Abstract
Inflammation may play an important role in the pathogenesis of cardiac fibrosis in heart failure (HF) after myocardial infarction (MI). N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a naturally occurring antifibrotic peptide whose plasma concentration is increased 4- to 5-fold by angiotensin-converting enzyme inhibitors. We tested the hypothesis that in rats with HF after MI, Ac-SDKP acts as an anti-inflammatory cytokine, preventing and also reversing cardiac fibrosis in the noninfarcted area (reactive fibrosis), and thus affording functional improvement. We found that Ac-SDKP significantly decreased total collagen content in the prevention group from 23.7±0.9 to 15.0±0.7 μg/mg and in the reversal group from 22.6±2.2 to 14.4±1.6 (P<0.01). Interstitial collagen volume fraction and perivascular collagen were likewise significantly reduced. We also found that infiltrating macrophages were reduced from 264.7±8.1 to 170.2±9.2/mm2, P<0.001 (prevention), and from 257.5±9.1 to 153.1±8.5 mm2, P<0.001 (reversal), while transforming growth factor (TGF)-β-positive cells were decreased from 195.6±8.4 to 129.6±5.7/mm2, P<0.01 (prevention), and from 195.6±8.4 to 130.7±10.8/mm2, P<0.01 (reversal). Ac-SDKP did not alter either blood pressure or left ventricular hypertrophy (LVH); however, it depressed systolic cardiac function in the prevention study while having no significant effect in the reversal group. We concluded that Ac-SDKP has an anti-inflammatory effect in HF that may contribute to its antifibrotic effect; however, this decrease in fibrosis without changes in LVH was not accompanied by an improvement in cardiac function.
Keywords: rat, myocardial infarction, cardiac function, collagen, macrophages, transforming growth factor-β
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a naturally occurring antifibrotic peptide whose plasma concentration is increased 4- to 5-fold by angiotensin-converting enzyme inhibitors. Ac-SDKP is released from its precursor thymosin-β4, which is present in most cells.1 It inhibits pluripotent hematopoietic stem cell and hepatocyte proliferation by halting entry into the S phase of the cell cycle, maintaining cells in the G0/G1 phase and thereby helping control their proliferation.2–4 We have shown that in vitro Ac-SDKP inhibited cardiac fibroblast proliferation and collagen synthesis,5 while in vivo it prevented collagen deposition in the left ventricle (LV) and kidneys in rats with aldosterone-salt hypertension and renovascular hypertension.5,6 This decrease in collagen deposition was associated with a reduced number of proliferating cell nuclear antigen (PCNA)-positive cells, a marker of cell proliferation. These effects of Ac-SDKP occurred without changes in blood pressure or cardiomyocyte hypertrophy. Our studies suggest that one of the mechanisms by which Ac-SDKP prevents fibrosis is by inhibiting fibroblast proliferation and collagen synthesis. It is also known that inflammation plays a central role in the pathogenesis of interstitial and perivascular cardiac fibrosis in heart failure (HF) post-myocardial infarction (MI). Fibrosis is often co-localized with macrophages, which release cytokines such as transforming growth factor-β (TGF-β) that play a crucial role in myocardial fibrosis.7 There is evidence that Ac-SDKP inhibits TGF-β signal transduction through suppression of Smad2 phosphorylation.8,9 However, it is not known whether it also inhibits the expression of TGF-β.
In the present study, we tested the hypothesis that in HF post-MI, Ac-SDKP in the noninfarcted region of the myocardium acts as an anti-inflammatory cytokine, decreasing macrophage infiltration and TGF-β expression, and thereby reducing reactive cardiac fibrosis, resulting in improved cardiac function. We also tested the hypothesis that Ac-SDKP not only prevents the development of inflammation and fibrosis but also can reverse these processes when they are already established.
Methods
Eight-week-old male Lewis inbred rats weighing 255 to 275 g (Charles River Laboratories, Wilmington, Mass) were housed in an air-conditioned room with a 12-hour light/dark cycle, received standard laboratory chow (0.4% sodium), and drank tap water. Animals were given 5 to 7 days to adjust to their new environment before surgery and then were subjected to MI by ligating the left anterior descending coronary artery (LAD) or sham operation as described previously.10,11 Ac-SDKP or vehicle (saline) was administered into the abdominal cavity via a miniosmotic pump inserted either 7 days before induction of MI (prevention protocol) or 2 months post-MI (reversal protocol). At the end of the experiment, the heart was stopped at diastole by injecting 10% KCl, then excised, weighed, and prepared for histology and determination of collagen content, macrophage infiltration, and TGF-β-positive cells. All procedures were approved and followed the guidelines of the Henry Ford Hospital Institutional Animal Care and Use Committee.
Experimental Protocols
Rats with MI were divided into two protocols: prevention and reversal.
Prevention Protocol
The prevention control group (n=10) received vehicle starting 7 days before MI induction and continuing for up to 4 months post-MI. This control group was also important for the reversal protocol, because it demonstrated that the inflammatory process did not decline by itself (time control). The prevention Ac-SDKP group (n=9) received Ac-SDKP starting 7 days before MI induction and continuing until 4 months post-MI.
Reversal Protocol
The reversal time control group (n=10) received vehicle starting 2 months after MI induction and continuing for up to 4 months post-MI. Because all parameters measured in these groups were similar to the prevention control group, they were combined and served as a 4-month control (MI-vehicle/4 months) for the prevention study and also as a time control for the reversal studies. In the reversal baseline control group (n=10), the rats were killed 2 months after MI and served as a baseline control for the reversal Ac-SDKP group (MI-vehicle/2 months). The reversal Ac-SDKP group (n=9) received Ac-SDKP starting 2 months after MI and continuing for 2 months. This time was selected because at 2 months, chronic inflammation and fibrosis are present in the LV. Rats in the sham MI group (n=11) underwent a surgical procedure similar to that performed in those with MI, except that the suture around the coronary artery was not tightened.
Parameters Measured
In all groups, we measured systolic blood pressure by tail cuff12 and cardiac function by echocardiography.12,13 At the end of the experiments, the heart was stopped at diastole by injecting 10% KCl, then excised and weighed. The LV was sectioned transversely into four slices from apex to base. One slice was stored for hydroxyproline assay and the other three were rapidly frozen in isopentane solution precooled in liquid nitrogen for morphometric analysis of infarct size as well as histological and immunohistochemical examination of infarct size, myocyte cross-sectional area, interstitial and perivascular collagen, macrophages, and TGF-β-positive cells in the noninfarcted myocardium (Extended details can be found in an online supplement available at http://www.hypertensionaha.org).
Data Analysis
Data are expressed as mean±SE. One-way ANOVA was used to test for differences among groups, such as sham versus MI-vehicle 2 months or MI-vehicle 4 months; MI-vehicle 4 months versus MI-Ac-SDKP prevention or MI-Ac-SDKP reversal; and MI-vehicle 2 months versus MI-Ac-SDKP reversal. Tukey's method of post hoc testing was used for pairwise comparisons. Homogeneity of variance was checked for each comparison, with appropriate adjustments made when warranted.
Results
Of the 60 rats that underwent coronary ligation, 5 died after surgery, for a surgical mortality rate of 8%. In addition, 4 died within 2 months (2 in the HF-vehicle group and 2 in the Ac-SDKP prevention group) and 3 died within 3 months (1 in the HF-vehicle group and 2 in the Ac-SDKP reversal group). No deaths involved cardiac rupture, and no rats in the sham-ligated group died.
Blood Pressure and Heart Rate
Systolic blood pressure was not significantly different among groups (Table). Heart rate was also similar in all groups, suggesting that Ac-SDKP has no significant influence on blood pressure and heart rate either in normal rats or in rats with MI.
Effect of Ac-SDKP on Blood Pressure, Infarct Size, Left Ventricular Weight, Myocyte Size, and Cardiac Function
| SBP (mm Hg) | HR (bpm) | IS (% LV) | LVW/BW (mg/10 g) | MCSA (μm2) | LVDd (mm) | EF (%) | E/A | |
|---|---|---|---|---|---|---|---|---|
| Sham MI | 115.2±2.5 | 346±8 | ... | 17.0±0.2 | 205±4 | 5.9±0.1 | 59.6±3.1 | 1.64±0.06 |
| MI–vehicle (2 mo) | 110.5±3.2 | 334±11 | 42±2 | 20.5±0.4† | 329±6† | 8.5±0.2† | 28.6±2.1† | 2.35±0.22* |
| MI–vehicle (4 mo) | 105.5±2.7 | 328±6* | 42±2 | 19.7±0.5† | 343±7† | 8.0±0.1† | 30.4±1.5† | 2.24±0.13† |
| MI–Ac-SDKP (prevention) | 104.1±4.4 | 314±28 | 44±3 | 20.2±0.3¶ | 326±7¶ | 8.9±0.4§ | 25.0±0.8‡ | 2.42±0.15¶ |
| MI–Ac-SDKP (reversal) | 113.9±3.1 | 331±7 | 45±3 | 19.6±0.3¶∥ | 312±11¶∥ | 7.9±0.2 | 31.8±1.3 | 2.25±0.16¶∥ |
SBP, indicates systolic blood pressure; HR, heart rate; IS, infarct size; LVW/BW, left ventricular weight/body weight; MCSA, myocyte cross-sectional area; LVDd, left ventricular diastolic dimension; EF, ejection fraction; E/A, ratio of E and A waves.
P<0.01 vs sham
P<0.001 vs sham
P=0.004 vs MI-vehicle (4 months)
P=0.057 vs MI-vehicle (4 months).
P=ns vs MI-vehicle (4 months)
P=ns vs MI-vehicle (2 months).
Cardiac Function and Infarct Size
Infarct size was similar among groups, indicating that early treatment with Ac-SDKP (prevention protocol) did not affect infarct size (Table). MI caused cardiac hypertrophy and chamber dilatation, as evidenced by increased LV weight, myocyte cross-sectional area (MCSA), and left ventricular diastolic dimension (LVDd). Ac-SDKP administered either early or late had no significant effect on these parameters. Ejection fraction (EF), an index of cardiac performance, was significantly decreased after MI; in the Ac-SDKP prevention group, EF tended to be lower compared with MI-vehicle (P=0.057) but it was not affected in the reversal group (Table). Ratio of E and A waves (E/A), an index of diastolic function, was significantly increased in both vehicle groups and was not affected by either early or late treatment with Ac-SDKP.
Macrophage Infiltration
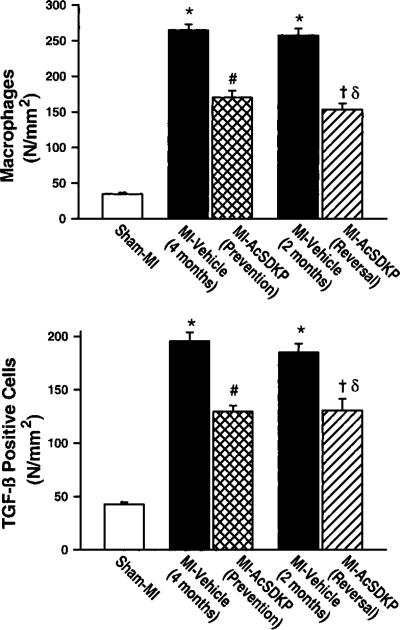
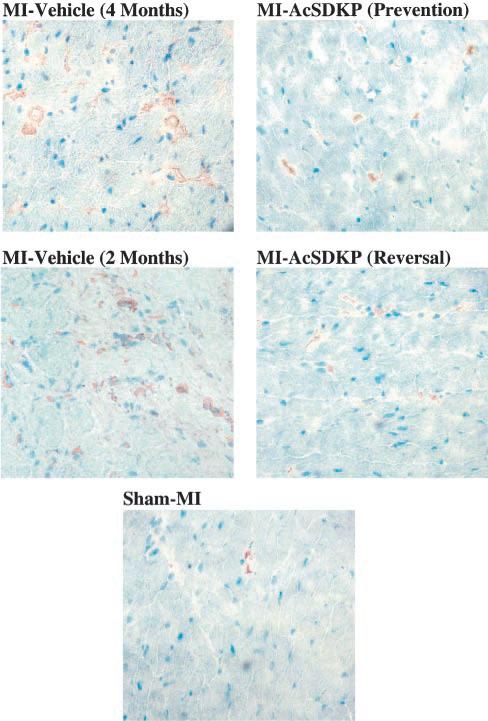
A few macrophages were seen in the interstitial space of sham-MI rats (34.7±2.1 per mm2). The number of macrophages increased significantly at 2 months post-MI (257.5±9.1 per mm2; P<0.001 versus sham MI) and remained elevated at 4 months (264.7±8.1 per mm2; P<0.001 versus sham MI). In the vehicle groups there were no significant differences in macrophage number between 2 and 4 months post-MI. Ac-SDKP attenuated the increase in macrophages in the prevention protocol (170.2±9.2 per mm2; P<0.001 versus MI-vehicle/4 months) and decreased macrophage infiltration in the reversal protocol (153.1±8.5 per mm2; P<0.001 versus MI-vehicle/2 months and MI-vehicle/4 months) (Figure 1, upper panel; Figure 2).
Figure 1.
Effect of Ac-SDKP on macrophage infiltration (upper panel) and TGF-β expression (lower panel) in rat hearts with sham myocardial infarction (MI) or MI treated with vehicle or Ac-SDKP. *P<0.01 vs sham; # and δP<0.01 vs MI-vehicle (4 months); †P<0.01 vs MI-vehicle (2 months).
Figure 2.
Representative images showing macrophage infiltration (brown staining) in rat hearts with sham myocardial infarction (MI) or MI treated with vehicle or Ac-SDKP (immunohisto-chemical staining and hematoxylin counterstaining, ×400).
TGF-β-Positive Cells
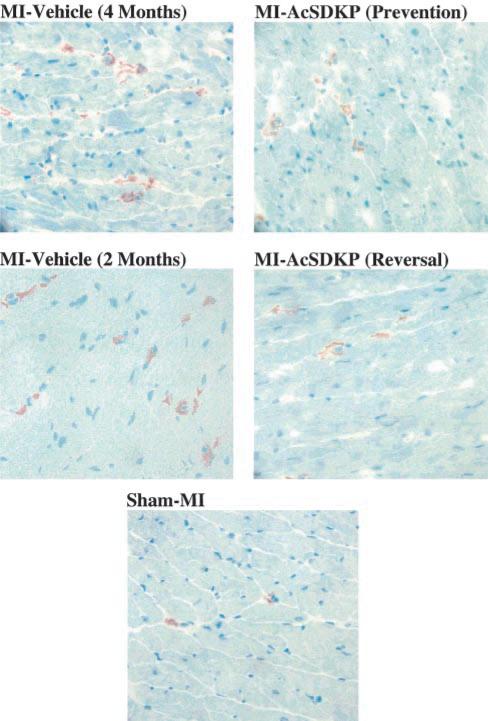
A few TGF-β-positive cells were seen in the heart in the sham MI group (42.8±1.9 per mm2). The number of TGF-β-positive cells increased markedly 2 months post-MI (184.9±8.0 per mm2; P<0.001) and remained elevated at 4 months (195.6±8.4 per mm2; P<0.001 versus sham MI). In the vehicle groups there were no significant differences in TGF-β-positive cell number between 2 and 4 months post-MI. Ac-SDKP attenuated the increase in TGF-β-positive cells in the prevention protocol (129.6±5.3 per mm2; P<0.001 versus MI-vehicle/4 months) and significantly decreased TGF-β-positive cells in the reversal protocol (130.7±10.2 per mm2; P<0.001 versus MI-vehicle/2 months and MI-vehicle/4 months) (Figure 1, lower panel; Figure 3). Histo-logically, TGF-β-positive cells were very similar to macrophages: large and round with an elliptical or kidney-shaped nucleus and located mainly in the interstitial space, suggesting that TGF-β is expressed mainly in macrophages in the rat heart post-MI.
Figure 3.
Representative images showing TGF-β-positive cells (brown staining) in rat hearts with sham MI or MI treated with vehicle or Ac-SDKP (immunohistochemical staining and hematoxylin counterstaining, ×400).
Collagen Content
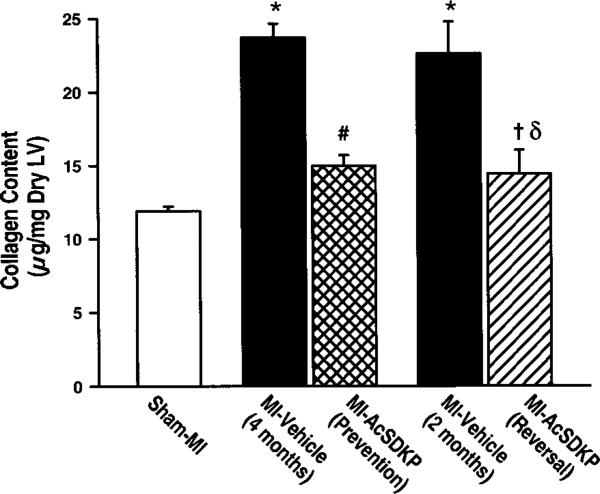
In the sham MI group, cardiac collagen content was 11.9±0.3 μg/mg LV dry weight, and it increased significantly and similarly at 2 and 4 months post-MI (22.6±2.0 and 23.7±0.9 μg/mg, respectively; P<0.001 versus sham MI). In the vehicle groups there were no significant differences in cardiac collagen content between 2 and 4 months post-MI. Ac-SDKP significantly attenuated the increase in collagen content in the prevention protocol to 15.0±0.7 μg/mg (P<0.001 versus MI-vehicle/4 months) and significantly decreased collagen content in the reversal protocol (14.4±1.5 μg/mg LV dry weight; P=0.006 versus MI-vehicle/2 months; P<0.001 versus MI-vehicle/4 months) (Figure 4).
Figure 4.
Effect of Ac-SDKP on total collagen content in the left ventricle as measured by hydroxyproline assay. *P<0.01 vs sham; # and δP<0.01 vs MI-vehicle (4 months); †P<0.01 vs MI-vehicle (2 months).
Interstitial Collagen Fraction
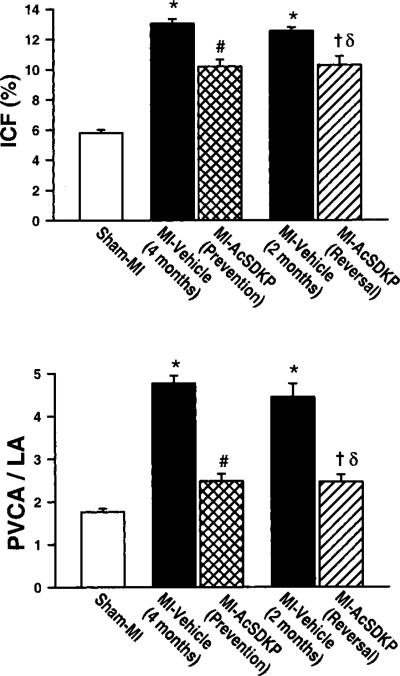
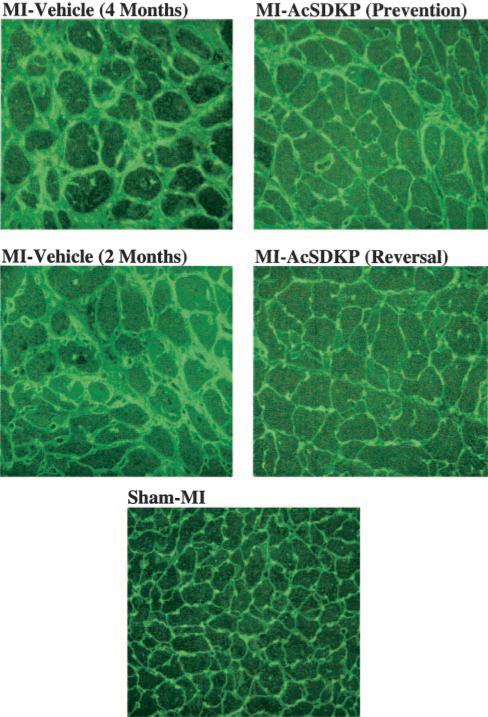
Cardiac interstitial collagen fraction (ICF) in the sham MI group was 5.8%±0.2% and increased significantly at 2 and 4 months post-MI (12.5±0.2% and 13.0±0.3%, respectively; P<0.001 versus sham MI). In the vehicle groups there were no significant differences in ICF between 2 and 4 months post-MI. Ac-SDKP attenuated the increase in ICF in the prevention protocol (10.2±0.4%; P<0.001 versus MI-vehicle/4 months) and decreased ICF in the reversal protocol (10.3±0.6 μg/mg LV dry weight; P=0.003 versus MI- vehicle/2 months; P<0.001 versus MI-vehicle/4 months) (Figure 5, upper panel; Figure 6).
Figure 5.
Effect of Ac-SDKP on left ventricular interstitial collagen fraction (ICF; upper panel) and perivascular fibrosis (lower panel), as indicated by the ratio of perivascular collagen area (PVCA) to luminal area (LA), in rats with sham MI or MI treated with vehicle or Ac-SDKP *P<0.01 vs sham; # and δP<0.01 vs MI-vehicle (4 months); †P<0.01 vs MI-vehicle (2 months).
Figure 6.
Representative images showing interstitial collagen deposition (green staining) and myocyte cross-sectional area (delineated by the interstitial space) in rats with sham MI or MI treated with vehicle or Ac-SDKP (fluorescence-labeled peanut agglutinin staining, ×400).
Perivascular Collagen
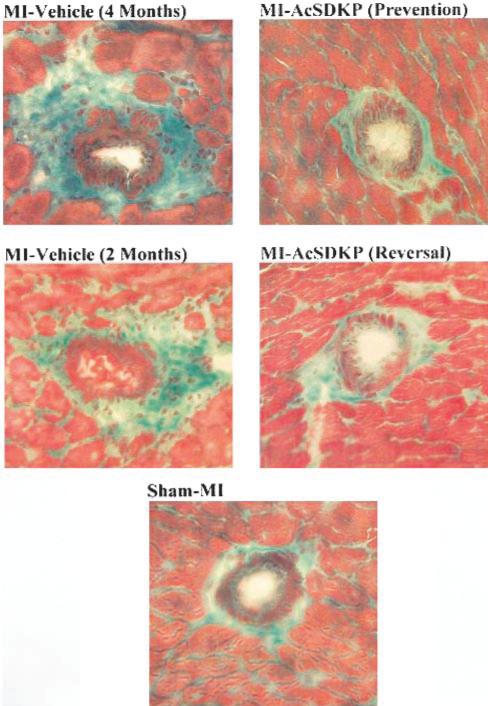
The ratio of perivascular collagen area to luminal area of the coronary arterioles (PVCA/LA) was 1.76±0.1 in the sham MI group, and it increased significantly at 2 and 4 months post-MI (4.5±0.5 and 4.8±0.2, respectively; P<0.001 versus sham MI). In the vehicle groups, there were no significant differences in PVCA/LA between 2 and 4 months post-MI. Ac-SDKP significantly attenuated the increase in perivascular collagen in the prevention protocol (2.5±0.2; P<0.001 versus MI-vehicle/4 months) and significantly decreased perivascular collagen in the reversal protocol (2.5±0.2; P=0.002 versus MI-vehicle/2 months; P<0.001 versus MI-vehicle/4 months) (Figure 5, lower panel; Figure 7).
Figure 7.
Representative images showing perivascular collagen deposition (green staining) in rat hearts with sham MI or MI treated with vehicle or Ac-SDKP (Gomori trichrome, ×400).
Discussion
Previously we have shown that Ac-SDKP prevents macrophage infiltration and cardiac fibrosis in rats with renovascular and mineralocorticoid-salt hypertension.5,14 In the present work, using a rat model of cardiac remodeling post-MI, we found that treatment with Ac-SDKP not only prevented but also reversed reactive cardiac fibrosis in the noninfarcted region of the myocardium (increase in total collagen, ICF, and perivascular collagen). In addition, Ac-SDKP partially prevented and also partially reversed the inflammatory process in the heart (increase in macrophages and TGF-β-positive cells). Cardiac fibrosis and chronic inflammation were similar at 2 and 4 months after MI in the vehicle groups. We also found that Ac-SDKP did not change blood pressure. Thus, the effects of Ac-SDKP on inflammation and fibrosis do not appear to be secondary to hemodynamic changes. Also, as we found in the hypertensive models, Ac-SDKP did not decrease MCSA or left ventricular hypertrophy (LVH).5,14
LV remodeling after MI is a complex process that involves both the infarcted and noninfarcted areas and contributes significantly to LV dilatation, development of HF, and death. Reparative fibrosis occurs at the site of the MI and is essential to rebuilding the necrotic myocardium to preserve structural integrity of the heart and to avoid cardiac rupture;15 however, scar formation was not the focus of this study. In the present work, we focused on the noninfarcted myocardium, where reactive and reparative interstitial fibrosis occur. Collective evidence indicates that the interstitial collagen matrix in the noninfarcted area plays a major role in healing and remodeling after MI.16–18 In this area there is some controversy over whether cardiac stem cells and inflammatory cells (after infiltrating the tissue) are able to proliferate.17,19,20 It has been shown that infiltrating macrophages, fibroblasts, and myofibroblasts are abundant in the interstitium of the heart post-MI.20–22 Chemotactic factors (including monocyte chemoattractant protein-1 [MCP-1]) stimulate macrophage infiltration in the myocardium and other tissues.22–25 These infiltrating macrophages release cytokines such as TGF-β, which stimulates fibroblast proliferation and interstitial matrix accumulation and promotes cardiac fibrosis.21,26,27 It has been well documented that TGF-β is a key cytokine that promotes accumulation of collagen and other major components of the extracellular matrix (ECM) in many fibrotic disorders, including cardiac and pulmonary fibrosis, glomerulonephritis, and vascular restenosis.28,29 Also, high plasma TGF-β1 levels were found in hypertensive patients with LVH and microalbuminuria.28 The antifibrotic effect of Ac-SDKP may be at least partially due to its effect on TGF-β1, which in turn could decrease fibroblast proliferation and collagen synthesis. In the present study, we found that after MI, rat hearts had considerable collagen deposition associated with markedly increased TGF-β1. We also found that TGF-β-positive cells and macrophages were co-localized mainly in the interstitial space, suggesting that TGF-β may be expressed mainly in infiltrating macrophages in this model. However, we are not sure the TGF-β1-positive cells are macrophages, because TGF-β1 is expressed not only in macrophages but also in fibroblasts, myofibroblasts, and other cells. In addition, TGF-β1 is released from the cells into the interstitial space, where it acts in an autocrine and paracrine fashion via a plasma membrane serine/threonine kinase receptor that phosphorylates Smad signaling proteins; these proteins are then translocated to the nucleus. In the heart during the chronic phase of MI, TGF-β1 activation was associated with increased expression of Smad 2 and 4 and decreased expression of Smad 7, and these events correlated positively with ongoing cardiac fibrosis.30,31 Recently, Pokharel et al8 reported that in cardiac fibroblasts in culture, Ac-SDKP inhibits expression and nuclear translocation of Smad 2, which may be partially responsible for its antifibrotic action. Also, Kanasaki et al9 showed that Ac-SDKP inhibits TGF-β signal transduction through suppression of R-Smad activation via nuclear export of Smad 7. In addition, our study demonstrates that Ac-SDKP decreases expression of TGF-β1, which has been proposed as the stimulus for differentiation of fibroblasts to myofibro-blasts.32 Ac-SDKP acting via these mechanisms may inhibit differentiation of cardiac fibroblasts to myofibroblasts, which are largely responsible for collagen production during cardiac remodeling after MI.27,33–37 Previously, we have shown that Ac-SDKP inhibits fibroblast proliferation and collagen synthesis in vitro.6 This direct effect on proliferation and synthesis may also be partially responsible for its antifibrotic effect in vivo. In another study, we found that in renovascular and mineralocorticoid hypertension, enhanced cardiac expression of PCNA, a marker of cell proliferation (probably cardiac fibroblasts), was markedly reduced by Ac-SDKP. This reduction in PCNA was associated with reduced collagen deposition,5,14 supporting the hypothesis that the antifibrotic effect of Ac-SDKP could result from inhibition of both fibroblast proliferation and collagen synthesis.
It is generally believed that increased collagen in the heart will increase diastolic stiffness and impair relaxation, ultimately promoting LV dysfunction. Thus, we expected that preventing or reversing cardiac fibrosis would benefit cardiac function. However, we found that even though Ac-SDKP decreased cardiac fibrosis, it did not improve the EF and E/A ratio, indexes of systolic and diastolic function. On the contrary, in the prevention protocol EF decreased and LVDd tended to increase compared with the MI-vehicle group (4 months). It could be that preventing collagen deposition soon after MI alters cardiac remodeling by causing cardiocyte slippage and increasing infarct expansion. These findings are in agreement with Baicu et al,38 who found that in hypertrophied papillary muscles from cats, acute regression of fibrosis with plasmin through activation of matrix metalloproteinases can negatively affect systolic function. Contractility in isolated myocytes exposed to the same treatment was no different from nontreated myocytes, suggesting that collagen may play an important role in myocyte-to-myocyte interaction, optimizing ventricular systolic function; hence, targeting collagen early in the context of post-MI remodeling can be deleterious. In our reversal protocol, despite a decrease in cardiac fibrosis, Ac-SDKP did not modify cardiac function, suggesting that the extent of cardiac fibrosis may not be an important determinant of function, which may be mainly due to the quality of fibrosis, such as changes in collagen cross-linking.39 Other components of LV remodeling besides collagen are crucial to ventricular performance. It could be that in this rat model with a large infarct (≥42% of the LV), a decrease in viable LV mass and an alteration in the geometry of the heart may be more important.
It has long been demonstrated that ACE inhibitors improve cardiac function, often associated with reduced collagen deposition.40–43 The antifibrotic effect of ACE inhibitors may be partially mediated by inhibiting degradation of Ac-SDKP, which is hydrolyzed almost exclusively by ACE.1,44 It has been shown that ACE inhibitors increase plasma Ac-SDKP 4-to 5-fold in vivo, while in vitro they inhibit hydrolysis of [3H]Ac-SDKP by 90% to 99%.1 Thus, increased Ac-SDKP may contribute significantly to the antifibrotic effect of ACE inhibitors. However, in this model of heart failure after MI, ACE inhibitors, unlike Ac-SDKP, improved cardiac function.12,40,45–49 The mechanism of action of ACE inhibitors in heart failure is complex. In addition to blocking the conversion of angiotensin I to II, they also inhibit kinin hydrolysis and stimulate the release of nitric oxide, effects not seen with Ac-SDKP.12,40,45–49
In summary, Ac-SDKP prevented and reversed interstitial collagen and perivascular collagen deposition in the rat heart post-MI, associated with reduced macrophage infiltration and TGF-β–positive cell expression. Our study suggests that the antifibrotic and anti-inflammatory effects of Ac-SDKP may be mediated in part by inhibition of macrophage infiltration and TGF-β expression. However, these effects per se do not necessarily translate into improved cardiac function.
Perspective
LV remodeling after MI is a complex process that involves both the infarcted and noninfarcted area. In the noninfarcted area, remodeling involves myocyte hypertrophy and an increase in ECM and nonmyocyte cells. Remodeling of the ECM may participate in the development of cardiac dysfunction;16–18 however, myocyte growth is accompanied by coordinated increases in ECM that provide a scaffolding for the myocytes and vasculature, maintaining the appropriate geometric structure of the heart and proper alignment of myocytes and enabling optimal transduction of coordinated force generated by cardiac contraction.18,50 The collagen matrix in the heart has a three-dimensional configuration similar to a honeycomb; the perimysium envelops groups of myocytes, whereas the endomysium forms a fine fibrillar collagen weave that surrounds and supports the myocytes. Also, fine lateral struts connect adjacent myocytes. How Ac-SDKP alters this fine structure of the fibrillar collagen is not known. Here, we found that Ac-SDKP prevented and reversed total collagen deposition (the main component of the ECM) and decreased inflammation in the heart, but despite these changes neither diastolic nor systolic function improved. On the contrary, in the prevention group systolic cardiac function was slightly decreased and LVDd was somewhat increased. The decrease in systolic function is in agreement with previous work by Baicu et al,38 who found that regression of fibrosis negatively affected systolic function, suggesting that collagen may play an important role in myocyte-to-myocyte interaction, thereby optimizing ventricular systolic function. Also, disruption of the ECM could interfere with cellular signaling processes mediated via ECM-integrins-cytoskeleton, thus affecting cardiac function.51 ACE inhibitors increase plasma Ac-SDKP 4- to 5-fold, and we have found recently that this concentration of Ac-SDKP has an antifibrotic effect (Rasoul et al, unpublished data, 2003). Yet unlike Ac-SDKP, ACE inhibitors improve myocyte and ECM remodeling and cardiac function in HF and are among the drugs of choice for treatment of this disease. One possibility is that Ac-SDKP participates in the antifibrotic effect of ACE inhibitors, but coordinated reduction of cardiac hypertrophy, fibrosis, and afterload needs to occur to improve cardiac function. Further work is needed to determine whether Ac-SDKP participates in the therapeutic benefit and in particular the antifibrotic effect of ACE inhibitors.
Acknowledgments
This work was supported by NIH grant HL28982.
References
- 1.Azizi M, Rousseau A, Ezan E, Guyene T-T, Michelet S, Grognet J-M, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci U S A. 1989;86:779–782. doi: 10.1073/pnas.86.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A, Guigon M. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Seraspenide) on the growth of human CD34+ subpopulations in response to growth factors. Blood. 1993;82:3307–3314. [PubMed] [Google Scholar]
- 4.Lombard M-N, Sotty D, Wdzieczak-Bakala J, Lenfant M. In vivo effect of the tetrapeptide, N-acetyl-Ser-Asp-Lys-Pro, on the G1-S transition of rat hepatocytes. Cell Tissue Kinet. 1990;23:99–103. doi: 10.1111/j.1365-2184.1990.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb N-E. Anti-fibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800. doi: 10.1161/01.hyp.37.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhaleb N-E, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.hyp.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB. Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res. 1996;32:1096–1107. doi: 10.1016/s0008-6363(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 8.Pokharel S, Rasoul S, Roks AJM, van Leeuwen REW, van Luyn MJA, Deelman LE, Smits JF, Carretero O, van Gilst WH, Pinto YM. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibro-blasts. Hypertension. 2002;40:155–161. doi: 10.1161/01.hyp.0000025880.56816.fa. [DOI] [PubMed] [Google Scholar]
- 9.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-b-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863–872. doi: 10.1097/01.asn.0000057544.95569.ec. [DOI] [PubMed] [Google Scholar]
- 10.Yang X-P, Sabbah HN, Liu Y-H, Sharov VG, Mascha EJ, Alwan I, Carretero OA. Ventriculographic evaluation in three rat models of cardiac dysfunction. Am J Physiol. 1993;266:H1946–H1952. doi: 10.1152/ajpheart.1993.265.6.H1946. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y-H, Yang X-P, Nass O, Sabbah HN, Peterson E, Carretero OA. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am J Physiol. 1997;272:H722–H727. doi: 10.1152/ajpheart.1997.272.2.H722. [DOI] [PubMed] [Google Scholar]
- 12.Yang X-P, Liu Y-H, Mehta D, Cavasin MA, Shesely E, Xu J, Liu F, Carretero OA. Diminished cardioprotective response to inhibition of angiotensin-converting enzyme and angiotensin II type 1 receptor in B2 kinin receptor gene knockout mice. Circ Res. 2001;88:1072–1079. doi: 10.1161/hh1001.090759. [DOI] [PubMed] [Google Scholar]
- 13.Yang X-P, Liu Y-H, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 14.Rhaleb N-E, Peng H, Yang X-P, Liu Y-H, Mehta D, Ezan E, Carretero OA. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation. 2001;103:3136–3141. doi: 10.1161/01.cir.103.25.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber KT, Sun Y, Katwa LC. Myofibroblasts and local angiotensin II in rat cardiac tissue repair. Int J Biochem Cell Biol. 1997;29:31–42. doi: 10.1016/s1357-2725(96)00116-1. [DOI] [PubMed] [Google Scholar]
- 16.Weber KT. Cardiac interstitium: extracellular space of the myocardium. In: Fozzard HA, editor. The Heart and Cardiovascular System. 2nd Ed. Raven Press; New York: 1992. pp. 1465–1480. [Google Scholar]
- 17.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 18.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix. When is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Zhang JQ, Zhang J, Ramires FJA. Angiotensin II, transforming growth factor-b1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 21.Knowlton KU, Chien KR. Inflammatory pathways and cardiac repair: the affliction of infarction. Nat Med. 1999;5:1122–1123. doi: 10.1038/13441. [DOI] [PubMed] [Google Scholar]
- 22.Mann DL. Inflammatory mediators and the failing heart. Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 23.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–1136. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 24.Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102:2243–2248. doi: 10.1161/01.cir.102.18.2243. [DOI] [PubMed] [Google Scholar]
- 25.Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S. Increased expression of interleukin-1b and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997;81:664–671. doi: 10.1161/01.res.81.5.664. [DOI] [PubMed] [Google Scholar]
- 26.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-b1. Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 28.Lijnen PJ, Petrov VV, Fagard RH. Association between transforming growth factor-β and hypertension. Am J Hypertens. 2003;16:604–611. doi: 10.1016/s0895-7061(03)00847-1. [DOI] [PubMed] [Google Scholar]
- 29.O'Kane S, Ferguson MWJ. Transforming growth factor bs and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 30.Hao J, Wang B, Jones SC, Jassal DS, Dixon IMC. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H3020–H3030. doi: 10.1152/ajpheart.2000.279.6.H3020. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Hao J, Jones SC, Yee M-S, Roth JC, Dixon IMC. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1685–H1696. doi: 10.1152/ajpheart.00266.2001. [DOI] [PubMed] [Google Scholar]
- 32.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-b1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 33.Wünsch M, Sharma HS, Markert T, Bernotat-Danielowski S, Schott RJ, Kremer P, Bleese N, Schaper W. In situ localization of transforming growth factor b1 in porcine heart: enhanced expression after chronic coronary artery constriction. J Mol Cell Cardiol. 1991;23:1051–1062. doi: 10.1016/0022-2828(91)91640-d. [DOI] [PubMed] [Google Scholar]
- 34.Yue P, Massie BM, Simpson PC, Long CS. Cytokine expression increases in nonmyocytes from rats with postinfarction heart failure. Am J Physiol. 1998;275:H250–H258. doi: 10.1152/ajpheart.1998.275.1.H250. [DOI] [PubMed] [Google Scholar]
- 35.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 36.Anversa P. Myocyte death in the pathological heart. Circ Res. 2000;86:121–124. doi: 10.1161/01.res.86.2.121. [DOI] [PubMed] [Google Scholar]
- 37.Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, Yamamoto H, Tamaki K, Shimokawa H, Takeshita A. Early induction of transforming growth factor-b via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 1998;32:273–279. doi: 10.1161/01.hyp.32.2.273. [DOI] [PubMed] [Google Scholar]
- 38.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–H132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- 39.Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res. 2003;57:632–641. doi: 10.1016/s0008-6363(02)00733-2. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y-H, Yang X-P, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure: role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks WW, Bing OH, Robinson KG, Slawsky MT, Chaletsky DM, Conrad CH. Effect of angiotensin-converting enzyme inhibition on myocardial fibrosis and function in hypertrophied and failing myocardium from the spontaneously hypertensive rat. Circulation. 1997;96:4002–4010. doi: 10.1161/01.cir.96.11.4002. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Ratajska A, Zhou G, Weber KT. Angiotensin-converting enzyme and myocardial fibrosis in the rat receiving angiotensin II or aldosterone. J Lab Clin Med. 1993;122:395–403. [PubMed] [Google Scholar]
- 43.Brown L, Duce B, Miric G, Sernia C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol. 1999;10:S143–S148. [PubMed] [Google Scholar]
- 44.Rousseau A, Michaud A, Chauvet M-T, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 45.McDonald KM, Mock J, D'Aloia A, Parrish T, Hauer K, Francis G, Stillman A, Cohn JN. Bradykinin antagonism inhibits the antigrowth effect of converting enzyme inhibition in the dog myocardium after discrete transmural myocardial necrosis. Circulation. 1995;91:2043–2048. doi: 10.1161/01.cir.91.7.2043. [DOI] [PubMed] [Google Scholar]
- 46.Carretero OA. Kinins in the heart. In: Share L, editor. Contemporary Endocrinology: Hormones and the Heart in Health and Disease. Humana Press; Totowa, NJ: 1999. pp. 137–158. [Google Scholar]
- 47.Liu Y-H, Yang X-P, Mehta D, Bulagannawar M, Scicli GM, Carretero OA. Role of kinins in chronic heart failure and in the therapeutic effect of ACE inhibitors in kininogen-deficient rats. Am J Physiol Heart Circ Physiol. 2000;278:H507–H514. doi: 10.1152/ajpheart.2000.278.2.H507. [DOI] [PubMed] [Google Scholar]
- 48.Carretero OA, Rhaleb N-E. Kinins and Ac-SDKP in the therapeutic effects of angiotensin-converting enzyme (ACE) inhibitors. In: Giles TD, editor. Angiotensin-Converting Enzyme (ACE): Clinical and Experimental Insights. Health Care Communications; Fort Lee, N. J.: 2001. pp. 147–155. [Google Scholar]
- 49.Liu Y-H, Xu J, Yang X-P, Yang F, Shesely E, Carretero OA. Effect of ACE inhibitors and angiotensin II type 1 receptor antagonists on endothelial NO synthase knockout mice with heart failure. Hypertension. 2001;39:375–381. doi: 10.1161/hy02t2.102796. [DOI] [PubMed] [Google Scholar]
- 50.Ju H, Dixon IMC. Extracellular matrix and cardiovascular diseases. Can J Cardiol. 1996;12:1259–1267. [PubMed] [Google Scholar]
- 51.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D'Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dys-function. J Clin Invest. 2000;106:857–866. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]