Abstract
Synthetic atrial natriuretic peptide (carperitide) and B-type natriuretic peptide (BNP; nesiritide) are used to treat congestive heart failure. However, despite beneficial cardiac unloading properties, reductions in renal perfusion pressures limit their clinical effectiveness. Recently, CD-NP, a chimeric peptide composed of C-type natriuretic peptide (CNP) fused to the C-terminal tail of Dendroaspis natriuretic peptide (DNP), was shown to be more glomerular filtration rate-enhancing than BNP in dogs. However, the molecular basis for the increased responsiveness was not determined. Here, we show that the DNP tail has a striking effect on CNP, converting it from a non-agonist to a partial agonist of natriuretic peptide receptor (NPR)-A while maintaining the ability to activate NPR-B. This effect is specific for human receptors because CD-NP was only a slightly better activator of rat NPR-A due to the promiscuous nature of CNP in this species. Interesting, the DNP tail alone had no effect on any NPR even though it is effective in vivo. To further increase the potency of CD-NP for NPR-A, we converted two different triplet sequences within the CNP ring to their corresponding residues in BNP. Both variants demonstrated increased affinity and full agonist activity for NPR-A, whereas one was as potent as any NPR-A activator known. In contrast to a previous report, we found that DNP binds the natriuretic peptide clearance receptor (NPR-C). However, none of the chimeric peptides bound NPR-C with significantly higher affinity than endogenous ligands. We suggest that bifunctional chimeric peptides represent a new generation of natriuretic peptide therapeutics.
The human natriuretic peptide system consists of atrial natriuretic peptide (ANP),2 B-type natriuretic peptide (BNP), C-type natriuretic peptide (CNP), and an N-terminally extended renal form of ANP called urodilatin (1). ANP, BNP, and urodilatin bind natriuretic peptide receptor (NPR)-A, whereas CNP binds NPR-B. Both receptors are transmembrane guanylyl cyclases, which when activated catalyze the synthesis of cGMP. The natriuretic peptide clearance receptor (NPR-C) lacks guanylyl cyclase activity and functions primarily to clear natriuretic peptides from the circulation. However, a signaling function has been proposed for this receptor as well (2).
Both ANP and BNP reduce blood pressure by increasing natriuresis, diuresis, vasodilation, and endothelial permeability. They also prevent cardiac hypertrophy and suppress the renin-angiotensin-aldosterone systems. In 2001, BNP was approved by the United States Food and Drug Administration for the treatment of acutely decompensated heart failure. However, recent reports have suggested impaired renal function, which may be related to excessive hypotension (3, 4).
CD-NP represents a new class of natriuretic peptide drug that may circumvent the hypotensive nature of BNP and preserve or augment renal function in the congestive heart failure setting. CD-NP contains the full-length 22-amino acid human CNP fused to the 15-amino acid C-terminal tail of Dendroaspis natriuretic peptide (DNP), an endogenous vasoactive peptide found in the venom of the green mamba. The benefits of CNP are that it is venodilatory; less hypotensive than ANP and BNP; and unlike ANP and BNP, remains active in the failing heart (5). However, CNP lacks the diuretic and natriuretic actions of ANP and BNP, which are critical for the improvement of patients with congestive heart failure. Because DNP possesses both natriuretic and hypotensive activities (6), CD-NP was engineered to combine the unique venodilatory and cardiac properties of CNP with the beneficial renal properties of DNP.
In vivo application of CD-NP is promising because infusion of CD-NP into dogs increases natriuresis and diuresis while being less hypotensive than BNP. Furthermore, CD-NP increases the glomerular filtration rate (GFR) more than BNP (7). Although CD-NP infusion significantly increases cGMP concentrations in plasma, urine, and cultured fibroblasts, the ability of CD-NP to bind and activate each NPR was not determined. Here, we describe the molecular basis for the unique properties of CD-NP. In addition, we created two new variants of CD-NP that are even more potent activators of NPR-A. Surprisingly, the mutation of two amino acids in the ring of CNP transformed CD-NP into a superagonist of NPR-A. To our knowledge, this is the first description of peptides that are capable of activating both NPR-A and NPR-B at less than micromolar concentrations.
EXPERIMENTAL PROCEDURES
Peptides and Reagents—Human ANP and CNP were purchased from Sigma. Human BNP, DNP, B-CDNP, and CDNP-B were synthesized by Phoenix Pharmaceuticals, Inc. (Burlingame, CA). CD-NP was manufactured by Clinalfa (Weil am Rhein, Germany). The lyophilized peptides were reconstituted in deionized water, aliquoted, and stored at -80 °C until used. cGMP radioimmunoassay kits were purchased from PerkinElmer Life Sciences.
Cells—Human embryonic kidney 293 cells stably expressing rat or human NPR-A or NPR-B were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 200 μg/ml G418 or hygromycin B.
Whole Cell cGMP Elevation Assays—Cells were plated in 48-well plates. The day of the assay, the cells were incubated in serum-free medium for 4 h. The cells were incubated for 10 min at 37 °C in Dulbecco's modified Eagle's medium containing 25 mm HEPES (pH 7.4) and 1 mm 1-methyl-3-isobutylxanthine. This medium was then replaced with the same medium containing various concentrations of natriuretic peptides. The cells were stimulated for 1 or 3 min, and then the assay was terminated by aspirating the medium and adding 200 μl of ice-cold 80% ethanol. cGMP concentrations were estimated by radioimmunoassay as described previously (8).
Membrane Guanylyl Cyclase Assays—Crude membranes were prepared and assayed as described previously (9). Briefly, cells from a 10-cm tissue culture plate were washed twice with ice-cold phosphate-buffered saline and then scraped in cold buffer containing phosphatase inhibitors. The cells were lysed by sonicating 1 to 2 s and membranes were precipitated by centrifugation. The crude membranes were resuspended in phosphatase inhibitor buffer. 20 μl of membranes containing 1 mm MgGTP were assayed for 3 min at 37 °C in the presence or absence of various concentrations of natriuretic peptide. The assayed were stopped with the addition of 400 μl of cold 50 mm sodium acetate buffer containing 5 mm EDTA and placed on ice. cGMP concentrations were determined on a fraction of the resulting solution by radioimmunoassay.
Whole Cell Binding Assays—Cells were plated on 24-well plates precoated with polylysine. When the cells were 75–90% confluent, the growth medium was replaced with 0.2% bovine serum albumin in Dulbecco's modified Eagle's medium. Cells were incubated for 1–2 h in this medium at 37 °C. Binding medium containing 1% bovine serum albumin and 125I-ANP was prepared on ice. Binding media containing increasing concentrations of unlabeled ligand were added to the cells and incubated at 4 °C for 1 h. The cells were washed with ice-cold phosphate-buffered saline to remove nonspecifically bound tracer. 0.5 ml of 1 n NaOH was added to each well and incubated at room temperature to solubilize the cells. Radioactivity was determined in a Beckman 5500 γ-counter.
Statistics—Each experiment was performed in triplicate in three separate assays. The data were graphed with Prism software and are presented as the average of all assays combined or as a representative experiment with error bars representing the S.E. of the individual experiment.
RESULTS
Our goal is to develop natriuretic peptide variants that are unique co-activators of NPR-B and NPR-A and therefore may have therapeutic advantage beyond native natriuretic peptides that are specific for NPR-B (CNP) or NPR-A (ANP and BNP) for the treatment of cardiorenal disease syndromes such as heart failure. As such, new peptides should reduce cardiac load, decrease cardiac remodeling, and most importantly, enhance renal function. Recently, CD-NP was shown to be a more effective stimulator of GFR, natriuresis, and diuresis than BNP (sequence in Fig. 1) (7). However, the pharmacological basis for how CD-NP achieves this result has not been described. There are several possible explanations for why CD-NP elicits a greater GFR-enhancing effect than BNP. CD-NP could have a greater half-life than BNP, it could activate both NPR-A and NPR-B, or it could increase local concentrations of endogenous natriuretic peptides by binding more tightly to NPR-C, thereby reducing receptor-mediated degradation. These pharmacological questions related to the NPRs are addressed here.
FIGURE 1.
Primary structures of various natriuretic peptides. The sequences of the human natriuretic peptides and chimeric peptides are shown with identical amino acids shaded.
Activation of Human NPR-A—To unequivocally determine the ability of various ligands to activate human NPR (hNPR)-A or hNPR-B, we stably expressed each individual receptor in human embryonic kidney 293 cells devoid of endogenous NPRs. Using these cells, we could be confident that any increase in natriuretic peptide-dependent cGMP concentrations results from the activation of the single stably expressed receptor.
We first tested the ability of CD-NP to activate hNPR-A compared with the original parent peptides, CNP and DNP. We found that addition of the C-terminal portion of DNP to CNP resulted in a peptide that was a dramatically more potent activator of hNPR-A than CNP (Fig. 2A). Although extremely high concentrations of CNP (10 μm) yielded just detectable increases in cellular cGMP concentrations, CD-NP produced equivalent responses at concentrations that were >2 orders of magnitude lower. The calculated EC50 for CD-NP was 723 nm. Surprisingly, saturating concentrations of CD-NP produced significantly less cGMP elevations than saturating amounts of ANP, indicating that CD-NP is a partial agonist of hNPR-A. Because infusion of the linear C-terminal portion of DNP significantly increased urinary cGMP excretion in dogs, we tested whether this same fragment could elevate cGMP levels in NPR-A-expressing cells. We found that this peptide at 10 μm did not elevate cGMP concentrations in cells expressing hNPR-A, consistent with a previous study showing that an intact ring structure is required for NPR-A activation (10).
FIGURE 2.
Chimeric peptide CD-NP, but not CNP, activates both NPR-A and NPR-B. Confluent 293neo cells stably expressing either hNPR-A (A) or hNPR-B (B) were incubated with increasing concentrations of ligand for 3 min. Cellular cGMP concentrations were then measured by radioimmunoassay. Graphs are representative experiments from three separate assays; each point was assayed in triplicate.
Activation of hNPR-B—Similar experiments were conducted on 293 cells stably expressing hNPR-B. In these cells, CD-NP was ∼5-fold less potent than the natural ligand for NPR-B, CNP. The EC50 for CD-NP was 202 nm compared with 38 nm for CNP (Fig. 2B). Hence, compared with CNP, CD-NP is a >200-fold better agonist for NPR-A but a 5-fold worse agonist for NPR-B. Interestingly, the activity of CD-NP on NPR-B was much higher than the other NPR-A-specific ligands, ANP and DNP, which contain residues C-terminal to the ring structure. As with hNPR-A, the linear C-terminal portion of DNP (C-terminal tail) was unable to stimulate hNPR-B (Fig. 2B).
Effect of CD-NP on Guanylyl Cyclase Activity—To determine whether the cGMP elevations observed in whole cells were due to direct activation of the receptor by CD-NP, guanylyl cyclase assays were performed. Crude membranes prepared from 293 cells expressing hNPR-A or hNPR-B were incubated with 1 mm GTP and 5 mm MgCl2 in the presence or absence of various concentrations of natriuretic peptide, and the amount of cGMP formed over a 3-min period was determined (Fig. 3). Similar to the whole cell assays, CD-NP was a dramatically more potent activator of hNPR-A than CNP (Fig. 3A). In contrast, the addition of the DNP tail only slightly reduced the ability of CNP to activate hNPR-B (Fig. 3B).
FIGURE 3.
Effect of CD-NP on the guanylyl cyclase activity of NPR-A or NPR-B. Crude membranes from 293neo cells stably expressing hNPR-A (A) or hNPR-B (B) were incubated for 3 min at 37 °C with increasing concentrations of ANP, CNP, or CD-NP in the presence of magnesium, ATP, and GTP. cGMP production was measured by radioimmunoassay.
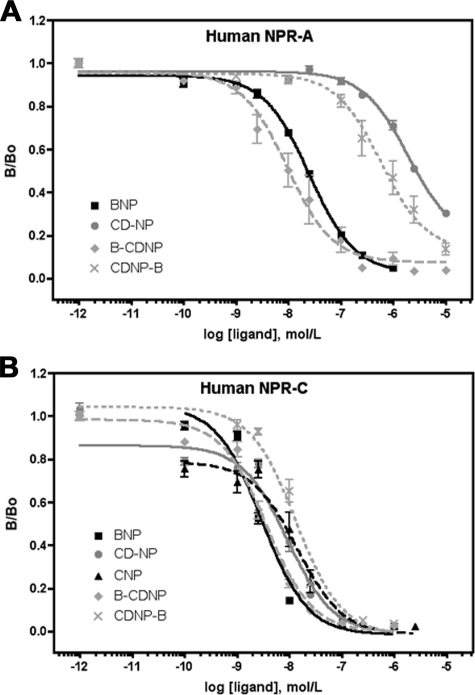
Competition Binding of CD-NP in hNPR-A-expressing Cells—To determine whether the elevated sensitivity of hNPR-A to CD-NP was due to an increase in receptor binding affinity, competition 125I-ANP binding experiments were performed on whole hNPR-A-expressing 293 cells. CD-NP was not a particularly efficient competitor for binding to hNPR-A because ∼1000-fold more CD-NP than ANP was required to compete for 125I-ANP (Fig. 4A). However, much more striking was the complete inability of CNP or the C-terminal portion of DNP to compete for 125I-ANP binding to hNPR-A even at 10 μm concentrations. Hence, the addition of the C-terminal tail of DNP to the C terminus of CNP dramatically increases its affinity for hNPR-A, consistent with the idea that CD-NP, but not CNP, is a NPR-A agonist. Similar experiments were attempted using [125I-Tyr0]CNP binding to hNPR-B. Unfortunately, the purchased tracer lacked the specificity necessary for accurate interpretation of the experiments.
FIGURE 4.
Addition of the C-terminal tail of DNP to CNP increases binding to NPR-A, but not NPR-C. 293neo cells stably expressing hNPR-A (A) or hNPR-C (B and C) were incubated for 1 h at 4 °C with 50 pm 125I-ANP in the presence or absence of increasing concentrations of unlabeled ligand. A, hNPR-A, average of three experiments with each point assayed in triplicate; B, hNPR-C, average of four experiments with each point assayed in triplicate; C, hNPR-C, average of two experiments with each point assayed in triplicate.
Competition Binding of CD-NP in hNPR-C-expressing Cells—The above studies indicate that CD-NP directly binds and activates hNPR-A. However, that does not mean that the renal and hemodynamic effects of CD-NP result solely from the direct activation of hNPR-A. Another possible explanation is that infusion of CD-NP prolongs the half-life of endogenous ANP and/or BNP by competing for its degradation by the clearance receptor. To address this possibility, we measured the ability of CD-NP, ANP, and CNP to compete for 125I-ANP binding to the clearance receptor (Fig. 4B). In contrast to the dramatic effect that the DNP tail had on the ability of CNP to bind NPR-A, it had little if any effect on binding to NPR-C because similar concentrations of all three peptides were required to block binding of 125I-ANP to this receptor. Thus, the addition of the C-terminal portion of DNP to CNP increases affinity for NPR-A but not NPR-C.
A previous report using 125I-DNP binding assays suggested that DNP does not bind NPR-C (11), whereas another group using 125I-ANP competition binding assays concluded that DNP does bind NPR-C (12). To clarify this issue, we tested the ability of DNP to displace 125I-ANP binding to NPR-C compared with ANP, BNP, and CNP. As shown in Fig. 4C, DNP bound 3–5-fold less avidly than CNP and 20–25-fold less avidly than ANP and BNP to the clearance receptor. Hence, our data resolve the debate in the literature concerning the ability of DNP to bind NPR-A versus NPR-C; it clearly does bind NPR-C, but it binds NPR-C less tightly than it binds NPR-A.
Activation of Rat Receptor Isoforms by CD-NP—The ability of CD-NP to activate the rat versions of NPR-A and NPR-B was also determined. Here, we made two surprising observations. First, unlike the human scenario, in which high concentrations of CNP had very little effect on hNPR-A (Fig. 5A), high concentrations of CNP were able to maximally activate rat NPR-A (Fig. 5B). Second, whereas CD-NP was a partial agonist of hNPR-A, it was a full agonist of rat NPR-A (Fig. 5B). In contrast, CD-NP had similar effects on rat and human NPR-B (data not shown). Hence, the addition of the C-terminal tail of DNP to CNP has a greater effect on hNPR-A because this receptor is essentially unresponsive to CNP even at pharmacological concentrations.
FIGURE 5.
Differential effects of CD-NP on human and rat NPR-A. Confluent 293neo cells stably expressing either human (A) or rat (B) NPR-A were incubated with increasing concentrations of ligand for 1 or 3 min, respectively. Reactions were stopped with ice-cold ethanol, and cellular cGMP concentrations were estimated by radioimmunoassay. Data are from one experiment, which is representative of four separate experiments. Each point was assayed in triplicate.
Effect of Amino Acid Substitutions within the CD-NP Ring—We next tested whether substitutions within the cysteine ring would lead to further increases in the EC50 for hNPR-A activation. Alignment of ANP, BNP, and CNP shows four sections of amino acid divergence: the N terminus, a three-amino acid segment just inside the first cysteine residue of the ring, a second three-amino acid intra-ring sequence closer to the C-terminal cysteine, and the C-terminal tail (Fig. 1). The divergence of the C-terminal portions of the natriuretic peptides is addressed with the dramatic change in the pharmacological profile of CNP upon addition of the C-terminal portion of DNP. Previous studies tested the effect of adding the C-terminal tail of ANP (vasonatrin) or BNP to CNP; therefore, we will not address this region of the peptides further. Instead, we focused on modifications of the amino acids in the ring structure of CD-NP to further enhance its potency and/or affinity for hNPR-A. We substituted the corresponding sequence for BNP into the positions described above and measured the effect of the modifications on the resultant peptides' abilities to activate the hNPRs. The mutations were based on BNP, not ANP, because BNP and NPR-A coevolved (13).
We used the same assays to determine the ability of the new chimeric peptides to activate hNPR-A and hNPR-B as we did to characterize CD-NP. However, in these studies, we used BNP rather than ANP as the representative NPR-A agonist because the sequence modifications for the new peptides were derived from BNP. ANP and BNP have similar abilities to activate NPR-A, although human ANP is typically a slightly better activator than BNP (EC50 = 10.8 nm for ANP in Fig. 2 versus EC50 = 28.4 nm for BNP in Fig. 6A). As shown in Fig. 6A, the mutation of the first set of amino acids to comparable residues within BNP resulted in a dramatic leftward shift in the concentration of the peptide required to yield maximal cGMP elevations in NPR-A-expressing cells. This peptide, called B-CDNP, was slightly more potent than human BNP, and unlike CD-NP, it was a full agonist. Mutation of the second set of amino acids within the ring structure of CNP to yield CDNP-B also resulted in a peptide with increased full agonist potency over CD-NP, although the effect was much less dramatic. Notably, both variants retained significant potency for NPR-B (Fig. 6B) because their EC50 values for NPR-B were <1 μm, although the reduction in potency was greater than that observed for CD-NP.
FIGURE 6.
Substitution of two amino acids in the ring of CD-NP converts it to a potent NPR-A agonist. Confluent 293neo cells stably expressing either hNPR-A (A) or hNPR-B (B) were incubated with increasing concentrations of ligand for 3 min. Cellular cGMP concentrations were then measured by radioimmunoassay. Data are from one experiment that is representative of three and four separate experiments for hNPR-A and hNPR-B, respectively. Each point was assayed in triplicate.
Finally, competitive binding studies were conducted to determine whether the change in potency of the CD-NP analogs versus CD-NP was due to increased affinity of the peptides for the extracellular domain of NPR-A. The binding profile shown in Fig. 7A is strikingly correlated with the whole cell receptor activation profile shown in Fig. 6A. Hence, the increased potency of the CD-NP analogs for NPR-A results from increased affinity for the receptor. Finally, the ring mutations are specific for NPR-A because they had minimal or no effect on binding to NPR-C (Fig. 7B).
FIGURE 7.
Substitution of BNP ring residues into CD-NP increases binding to NPR-A, but not NPR-C. 293neo cells stably expressing hNPR-A (A) or hNPR-C (B) were incubated for 1 h at 4 °C with 50 pm 125I-ANP in the presence or absence of increasing concentrations of unlabeled ligand. After washing to remove nonspecific binding, the amount of bound radio-ligand was quantitated in a γ-counter. Data are the average from three separate experiments.
DISCUSSION
The addition of the C-terminal tail of DNP to CNP, which is normally not natriuretic, resulted in a peptide (CD-NP) that is substantially natriuretic, diuretic, and GFR-enhancing while being less hypotensive than BNP (7). The retention of renal activities by the chimeric peptide is particularly important for therapeutic benefit. Synthetic BNP (nesiritide) is currently approved for the treatment of acutely decompensated heart failure. However, infusion of nesiritide can cause dramatic decreases in blood pressure in some patients, which reduces renal perfusion pressure and ultimately may reduce GFR (3). Therefore, the ability of CD-NP to retain these activities without eliciting extensive hypotension may be extremely beneficial in the clinical setting. Determining the molecular mechanism of action of CD-NP was the goal of this study, with specific focus on hNPRs.
Our experiments unequivocally determined that CD-NP is a direct activator of both hNPR-A (Fig. 2A) and hNPR-B (Fig. 2B). This is in direct contrast to CNP, which essentially has no ability to activate the human form of NPR-A at concentrations up to 10 μm (Fig. 2A). The potency of CD-NP compared with ANP and BNP is reduced by ∼60-fold, but at pharmacological concentrations, it would still likely activate the receptor. The mutation of amino acids in the ring portion of the peptide to corresponding residues in BNP resulted in a chimeric peptide, B-CDNP, which activated hNPR-A with a potency similar to human BNP (Fig. 6A) and still maintained some ability to activate NPR-B, although higher concentrations were required to achieve similar levels of activation (Fig. 6B). Thus, determining which residues within these derivative chimeric peptides mediate selective binding to NPR-A and NPR-B will be the focus of future studies.
The reduced potency of CD-NP for NPR-A could explain the less hypotensive effects observed upon infusion of CD-NP in dogs (7) and could be an important distinction for its therapeutic use over ANP (carperitide) and BNP (nesiritide). This characteristic may make CD-NP more beneficial than its derivatives, B-CDNP and CDNP-B, in normotensive patients, but the latter peptides may be therapeutically useful to hypertensive patients. Additionally, several reports suggest that NPR-B is involved in preventing cardiac hypertrophy in heart failure (14, 15). The ability of CD-NP to activate both hNPR-A and hNPR-B may prove doubly beneficial in regards to inhibiting cardiac remodeling in heart failure patients. Activation of NPR-A should induce natriuresis, diuresis, and arterial vasodilation to reduce blood pressure and load on the heart, and activation of NPR-B should unload the heart by stimulating venodilation (16) as well as by acting directly on the heart to decrease hypertrophy and fibrosis.
Although our activation studies showed that CD-NP directly activates hNPR-A, they did not rule out additional causes for the physiological effects of CD-NP infusion in whole animal studies. The increase in cGMP could be explained by direct activation of NPR-A or by an increased half-life of ANP or BNP due to competition with CD-NP for degradation. Natriuretic peptides are removed from the circulation by NPR-C-mediated internalization and degradation as well as by neutral endopeptidase 24.11-dependent and possibly other protease-dependent degradation. We determined that CD-NP binds the clearance receptor with similar affinity to ANP and CNP (Fig. 4B); thus, CD-NP competes for degradation by NPR-C but not any better than CNP. Previous reports suggest that DNP is resistant to degradation by neutral endopeptidase (17); therefore, addition of the C-terminal tail of DNP to CNP may render the peptide less susceptible to neutral endopeptidase degradation and increase its half-life. However, we did not make any direct measurements of the ability of CD-NP to be degraded by neutral endopeptidase. To be most effective clinically, CD-NP should have a long half-life; thus, this topic warrants future investigation.
Similar strategies for producing chimeric natriuretic peptides have been attempted previously. In 1993, Wei et al. (18) described the development of vasonatrin peptide. This chimeric peptide contains the five-amino acid C-terminal tail of ANP appended to full-length CNP. Vasonatrin induces natriuresis similarly to ANP and is more potent than CNP in inducing venodilation. One might speculate that the strong venodilation combined with a potent arterial vasodilation response may produce hypotension similar to that observed with infusion of ANP or BNP. Therefore, vasonatrin would have little advantage over infusion of synthetic ANP or BNP in heart failure patients. Conversely, by being a less potent activator of NPR-A, CD-NP may eliminate or reduce some of the side effects of NPR-A agonists but still be potent enough to produce the beneficial renal actions reported with DNP.
The species difference in CD-NP activation of NPR-A (Fig. 5) is similar to that reported by Cunningham et al. (19) in their attempt to identify NPR-A-specific analogs and emphasizes the importance of using the correct model system when evaluating compounds for therapeutic potential. NPR-A is 91% identical in rat and human. Thus, the full agonist versus partial agonist responses to CD-NP shown in Fig. 5, B versus A, are clearly a function of the subtle differences between the human and rat receptors because CNP is identical in rats, mice, dogs, and humans.
The structures of all three human natriuretic peptides (ANP, BNP, and CNP) are very similar (Fig. 1) (10). CNP differs from the other peptides in that it lacks a C-terminal tail. Although the ring structure appears to be most critical for activity, numerous reports have determined that deletion of amino acids C-terminal to the ring reduces the potency of ANP (20, 21). Data from the crystallography studies by Ogawa et al. (22) using the extracellular domain of rat NPR-A bound to an ANP peptide identified some of the amino acids important for binding ANP and suggest that the C-terminal tail portion of ANP is especially important for stabilization of binding. Hence, it is likely that the C-terminal amino acids from DNP form critical interactions with residues found in the extracellular domain of human but not rat NPR-A.
Whether this species difference also applies to the dog receptor used as the model for the in vivo studies on CD-NP is not known. Sequence comparison of the extracellular ligand-binding domains of dog and rat NPR-A indicates that this region is 88 and 85% identical to the human receptor, respectively. Unfortunately, the critical interactions between hNPR-A and CD-NP are not known, so we cannot determine whether the important contacts are conserved in the dog receptor at this time. Comparison of the amino acids from the structure study of rat NPR-A by Ogawa et al. (22) indicates no significant amino acid difference between human, rat, and dog NPR-A. We are currently cloning the dog NPRs to directly compare the sensitivity of these receptors to the human receptors.
Interestingly, although our data explain the mechanism behind the physiological response following in vivo administration of CD-NP, we could not explain the response seen following infusion of the linear C-terminal portion of DNP. This C-terminal fragment was unable to induce a cGMP response in either NPR-A- or NPR-B-expressing cells at concentrations up to 10 μm. We also did not measure any appreciable binding of the peptide to NPR-A or NPR-C. Thus, we speculate that the linear peptide blocks degradation and inactivation of the endogenous peptides by neutral endopeptidase or other proteases, but this remains to be determined.
In summary, CD-NP is a novel natriuretic peptide that represents a potential new drug for the treatment of heart failure and other cardiorenal disease syndromes. The unique property of this chimeric peptide is that it activates both hNPR-A and hNPR-B. Additional changes in the amino acids located in the ring structure make the peptide a more potent NPR-A agonist. The availability of the three CD-NP peptides described here to distinguish between the benefits of a full NPR-A agonist versus a partial agonist in concert with the ability of these peptides to activate NPR-B may maximize the therapeutic potential of the NPRs and result in the development of a new group of drugs whose individual use could be selected for based on optimum treatment of the symptoms of patients with congestive heart failure with and without hypertension.
This work was supported by a Minnesota-Mayo Partnership grant (to L. R. P. and J. C. B.) from Medica. CD-NP has been licensed by the Mayo Clinic to Nile Therapeutics, and J. C. B. is Chair of its Scientific Advisory Board. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CNP, C-type natriuretic peptide; NPR, natriuretic peptide receptor; NPR-C, natriuretic peptide clearance receptor; DNP, Dendroaspis natriuretic peptide; GFR, glomerular filtration rate; hNPR, human NPR.
References
- 1.Potter, L. R., Abbey-Hosch, S., and Dickey, D. M. (2006) Endocr. Rev. 27 47-72 [DOI] [PubMed] [Google Scholar]
- 2.Rose, R. A., and Giles, W. R. (2008) J. Physiol. (Lond.) 586 353-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackner-Bernstein, J. D., Skopicki, H. A., and Aaronson, K. D. (2005) Circulation 111 1487-1491 [DOI] [PubMed] [Google Scholar]
- 4.Sackner-Bernstein, J. D., Kowalski, M., Fox, M., and Aaronson, K. (2005) J. Am. Med. Assoc. 293 1900-1905 [DOI] [PubMed] [Google Scholar]
- 5.Dickey, D. M., Flora, D. R., Bryan, P. M., Xu, X., Chen, Y., and Potter, L. R. (2007) Endocrinology 148 3518-3522 [DOI] [PubMed] [Google Scholar]
- 6.Lisy, O., Lainchbury, J. G., Leskinen, H., and Burnett, J. C., Jr. (2001) Hypertension 37 1089-1094 [DOI] [PubMed] [Google Scholar]
- 7.Lisy, O., Huntley, B. K., McCormick, D. J., Kurlansky, P. A., and Burnett, J. C., Jr. (2008) J. Am. Coll. Cardiol. 52 60-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbey, S. E., and Potter, L. R. (2002) J. Biol. Chem. 277 42423-42430 [DOI] [PubMed] [Google Scholar]
- 9.Bryan, P. M., Xu, X., Dickey, D. M., Chen, Y., and Potter, L. R. (2007) Am. J. Physiol. 292 F1636-F1644 [DOI] [PubMed] [Google Scholar]
- 10.Misono, K. S., Grammer, R. T., Fukumi, H., and Inagami, T. (1984) Biochem. Biophys. Res. Commun. 123 444-451 [DOI] [PubMed] [Google Scholar]
- 11.Singh, G., Kuc, R. E., Maguire, J. J., Fidock, M., and Davenport, A. P. (2006) Circ. Res. 99 183-190 [DOI] [PubMed] [Google Scholar]
- 12.Johns, D. G., Ao, Z., Heidrich, B. J., Hunsberger, G. E., Graham, T., Payne, L., Elshourbagy, N., Lu, Q., Aiyar, N., and Douglas, S. A. (2007) Biochem. Biophys. Res. Commun. 358 145-149 [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld, J. R., Sehl, P., Quan, C., Burnier, J. P., and Lowe, D. G. (1995) Mol. Pharmacol. 47 172-180 [PubMed] [Google Scholar]
- 14.Langenickel, T. H., Buttgereit, J., Pagel-Langenickel, I., Lindner, M., Monti, J., Beuerlein, K., Al-Saadi, N., Plehm, R., Popova, E., Tank, J., Dietz, R., Willenbrock, R., and Bader, M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 4735-4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soeki, T., Kishimoto, I., Okumura, H., Tokudome, T., Horio, T., Mori, K., and Kangawa, K. (2005) J. Am. Coll. Cardiol. 45 608-616 [DOI] [PubMed] [Google Scholar]
- 16.Wei, C. M., Aarhus, L. L., Miller, V. M., and Burnett, J. C., Jr. (1993) Am. J. Physiol. 264 H71-H73 [DOI] [PubMed] [Google Scholar]
- 17.Chen, H. H., Lainchbury, J. G., and Burnett, J. C., Jr. (2002) J. Am. Coll. Cardiol. 40 1186-1191 [DOI] [PubMed] [Google Scholar]
- 18.Wei, C. M., Kim, C. H., Miller, V. M., and Burnett, J. C., Jr. (1993) J. Clin. Investig. 92 2048-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham, B. C., Lowe, D. G., Li, B., Bennett, B. D., and Wells, J. A. (1994) EMBO J. 13 2508-2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarborough, R. M., Schenk, D. B., McEnroe, G. A., Arfsten, A., Kang, L. L., Schwartz, K., and Lewicki, J. A. (1986) J. Biol. Chem. 261 12960-12964 [PubMed] [Google Scholar]
- 21.Currie, M. G., Geller, D. M., Cole, B. R., Siegel, N. R., Fok, K. F., Adams, S. P., Eubanks, S. R., Galluppi, G. R., and Needleman, P. (1984) Science 223 67-69 [DOI] [PubMed] [Google Scholar]
- 22.Ogawa, H., Qiu, Y., Ogata, C. M., and Misono, K. S. (2004) J. Biol. Chem. 279 28625-28631 [DOI] [PubMed] [Google Scholar]