baseline” performance (nRP). This may be due to a sampling bias such that the sleep group was better at recalling nRP items or sleep may have actually benefited nRP items.
In spite of these differences, we also consider a parsimonious account of these seemingly conflicting reports. Whether REM sleep acts on weak memories may be determined by whether SWS acted on related memories. Specifically, memories that are acted upon during SWS, such as the RP+ pairs in our study, are the prime related memories to be consolidated and stored during subsequent REM. We assume that SWS processing did not occur in Racsmany’s study given that no sleep-related changes were observed for the practiced pairs. In the absence of SWS-processing of a related memory, memories may be deemed irrelevant and further suppressed.
It has been suggested that competitive forgetting is beneficial and adaptive. Forgetting of competing targets reduces the engagement of cognitive control processes during retrieval of the intended target (Kuhl et al. 2007). As such, reduced forgetting with sleep, on the surface, may seem to be un-adaptive, or a negative consequence of sleep. Payne et al. (2009) reported that increased memory of word lists following sleep was paralleled by an increase in false memories, presenting another seemingly harmful consequence of sleep-dependent memory processing. However, as those authors note, false memories may, in fact, be beneficial as it reflects the extraction of the gist of the list which is likely useful. We posit that the seemingly negative consequence of sleep enhancing competing memories may also be beneficial given that the processing steps are sequential. The target memory, reactivated during SWS, might not be reactivated with the competitor during REM, thus allowing the two memories to become more independent. As such, the target and competitor may not conflict at subsequent retrieval. Future work must address whether neural processing benefits are observed for practiced pairs following sleep.
Acknowledgments
We are grateful to Rich Ivry for his feedback. We also thank Brice Kuhl for his RIF stimulus set. This work was funded in part by National Institutes of Health K99/R00 AG29710 (PI: R. M. C. Spencer).
Contributor Information
Bengi Baran, Department of Psychology, University of Massachusetts, Amherst, USA.
Jessica Wilson, Department of Psychology, University of California, Berkeley, USA.
Rebecca M. C. Spencer, Department of Psychology, University of Massachusetts, Amherst, USA, rspencer@psych.umass.edu
References
- AASM. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- Danker-Hopfe H, Schafer M, Dorn H, Anderer P, Saletu B, Gruber G, Zeitlhofer J, Kunz D, Barbanoj M-J, Himanen SL, Kemp B, Penzel T, Roschke J, Dorffner G. Percentile reference charts for selected sleep parameters for 20- to 80-year-old healthy subjects from the SIESTA database. Somnologie. 2005;9:3–14. [Google Scholar]
- Hogge M, Adam S, Collette F. Retrieval-induced forgetting in normal ageing. J Neuropsychol. 2008;2:463–476. doi: 10.1348/174866407x268533. [DOI] [PubMed] [Google Scholar]
- Jones GV. Back to Woodworth: role of interlopers in the tip-of-the-tongue phenomenon. Mem Cognit. 1989;17:69–76. doi: 10.3758/bf03199558. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, MacHen MA, Petrie SR, Ritenour AM. The Pittsburgh sleep diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the Complementary Learning Systems model: oscillating inhibition and autonomous memory rehearsal. Neural Netw. 2005;18:1212–1228. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Detre G. A neural network model of retrieval-induced forgetting. Psychol Rev. 2007;114:887–953. doi: 10.1037/0033-295X.114.4.887. [DOI] [PubMed] [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA, Walker MP, Stickgold R. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. NeuroReport. 1999;10:2741–2747. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- Racsmany M, Conway MA, Demeter G. Consolidation of episodic memories during sleep: long term effects of retrieval practice. Psychol Sci. 2010;21:80–85. doi: 10.1177/0956797609354074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Sleep: off-line memory processing. Trends Cogn Sci. 1998;2:484–492. doi: 10.1016/s1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005;28:51–104. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]