Abstract
Prediction of export pathway specificity in prokaryotes is a challenging endeavor due to the similar overall architecture of N-terminal signal peptides for the Sec-, SRP- (signal recognition particle), and Tat (twin arginine translocation)-dependent pathways. Thus, we sought to create a facile experimental strategy for unbiased discovery of pathway specificity conferred by N-terminal signals. Using a limited collection of Escherichia coli strains that allow protein oxidation in the cytoplasm or, conversely, disable protein oxidation in the periplasm, we were able to discriminate the specific mode of export for PhoA (alkaline phosphatase) fusions to signal peptides for all of the major modes of transport across the inner membrane (Sec, SRP, or Tat). Based on these findings, we developed a mini-Tn5 phoA approach to isolate pathway-specific export signals from libraries of random fusions between exported proteins and the phoA gene. Interestingly, we observed that reduced PhoA was exported in a Tat-independent manner when targeted for Tat export in the absence of the essential translocon component TatC. This suggests that initial docking to TatC serves as a key specificity determinant for Tat-specific routing of PhoA, and in its absence, substrates can be rerouted to the Sec pathway, provided they remain compatible with the Sec export mechanism. Finally, the utility of our approach was demonstrated by experimental verification that four secreted proteins from Mycobacterium tuberculosis carrying putative Tat signals are bona fide Tat substrates and thus represent potential Tat-dependent virulence factors in this important human pathogen.
Despite recent advances in bioinformatic analysis of N-terminal protein export signals (1-6) prediction of export pathway specificity based on sequence information alone is complicated by the similar overall architecture of N-terminal export signals (Fig. 1a). Since bioinformatic tools for predicting pathway specificity typically rely on experimentally confirmed signal peptides, these approaches can be biased toward export signal whose primary structure does not differ greatly from those of the learning set. The TnphoA transposon probe developed by Manoil and Beckwith (7) has been an extraordinarily useful experimental tool for verifying and discovering signal peptides in numerous bacterial species on a genome-wide scale (8, 9). TnphoA is a derivative of the Tn5 transposon that enables the generation of protein fusions to Escherichia coli PhoA (alkaline phosphatase; EC3.1.3.1) devoid of its native amino-terminal export signal. The resulting fusions only confer phosphate hydrolase activity to cells if they are capable of export out of the cytoplasm. Consequently, TnphoA can be used to detect proteins localized to the periplasm, inner and outer membranes, or extracellularly. Living cells can be assayed for PhoA activity using the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate (BCIP)3 or by selective growth on medium containing a sole carbon or phosphate source that requires PhoA activity to be metabolized (10). The general utility of this genetic construct is exemplified by the large number of applications reporting its use, including, for example, identification of cell surface and secreted virulence factors (11-16), dissection of membrane protein topology (17-20), determination of sites within proteins that are permissive to large insertions without disrupting function (21), and epitope tagging of proteins at internal positions (8, 21).
FIGURE 1.
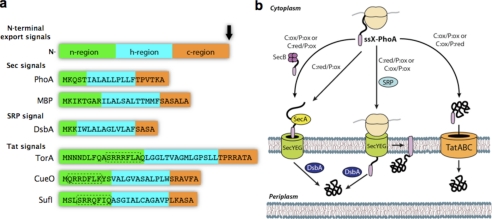
Translocation of proteins through the inner membrane of bacteria. a, composition of N-terminally fused signal peptides used in this study. Each is composed of three linearly arranged domains: an n-region, a hydrophobic h-region, and a c-region that is followed by the signal peptide cleavage site (black arrow). The targeting specificity of Sec- and SRP-dependent signals is governed by the relative hydrophobicity of the h-region; SRP binds preferentially to the more hydrophobic signals found on SRP substrates. By comparison, Tat signals are typically about 15 amino acids longer than their Sec or SRP counterparts and contain a less hydrophobic h-region that appears to aid in targeting specificity. In addition, E. coli Tat signals possess a consensus motif (underlined) of (S/T)RRXFLK (where X is any polar amino acid) that is absent in Sec- and SRP-dependent signals. Further specificity for the Tat pathway is imparted by the occurrence of an overall charge of +2 or greater for the c-region together with the N terminus of the mature protein, as opposed to the typically neutral charge found in the c-region of Sec and SRP signal peptides. b, schematic of pathway specificity in the context of cellular redox potential. Export of PhoA (alkaline phosphatase) depends on the targeting specificity of the signal peptide (ssX), which includes (from left to right) SecB-dependent, SecB-independent, SRP-dependent, and Tat-dependent export as well as the relative redox potential (oxidizing (ox) or reducing (red)) of the cytoplasm (C) and periplasm (P).
In general, the TnphoA strategy is very efficient at uncovering proteins that carry Sec signals peptides. This is because (i) PhoA itself is a native Sec-dependent substrate, and (ii) the majority of proteins that are exported out of the cytoplasm of bacteria are substrates of the Sec pathway (22, 23). The Sec pathway consists of the SecYEG translocase formed by the SecYEG integral membrane proteins and a molecular motor, SecA, which drives translocation of unfolded substrates in an ATP-dependent manner (22-24). The bulk of proteins targeted to SecYEG are routed in a post-translational manner with assistance from the dedicated molecular chaperone SecB (25, 26), although for some substrates, including PhoA, post-translational export can proceed without assistance from SecB (27). Alternatively, a subset of exported proteins are routed co-translationally by the signal recognition particle (SRP) (28, 29) that directs substrates to the FtsY receptor (30) and ultimately the SecYEG translocase. This mechanism is responsible for the export of both soluble periplasmic proteins (31, 32) and, with assistance from YidC, integral membrane proteins (29, 33).
Importantly, although the use of TnphoA in bacteria is capable of detecting proteins exported via the Sec and SRP pathways, it does not provide sufficient resolution to distinguish between these different modes of SecYEG targeting. To address this limitation, previous studies used TnphoA in combination with Escherichia coli lpp-5508 mutants that have a leaky outer membrane. Free periplasmic PhoA diffused away from these cells and hydrolyzed BCIP in the surrounding medium to yield a “blue halo” around the colony, whereas membrane-bound PhoA could only hydrolyze intracellular BCIP, and the resulting blue color was localized within the colony (34, 35). This approach enabled discrimination between cells expressing PhoA fusions with cleavable signal peptides (mostly Sec substrates) from those that spanned the inner membrane (mostly SRP substrates) but did not shed light on the specific targeting route. A further shortcoming of the TnphoA system is its limited ability to detect export via the Tat (twin-arginine translocation) pathway, whose substrates contain unique Arg-Arg signal peptides and are known to fold in the cytoplasm prior to transiting the inner membrane (36-38). For instance, in a recent genome-wide screen for N-terminal signal peptides in Psuedomonas aeruginosa, 310 PhoA fusions were identified, of which only one (RnfG, PA3493) was predicted to be a Tat substrate (39) despite the fact that as many as 57 Tat-dependent substrates have been predicted for this organism (4). The reason for this is that the bacterial Tat transporter accepts only those proteins that have attained a native or nearly native structure in the cytoplasm (40-42). Since PhoA folding is dependent upon disulfide bonds that can only form in the periplasm (43), PhoA fusions misfold in the cytoplasm of wild type bacteria and thus are incapable of transiting the Tat pathway (41). However, PhoA can be exported by the Tat pathway under specific conditions, such as when the cytoplasm is rendered more oxidizing by deletion of the trxB gor genes that encode the two major cytoplasmic reductases in E. coli (41).
In this study, we sought to expand the utility of TnphoA screening for (i) resolving the targeting specificity (e.g. SecB-independent versus SecB-dependent versus SRP-dependent) of exported proteins and (ii) detecting export via the Tat pathway. Using mini-Tn5 phoA-generated libraries in combination with a limited collection of isogenic E. coli strains derived from strain DR473 that allow toggling of PhoA folding, we have isolated PhoA fusions for all major modes of inner membrane transport in E. coli. Additionally, we have employed this strategy to experimentally verify that four secreted proteins from Mycobacterium tuberculosis carrying putative Tat signal peptides are bona fide Tat substrates and thus represent potential Tat-dependent virulence factors in this important human pathogen.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids—The strains and plasmids used in this study are listed in Table 1. E. coli strains were routinely grown in LB medium (Difco) broth at 30 or 37 °C with antibiotics added at the following concentrations: 100 μg/ml ampicillin, 20 μg/ml chloramphenicol, 10 μg/ml gentamycin, 50 μg/ml hygromycin, 50 μg/ml kanamycin, and 50 μg/ml spectinomycin. Mycobacterium smegmatis strains were grown in Middlebrook 7H9 medium (Difco) supplemented with 0.2% glycerol and 0.05% Tween 80; l-lysine was added at a concentration of 80 μg/ml for the strains MB692, PM759, and JM578, and all cells were grown at 37 °C with antibiotics added at the same concentrations as listed above. M. smegmatis cells were also grown on solid Middlebrook 7H10 medium (Difco) supplemented as indicated above but with 40 μg/ml l-lysine when necessary.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype/phenotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| MC4100 | F ΔlacU169 araD139 rpsL150 relA1 ptsF rbs flbB5301 | Laboratory stock |
| SM10 λ-pir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Kmrpir | Ref. 77 |
| DHB4 | MC1000 phoR Δ(phoA) PvuII (malF)3 F′[lacIqZYA pro] | Laboratory stock |
| JW3584 | secB::kan | Ref. 78 |
| JW5580 | tatB::kan | Ref. 78 |
| JW3815 | tatC::kan | Ref. 78 |
| JW3813 | tatA:::kan | Ref. 78 |
| JW0622 | tatE::kan | Ref. 78 |
| DHAE | DHB4 tatA tatE | This work |
| DHB | DHB4 tatB | This work |
| DHC | DHB4 tatC | This work |
| DR473 | DHB4 ΔtrxB gor552 Tn10Tet ahpC* Tn10Cm (araC Para-trxB) | Ref. 41 |
| DRA | DR473 dsbA::kan | Ref. 41 |
| DRAE | DR473 tatA tatE | This work |
| DRB | DR473 tatB::kan | Ref. 41 |
| DRC | DR473 tatC::spec | Ref. 41 |
| DRS | DR473 secB | This work |
|
TOP10
|
F−mcrA Δ(mrr-hsdRMS-mcrBC)
f80lacZDM15 DlacX74 recA1 araD139 Δ(ara leu)
7697 galU galK rpsL (StrR) endA1 nupG |
Laboratory stock
|
| M. smegmatis strains | ||
| mc2155 | ept-1 | Laboratory stock |
| PM759 | mc2155 ΔblaS1 ΔlysA4 rpsL6 | Ref. 79 |
| MB692 | mc2155 ΔtatA | Ref. 68 |
|
JM578
|
PM759 ΔtatA |
Ref. 68
|
| Plasmids | ||
| pCP20 | Apr, Cmr | Ref. 44 |
| pBR322 | Apr, Tcr | Laboratory stock |
| pBR322-Gm | Apr, Gmr | This study |
| pBR-BamHI-Gm | BamHI site cloned into pBR322-Gm | This study |
| pBRGC.1 | Gateway cassette RfC.1 in pBR322-Gm | This study |
| pUTphoA | Apr, Kmr, mini-Tn5 phoA cassette in pUT-based plasmid | Ref. 55 |
| pPhoA | E. coli phoA gene cloned in pTrc99A | Laboratory stock |
| pTorA-PhoA | E. coli ssTorA fused to Δ(1-22)PhoA in pTrc99A | Ref. 41 |
| pMCS-ΔssPhoA | Δ(1-22)PhoA cloned in pBR322-Gm | This study |
| pssMBP-PhoA | E. coli ssMBP fused to Δ(1-22)PhoA in pMCS-DssAP | This study |
| pCueO-PhoA | E. coli CueO fused to Δ(1-22)PhoA in pMCS-DssAP | This study |
| pSufI-PhoA | E. coli SufI fused to Δ(1-22)PhoA in pMCS-DssAP | This study |
| pΔssPhoA | Δ(1-22)PhoA in pBRGC.1 | This study |
| pssCueO-PhoA | E. coli ssCueO fused to Δ(1-22)PhoA in pBRGC.1 | This study |
| pssSufI-PhoA | E.coli ssSufI fused to Δ(1-22)PhoA in pBRGC.1 | This study |
| pCueO | E. coli cueO gene in pBRGC.1 | This study |
| pSufI | E. coli sufI gene in pBRGC.1 | This study |
| pMalE | E. coli malE gene in pBRGC.1 | This study |
| pDsbA | E. coli dsbA gene in pBRGC.1 | This study |
| pAg85A | M. tuberculosis Rv3804c in pBRGC.1 | This study |
| pAg85C | M. tuberculosis Rv0129c in pBRGC.1 | This study |
| pModD | M. tuberculosis Rv1860 in pBRGC.1 | This study |
| pPepA | M. tuberculosis Rv0125 in pBRGC.1 | This study |
| pVV16 | Hmr, Kmr | Ref. 45 |
| pVV-GC.1-FH | Gateway cassette RfC.1 in pVV16; introduces C-terminal FLAG and His6 epitope tags | This study |
| pVV-Ag85A-FH | M. tuberculosis Rv3804c in pVV-GC.1-FH | This study |
| pVV-Ag85C-FH | M. tuberculosis Rv0129c in pVV-GC.1-FH | This study |
| pVV-ModD-FH | M. tuberculosis Rv1860 in pVV-GC.1-FH | This study |
| pVV-PepA-FH | M. tuberculosis Rv0125 in pVV-GC.1-FH | This study |
| pVV-BlaC-FH | M. tuberculosis Rv2068c in pVV-GC.1-FH | This study |
| pSALect | Apr, Cmr | Ref. 80 |
| pMCS-ΔssBlaC | Δ(1-31)BlaC cloned in pSALect | This study |
| pVV-ssAg85A-BlaC-FH | ssAg85A fused to Δ(1-31)BlaC in pVV16 | This study |
| pVV-ssAg85C-BlaC-FH | ssAg85C fused to Δ(1-31)BlaC in pVV16 | This study |
| pVV-ssModD-BlaC-FH | ssModD fused to Δ(1-31)BlaC in pVV16 | This study |
| pVV-ssPepA-BlaC-FH | ssPepA fused to Δ(1-31)BlaC in pVV16 | This study |
Genetic disruption of DHB4 and DR473 cells was achieved using P1vir transducing phage. Briefly, donor cells containing the gene of interest with a selectable marker were grown in LB broth at 37 °C overnight and subcultured into fresh medium containing 5 mm CaCl2, 10 mm MgSO4, and 0.2% glucose at a 100-fold dilution and allowed to grow for 1.5 h at 37 °C. Lytic phage was then added, and the culture was grown until “clearing” occurred, after which the phage was harvested through centrifugation and stored at 4 °C with chloroform. Recipient DHB4 or DR473 cells were grown in appropriate antibiotics at 37 °C overnight and centrifuged at 5,000 × g for 3 min. Cells were then resuspended in fresh medium containing 5 mm CaCl2, 10 mm MgSO4, and 0.2% glucose, and lytic phage carrying the marked gene of interest was added at a 10-fold dilution. Cells and phage were grown at 37 °C for 30 min, subsequently supplemented with 100 mm sodium citrate, grown for an additional 1 h, and plated on LB agar containing the appropriate antibiotics and 100 mm sodium citrate overnight. Cells containing the genetic disruption were recovered, and the resistance marker was removed using the pCP20 helper plasmid, as previously described (44).
Construction of the plasmid pBR322-Gm was carried out by replacement of the Tcr cassette in pBR322 with the gentamycin resistance gene. pBR322-Gm was then converted into a destination vector with an insertion of the Gateway RfC.1 cassette (Invitrogen) into the SspI/ScaI sites, creating the vector pBRGC.1. Plasmid pVV16 (45) was converted into a destination vector named pVV-GC.1-FH by insertion of a modified version of the Gateway RfC.1 cassette containing a FLAG affinity epitope at the 3′-end into the NdeI/HindIII sites. All derivatives of pVV-GC.1-FH and pBRGC.1 were created using Gateway cloning technology according to the manufacturer's protocols (Invitrogen). The plasmids pCueO-AP and pSufI-AP were constructed by first inserting the 1,412-bp E. coli phoA gene with a modified 5′-end to include a mini multicloning site (NheI, PsiI, and XhoI sites) into the HindIII/SalI sites of pBR322, yielding vector pMCS-ΔssPhoA. Then DNA encoding E. coli cueO or E. coli sufI was PCR-amplified and inserted in the NheI/PsiI sites pMCS-ΔssPhoA. Plasmid pssMBP-PhoA was constructed by PCR amplification of the first 143 bp of malE from pMalE and insertion of the resulting product into the HindIII/XhoI sites of pMCS-ΔssPhoA. Plasmid pMCS-ΔssBlaC was constructed by inserting an 864-bp PCR product corresponding to M. tuberculosis blaC lacking the first 93 bp but with a 33-bp FLAG epitope tag added to the 3′-end into the SpeI/EcoRI sites of pSALect. To construct plasmid pVV-ssAg85A-BlaC-FH, a 120-bp PCR product encoding the M. tuberculosis Ag85A signal peptide was first cloned into the SalI/SpeI sites of pMCS-ΔssBlaC. Then a 14-bp ribosome binding site was appended to the 5′-end of the DNA encoding Ag85A-BlaC via PCR, and the entire 1010-bp construct was cloned into the NdeI/HindIII sites of pVV16. This same procedure was repeated to create pVV-ssAg85C-BlaC-FH, pVV-ssModD-BlaC-FH, and pVV-ssPepA-BlaC-FH by inserting DNA encoding the signal peptides (72, 117, and 96 bp, respectively) inserted at the 5′-end of ΔssBlaC. Plasmid pBR-BamHI-Gm was constructed by inserting the BamHI restriction site into the SspI/PstI sites of pBR322-Gm.
Subcellular Fractionation—Cytoplasmic and periplasmic fractions were prepared from cells that had been induced for protein expression at 30 °C for 8 h in the presence of either arabinose or glucose, as indicated. Following protein induction, cells were pelleted by centrifugation at 3,000 × g for 15 min at 4 °C and then subjected to the ice-cold osmotic shock procedure as previously described (46). The quality of all fractionations was determined by immunodetection of the cytoplasmic GroEL protein (41).
Western Blot Analysis—Proteins were separated by SDS-PAGE, and Western blotting was performed as described previously (47). Briefly, all lanes of SDS-12% polyacrylamide gels (Bio-Rad) were loaded with samples prepared from an equivalent number of cells harvested for each experiment. The following primary antibodies were used: monoclonal mouse anti-PhoA (Sigma) diluted 1:20,000; monoclonal mouse anti-FLAG (Stratagene) diluted 1:3,000, and polyclonal anti-GroEL (Sigma) diluted 1:10,000. Secondary antibodies were either goat anti-mouse (Promega) or goat anti-rabbit (Promega) diluted 1:2,500. Following development of blots using the Immun-Star horseradish peroxidase substrate kit (Bio-Rad) and visualized using x-ray film (Eastman Kodak Co.), membranes were stripped in a solution consisting of 2.0% SDS, 7.0% β-mercaptoethanol, 0.03% NaCl, and 0.0025% Tris, reblocked, and probed with anti-GroEL antibody. Monitoring of PhoA Activity in Intact Cells—E. coli cells were grown in LB supplemented with appropriate antibiotics at 37 °C overnight and streaked onto LB agar supplemented with appropriate antibiotics, 50 μg/ml 5-bromo-4-chloro-3-indolyl phosphate (Sigma), and 0.2% arabinose or 0.2% glucose and grown at 30 °C for 2 days. Streaks that attained a blue phenotype were classified as export competent, whereas cells that appeared white/colorless were classified as incapable of PhoA export from the cytoplasm.
Isolation of Mini-Tn5 phoA Insertions—SM10 λ-pir cells carrying pUTphoA and MC4100 cells carrying the expression vector of interest were grown overnight at 37 °C in LB supplemented with appropriate antibiotics. Cells were then pelleted, washed three times with fresh LB, mixed, and spotted in 40-μl aliquots onto a nitrocellulose membrane on LB agar and grown at 30 °C for 16 h. The membrane was then resuspended in 10 ml of fresh LB and used to inoculate 200 ml of LB supplemented with 300 μg/ml kanamycin and 10 μg/ml gentamycin, followed by growth at 37 °C for 16 h. Upon conjugation, ∼104 recipient cells containing both the marked transposon and the expression vector of interest were selected and pooled together. Plasmid DNA was then extracted from these cells and used to transform competent DR473 cells that were grown on LB agar supplemented with appropriate antibiotics, 50 μg/ml BCIP, and either 0.2% glucose or 0.2% arabinose at 30 °C for 2 days. For each library, 12 colonies that exhibited a strong blue phenotype were restreaked onto the same medium and grown at 30 °C for 2 days to confirm the phenotype. Plasmid DNA was then prepared from all positive clones and sequenced using a primer specific for the antisense strand of the 5′-end of the phoA gene.
Construction and Screening of Genomic Library—Genomic DNA was isolated from MC4100 cells and partially digested using Sau3AI, as previously described (39). Fragments between 0.75 and 3.0 kb were excised from a 1% agarose-Tris borate-EDTA gel and gel-purified (Qiagen) and then ligated into the BamHI site of pBR-BamHI-Gm. This library of genomic DNA was then electroporated into competent MC4100 cells and conjugated with SM10 λ-pir cells carrying pUTphoA as described above. The resulting library, composed of transposon insertions into genomic DNA, was isolated and electroporated into competent DR473 cells. Screening of the library was performed on BCIP indicator plates supplemented with either glucose or arabinose as described above. Cells displaying a bright blue phenotype were reconfirmed, after which their plasmid DNA was isolated and sequenced using a primer specific for the antisense strand of the 5′-end of the phoA gene.
Bioinformatic Prediction of Tat Substrates—Analysis was carried out as previously described (3). Briefly, a hidden Markov model (HMM) was developed using a training set constructed from experimentally confirmed Tat substrates present in E. coli and P. aeruginosa. The previously developed HMM for Tat motifs using hmmbuild (available on the World Wide Web) was calibrated with hmmcalibrate and used to search the annotated proteins from the chromosome of M. tuberculosis H37Rv (GenBank™ accession number NC_000962.2) with hmmsearch. Signal peptides were restricted to fall within the first 50 amino acids of the given protein sequence, and all putative signals were cross-checked using SignalP 3.0 (1) and TatP 1.0 (2).
Antimicrobial Susceptibility Testing—M. smegmatis cells were grown for 2 days at 37 °C in Middlebrook 7H9 medium and appropriate antibiotics as outline above. Cultures were then diluted 1000-fold in fresh medium, and 5 μl were pipetted onto LB agar supplemented with 0.2% glycerol and 75 μg/ml carbenicillin (Sigma). Cells were then grown at 37 °C for 4 days, and growth was determined by single colony formation within spots.
RESULTS
PhoA-based Screen for Discriminating Export Pathway Specificity—First, we sought to develop an experimental strategy using a Tn5-based phoA derivative for unbiased determination of pathway specificity conferred by N-terminal signals. To accomplish this, we exploited a limited collection of E. coli mutant strains that allow protein oxidation in the cytoplasm or, conversely, disable protein oxidation in the periplasm (41). Specifically, strain DR473 (Table 1) used in this study carries deletions in the genes trxB (thioredoxin reductase) and gor (glutaredoxin reductase) such that the cytoplasm of these cells favors the oxidation of protein thiols (41, 48). In addition, these cells lack phoA and have a chromosomal copy of the trxB gene under control of an arabinose-inducible promoter such that the addition of arabinose or glucose can be used to effectively toggle the cytoplasm as a reducing or oxidizing environment, respectively. In the presence of arabinose, this strain background is designated C:red/P:ox, because the cytoplasm is relatively reducing, whereas the periplasm is oxidizing. In the presence of glucose, the strain is designated C:ox/P:ox, since both the cytoplasm and periplasm are oxidizing. Following introduction of the dsbA::kan allele into DR473, protein oxidation in the periplasm is impaired, and the resulting strain is designated either C:red/P:red or C:ox/P:red, depending on whether arabinose or glucose, respectively, is supplemented (41). Finally, DR473 derivatives carrying an insertional deletion in the gene encoding the SecB chaperone or the essential Tat translocase component TatC were used to inactivate specific targeting routes.
We hypothesized that the use of these different strains and growth conditions in combination with the redox-dependent folding behavior of PhoA (41, 43) would reveal targeting specificity for different signal peptide-PhoA fusions, as shown schematically in Fig. 1b. For instance, we reasoned that in C:red/P:ox cells, PhoA with its native export signal (X represents PhoA in Fig. 1b) would be efficiently exported in a post-translational, SecB-independent manner, where rapid oxidation by DsbA in the periplasm would catalyze formation of the two disulfide bonds that are critical for the stability and activity of PhoA (43) (Fig. 1b). To test this, we monitored in vivo export of native PhoA in C:red/P:ox and C:red/P:red cells grown on LB agar plates supplemented with arabinose and BCIP, a colorimetric substrate that emits a visible blue color when hydrolyzed by PhoA. Since BCIP diffusion through the inner membrane of E. coli is very inefficient4 (see below), it is an excellent indicator of PhoA activity in the E. coli periplasm or extracellular medium. As expected, the C:red/P:ox cells exhibited a strong blue phenotype (Fig. 2a), whereas the C:red/P:red cells exhibited a colorless phenotype (Fig. 2a), consistent with the known DsbA dependence of PhoA folding and activity (49). The same construct was expressed in C:red/C:ox SecB_ cells and the blue phenotype remained (Fig. 2a), confirming that export of PhoA proceeded in a SecB-independent manner (50). For comparison, we assayed a fusion between mature PhoA and the 26-residue signal peptide derived from the SecB-dependent substrate maltose-binding protein (MBP; encoded by the malE gene) (27). As expected, export was SecB-dependent, because the expression of ssMBP-PhoA in C:red/C:ox cells elicited a strong blue phenotype that was completely abolished in cells lacking SecB (Fig. 2a).
FIGURE 2.
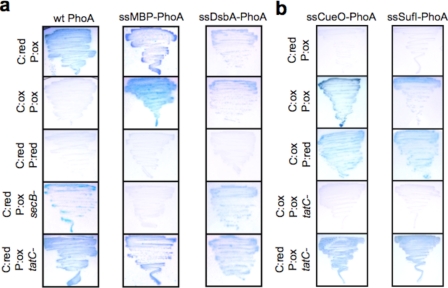
PhoA export monitored on BCIP indicator plates. a, growth of DR473 or DR473 mutant cells deficient in dsbA, secB, or tatC as indicated after 48 h at 30 °C on agar plates supplemented with BCIP and either arabinose (Ara+) or glucose (Glu+) as indicated. Cells were induced to express (from left to right) (i) native PhoA, (ii) the first 26 amino acids of MBP fused to the mature domain of PhoA, or (iii) the first 19 amino acids of DsbA fused to the mature domain of PhoA. b, growth of DR473 or DR473 mutant cells deficient in dsbA, secB, or tatC as indicated after 48 h at 30 °C on agar plates supplemented with BCIP and either arabinose or glucose, as indicated. Cells were induced to express either the first 29 amino acids of CueO (left) or the first 27 amino acids of SufI (right) fused to the mature domain of PhoA. wt, wild type.
Since native PhoA does not require the antifolding activity of SecB prior to export, we hypothesized that PhoA might fold prematurely in C:ox cells and fail to be exported. Indeed, expression of native PhoA in C:ox/P:ox cells resulted in colonies that appeared colorless, suggesting that the C:ox environment rendered PhoA translocation-incompetent. An identical colorless phenotype was observed in C:ox/P:ox secB- cells (data not shown), confirming that premature folding of PhoA and not SecB binding was the cause of translocation incompetence in the C:ox environment. On the contrary, we observed that C:ox/P:ox cells expressing ssMBP-PhoA produced a strong blue phenotype (Fig. 2a), suggesting that binding of ssMBP-PhoA by SecB is sufficient to maintain the protein in an export-competent conformation even in an oxidizing folding environment. As expected for Sec-dependent targeting signals, expression and localization of native PhoA and ssMBP-PhoA were unaffected by a deletion in tatC (Fig. 2a) that is known to completely abolish export via the Tat export pathway (51). In all of the above cases, Western blot analysis was used to confirm that the phenotypic data correctly reported the subcellular localization of each PhoA fusion (shown in Fig. 3a for native PhoA). As a control, mature PhoA lacking a signal peptide was expressed in C:red/P:ox and C:ox/P:ox cells. This resulted in a colorless phenotype (data not shown), indicating lack of export and confirming that the BCIP indicator was incapable of diffusing into the cytoplasm under the conditions tested here. Western blot analysis of subcellular fractions generated from these same cells confirmed the cytoplasmic location for mature PhoA irrespective of the cytoplasmic redox status (Fig. 3b).
FIGURE 3.
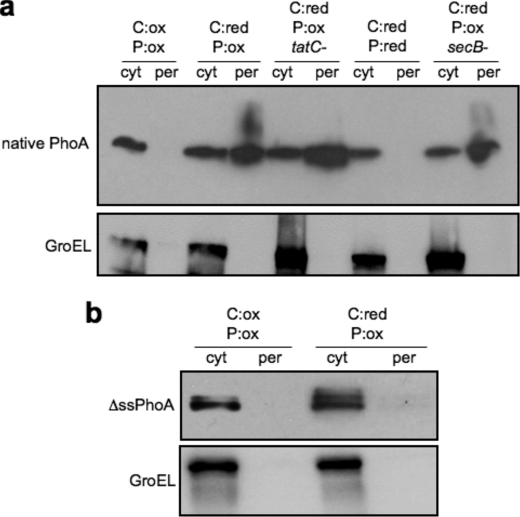
Subcellular distribution of full-length and truncated PhoA. a, subcellular fractionation of DR473 or DR473 mutant cells deficient in tatC, dsbA, or secB, as indicated, expressing native PhoA. Cells were grown in either the presence of arabinose (Ara+) or glucose (Glu+), as indicated, and cytoplasmic (cyt) and periplasmic (per) samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL. b, cytoplasmic and periplasmic fractions from DR473 cells expressing the mature domain of PhoA, lacking its native signal peptide and with an artificial initiation codon, grown in the presence of arabinose or glucose, as indicated, and immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL.
To further explore the ability of our PhoA-based screen to discriminate signal peptides based on their specific mode of export, we evaluated the phenotype of cells expressing a chimera between the E. coli DsbA signal peptide (ssDsbA) and PhoA. It was previously reported that ssDsbA is capable of routing passenger proteins, such as E. coli thioredoxin-1, to the co-translational SRP-dependent pathway (32). Thus, we hypothesized that co-translational export of ssDsbA-PhoA would effectively bypass the cytoplasm and, as a result, the chimera would be unaffected by the cytoplasmic redox status. In agreement with this notion, both C:red/P:ox and C:ox/P:ox cells expressing ssDsbA-PhoA appeared blue when grown on BCIP indicator plates (Fig. 2a). As expected for SRP-dependent export, the blue phenotype was unaffected by the absence of SecB or TatC, but deletion of DsbA resulted in the complete absence of blue coloration (Fig. 2a).
We next sought to develop our PhoA-based screen for verification of Tat-dependent protein export. As mentioned above, export of PhoA by the Tat pathway is observed only in strains that enable oxidative protein folding in the cytoplasm (41). This was corroborated by the observation that expression of fusions between mature PhoA and Tat-specific signal peptides derived from either E. coli CueO or SufI (ssCueO-PhoA or ssSufI-PhoA, respectively) in C:ox/P:ox cells conferred a blue phenotype (Fig. 2b). In a reduced, incorrectly folded state, PhoA is unable to be translocated, because, with few exceptions, the Tat pathway appears to export only native or native-like proteins (40, 41, 52). Indeed, C:red/P:ox cells expressing the same constructs resulted in a colorless phenotype, consistent with the fact that reduced PhoA is incompetent for Tat export (41). Unlike the SRP- or Sec-dependent PhoA fusions, expression of the ssCueO-PhoA and ssSufI-PhoA constructs in C:ox/P:red dsbA- cells resulted in a blue phenotype (Fig. 2b), indicating that oxidative folding of Tat-targeted PhoA occurs in the cytoplasm and is not dependent on DsbA for folding or activity (41). As expected, neither ssCueO-PhoA nor ssSufI-PhoA were localized to the periplasm in tatC- cells (Fig. 2b). Unexpectedly, in C:red/P:ox tatC- cells, both ssCueO-PhoA and ssSufI-PhoA were localized to the periplasm in an active conformation (Fig. 2b). This result suggests that in the absence of TatC, which has been shown to be the primary recognition component for Tat signal peptides (53), reduced PhoA is exported in a Tat-independent manner, perhaps via the Sec pathway, as suggested in previous studies (41, 54). However, this promiscuity was never observed when TatC was present or when PhoA was expressed under C:ox conditions, suggesting that both TatC and the folded state are specificity determinants in Tat-dependent PhoA export (see below for more details).
A Transposon-based Strategy for Isolating Export Pathway-specific PhoA Fusions—Collectively, the above data reveal a logic table for PhoA pathway specificity in DR473 and its derivatives grown in the presence of arabinose or glucose (Table 2; see also Fig. 1b). Based on this logic, we hypothesized that judicious selection of strain and growth conditions could be employed to isolate signal peptides according to their specific mode of inner membrane export. To test this notion, a library of random fusions of the gene encoding the SecB-dependent substrate MBP and the phoA gene were generated by mini-Tn5 phoA insertions (55). For mini-Tn5 phoA-generated hybrid proteins to have high enzymatic activity, the phoA gene must be fused to a target gene coding for a signal that promotes protein export (7, 55). To increase our chances of success, we “relaxed” the screening stringency by expressing the MBP-PhoA hybrids in C:ox/P:ox cells, a combination of host and growth conditions that can potentially yield signals for SecB-, SRP-, and Tat-dependent export (see Table 2). Following screening of cells on BCIP indicator plates supplemented with glucose, 12 blue colonies were isolated at random, each of which encoded an inframe fusion between the first 372 amino acids of MBP and the N terminus of TnphoA (ssMBP372-PhoA). Because library screening in C:ox/P:ox cells can produce clones directing SecB-, SRP-, or Tat-specific export, we further tested ssMBP372-PhoA in C:ox/P:ox cells lacking SecB or TatC and observed a colorless phenotype and blue phenotype, respectively (data not shown). Furthermore, Western blot analysis confirmed that ssMBP372-PhoA was localized in the periplasm when expressed in either C:ox or C:red cells (Fig. 4a). Collectively, these experiments revealed the potential of our strategy to isolate SecB-dependent substrates from transposon libraries.
TABLE 2.
PhoA pathway specificity in DR473 derivatives grown on glucose or arabinose
|
Genotypea |
PhoA
phenotypeb |
|||
|---|---|---|---|---|
| Native PhoA (SecB-independent) | ssMBP-PhoA (SecB-dependent) | ssDsbA-PhoA (SRP-dependent) | ssCueO/ssSufI-PhoA (Tat-dependent) | |
| C:red/P:ox | + | + | + | − |
| C:red/P:red | − | − | − | − |
| C:red/P:ox secB− | + | − | + | − |
| C:red/P:ox tatC− | + | + | + | − |
| C:ox/P:ox | − | + | + | + |
| C:ox/P:red | − | − | − | + |
| C:ox/P:ox secB− | − | − | + | + |
| C:ox/P:ox tatC− | − | + | + | − |
| C:ox/P:ox secB−tatC−c | − | − | + | − |
Arabinose and glucose were used to render DR473 cells as C:red and C:ox, respectively.
PhoA phenotype scored as blue (+) or white (−), as determined by plating cells on BCIP indicator plates (see “Experimental Procedures”).
Hypothetical genotype not tested in this study.
FIGURE 4.
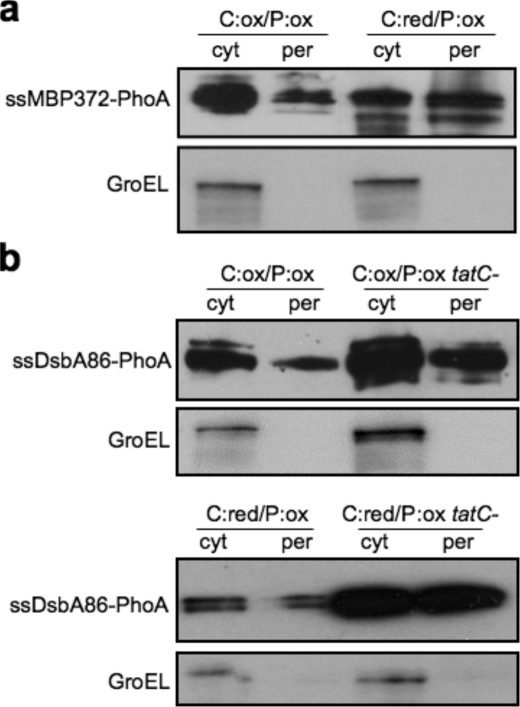
Sec- and SRP-dependent export of PhoA fusions. a, subcellular fractionation of DR473 cells expressing a chimeric fusion between the first 372 amino acids of MBP and mature PhoA. Cells were grown in either the presence of arabinose (Ara+) or glucose (Glu+), as indicated; cytoplasmic (cyt) and periplasmic (per) samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL. b, subcellular fractionation of DR473 or DR473 cells deficient in tatC expressing a chimeric protein fusion of the first 86 amino acids of DsbA to the N terminus of the mature domain of PhoA. Cells were grown in the presence of arabinose or glucose; cytoplasmic and periplasmic samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL.
To further explore the ability of our Tn-based approach to isolate signal peptides based on pathway specificity, we next sought to identify SRP-dependent export signals. This was achieved by generating random fusions between the gene encoding DsbA and the phoA gene using mini-Tn5 phoA and screening the resulting DNA library in C:ox/P:ox tatC- cells. According to Table 2, this combination of strain and growth conditions was more stringent than that used above as it could only produce SecB- or SRP-dependent export signals. Upon screening, we selected 12 blue colonies at random and found that each of these clones expressed an identical fusion comprised of the first 86 amino acids of DsbA fused in-frame to the N terminus of mature PhoA (ssDsbA86-PhoA). To verify whether this was an SRP-dependent export signal, we screened this clone in cells lacking SecB and found that, unlike ssMalE372-PhoA, the ssDsbA86-PhoA fusion conferred a blue phenotype to C:ox/P:ox secB- cells grown on glucose (data not shown). Western blot analysis further confirmed that ssDsbA86-PhoA localized in the periplasm regardless of the cytoplasmic oxidation state of DR473 cells (Fig. 4b). As expected for an SRP export signal, the absence of a functional Tat pathway did not prevent translocation of ssDsbA86-PhoA to the periplasm (Fig. 4b). These experiments revealed the potential to isolate SecB-independent signal peptides from transposon libraries.
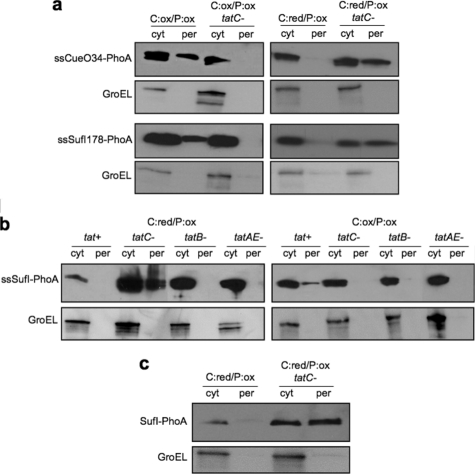
Isolation of Tat-dependent PhoA Fusions—As a final test of our transposon screening approach, we sought to isolate signal peptides for the Tat pathway. This represented a significant challenge because Tat export signals have largely been precluded from TnphoA-based screens (e.g. see Ref. 39) an observation that is best explained by the fact that PhoA is unable to obtain a correctly folded, Tat export-competent conformation in the cytoplasm of wild type bacteria (41). However, as seen above, C:ox/P:ox cells can efficiently translocate fusions between Tat signal peptides and mature PhoA in a Tat-specific manner. Encouraged by this finding, we used mini-Tn5 phoA to generate two libraries of random fusions between the gene encoding either CueO or SufI and the phoA gene and screened each of these libraries in C:ox/P:ox cells. Two unique clones were isolated, namely ssCueO34-PhoA and ssSufI178-PhoA. As mentioned above, screening C:ox/P:ox cells can yield SecB-, SRP-, and Tat-dependent export. Therefore, we tested ssCueO34-PhoA and ssSufI178-PhoA in the C:ox/P:red genotype, which can be used to easily discriminate between Sec/SRP and Tat signals (see Table 2). Consistent with Tat-dependent export, both ssCueO34-PhoA and ssSufI178-PhoA conferred a blue phenotype to C:ox/P:red cells (data not shown). Western blot analysis of subcellular fractions confirmed that these clones were localized to the periplasm only when TatC was present (Fig. 5a). In C:red/P:ox cells, ssCueO34-PhoA and ssSufI178-PhoA were not exported (Fig. 5a). Interestingly, as was seen above for ssCueO-PhoA and ssSufI-PhoA, Tat-independent export was observed for these two clones when each was expressed in cells with a reducing cytoplasm (C:red) that also lacked TatC (Fig. 5a). Although ssCueO and ssSufI have been shown to display some pathway promiscuity (41, 54), the specific contribution of TatC and of the folding state to this promiscuous export had not previously been reported. This phenomenon was explored in greater detail below.
FIGURE 5.
Tat-dependent export of PhoA fusions. a, subcellular fractionation of DR473 or DR473 mutant cells deficient in tatC expressing a protein fusion composed of either the first 34 amino acids of CueO (left) or the first 178 amino acids of SufI (right) and the mature domain of PhoA. Cells were grown in either the presence of arabinose (Ara+) or glucose (Glu+); cytoplasmic (cyt) and periplasmic (per) samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL. b, subcellular fractionation of DR473 or DR473 cells deficient in either tatC, tatB, or tatA/E, as indicated, expressing a protein fusion between the first 27 amino acids of SufI and the mature domain of PhoA. Cells were grown in either the presence of arabinose or glucose; cytoplasmic and periplasmic samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL. c, subcellular fractionation of DR473 or DR473 mutant cells deficient in tatC expressing a protein fusion between native SufI and the mature domain of PhoA. Cells were grown in the presence of arabinose, and cytoplasmic and periplasmic samples were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL.
Tat Pathway Specificity Is Maintained by the Substrate Binding Component TatC—Our rather surprising observation that Tat-targeted PhoA constructs were exported in a Tat-independent manner when both (i) PhoA was reduced and (ii) TatC was absent from cells, is probably due to the fact that TatC is the initial docking site for ssCueO-PhoA and ssSufI-PhoA. In its absence, both of these substrates can be rerouted to the Sec pathway, provided they are in a Sec export-competent conformation (i.e. reduced). If TatC were indeed the docking site, we reasoned that Tat-independent export of ssCueO-PhoA and ssSufI-PhoA, presumably via the Sec pathway, would only be observed in tatC- cells. In contrast, Tat-deficient mutants lacking TatB or TatA/E would not be expected to exhibit Tat-independent export of ssCueO-PhoA and ssSufI-PhoA, because, despite the inability of these cells to complete the entire Tat export process, the presence of the TatC component would ensure initial targeting of PhoA to the Tat pathway (53, 56, 57). Recently, in vivo genetic studies have yielded suppressor mutants of TatC that permit export of noncanonical Tat signals, further underscoring its role in signal peptide docking (58, 59). As seen in Fig. 5b, growth of C:red/P:ox tatC- cells resulted in Tat-independent export of ssSufI-PhoA, whereas C:red/P:ox tatB- and tatA/E- cells were incapable of localizing ssSufI-PhoA to the periplasm. For comparison, when the same Tat-deficient strains were grown on glucose (C:ox), no detectable PhoA export was observed (Fig. 5b), highlighting the fact that only reduced, and not oxidized, PhoA is capable of Tat-independent export. To determine whether the observed Tat-independent export was due to the lack of specificity cues that might be contained within the mature region of SufI, we created an in-frame fusion between the entire coding region of sufI and the sequence encoding the mature region of PhoA. As shown in Fig. 5c, C:red/P:ox cells expressing SufI-PhoA exhibited the same Tat-independent export as seen above for ssSufI-PhoA. Thus, taken together, our results suggest that Tat targeting specificity is maintained by the signal peptide binding site formed by TatC, the folding state of PhoA (oxidized versus reduced), and the N-terminal signal peptide but does not appear to be affected by regions within the mature SufI protein.
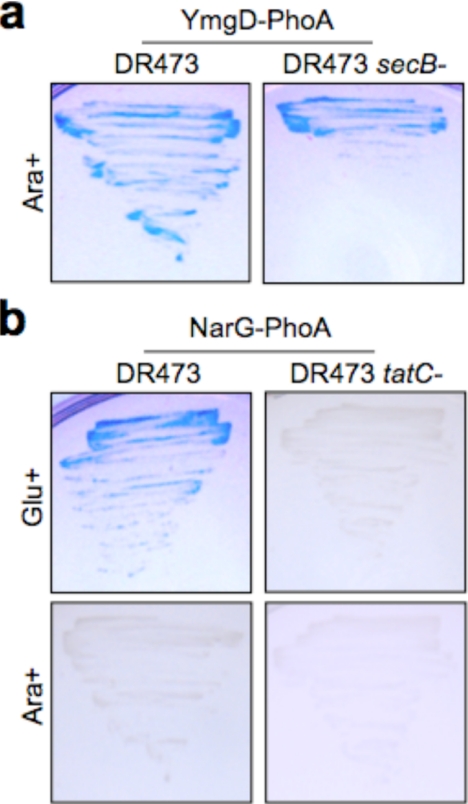
Genome-wide Identification of E. coli Export Signals Specific for a Given Pathway—To determine whether the method holds promise for genome-wide, unbiased screening of signal sequences specific for a given pathway, we constructed a library of random E. coli DNA fragments fused to TnphoA. DR473 cells were transformed with this library and plated on arabinose (C:red/P:ox) such that the cytoplasm was reducing. Recall that DR473 cells with a reducing cytoplasm allow for identification of Sec substrates exported in either a SecB-dependent or independent manner as well as SRP substrates but not Tat substrates (Fig. 1b and Table 2). Using these screening conditions, we identified five in-frame fusions to PhoA that were able to promote export as indicated by a strong blue phenotype when cells were grown on BCIP indicator plates. The five identified genes were (i) atoS, (ii) ydjX, (iii) yfhR, (iv) yjgX, and (v) ymgD. Consistent with the screening conditions, all five hits were predicted to be either integral membrane proteins (atoS, ydjX, yfhR, and yjgX) according to the TMHMM membrane protein topology prediction method of Sonnhammer and co-workers (61) or to carry N-terminal Sec export signals (ymgD) according to the signal peptide prediction tool SignalP 3.0 (1). As expected, no Tat substrates were identified. Moreover, atoS and ydjX were previously shown to encode for integral inner membrane proteins with two and five transmembrane helices (60). In contrast, the ymgD clone, which does not contain any transmembrane helices, was determined to be a SecB-independent substrate according to BCIP screening of secB+ and secB- cells expressing the YmgD-PhoA fusion (Fig. 6a). Importantly, all five clones were independently confirmed to localize PhoA out of the cytoplasm by blue/white screening and Western blot analysis (data not shown).
FIGURE 6.
Genome-wide screen for E. coli export signals yields YmgD and NarG. a, growth of DR473 and DR473 secB- cells expressing YmgD-PhoA after 48 h at 30 °C on agar plates supplemented with BCIP and arabinose (Ara+) to render the cytoplasm C:red. b, growth of DR473 and DR473 tatC- cells expressing NarG-PhoA after 48 h at 30 °C on agar plates supplemented with BCIP and either glucose (Glu+) or arabinose, as indicated.
The same DR473 cell library was also screened on glucose (C:ox/P:ox), which we expected would yield signals for SecB-dependent, SRP-dependent, and Tat-dependent export (Fig. 1b and Table 2). However, SecB-independent translocation of PhoA protein fusions would be excluded under these conditions. Upon screening, four in-frame fusions to PhoA were identified that corresponded to (i) narG, (ii) wzc, (iii) ybhR, and (iv) yqjF. As expected, three of these hits are inner membrane proteins: wzc encodes a membrane-bound protein-tyrosine kinase with two transmembrane helices (62); ybhR encodes an ABC transporter with six membrane helices (60); and yqjF is a quinol oxidase subunit with four transmembrane helices (60). The fourth clone, narG, encodes the α-subunit of nitrate reductase A (NarGHI) that was previously shown to be exported out of the cytoplasm via the Tat pathway (63). Indeed, NarG was found to export PhoA in a Tat-dependent manner (Fig. 6b). Interestingly, it did not exhibit the same promiscuous export as was seen for all the other Tat signals expressed in C:red/P:ox tatC- cells (Fig. 6b). It is noteworthy that this protein possesses only the vestige of a twin arginine motif (MSKFLDRFRYFKQKGET...; where the noncanonical Tat motif is underlined) (63), and as a result, it has escaped detection by current bioinformatic tools (54). Thus, the identification of the Tat substrate NarG exemplifies the potential of our approach for uncovering unexpected, noncanonical export signals that would otherwise be missed with classical TnphoA-based screening or bioinformatic analysis. As above, all four clones were independently confirmed to localize PhoA out of the cytoplasm (data not shown).
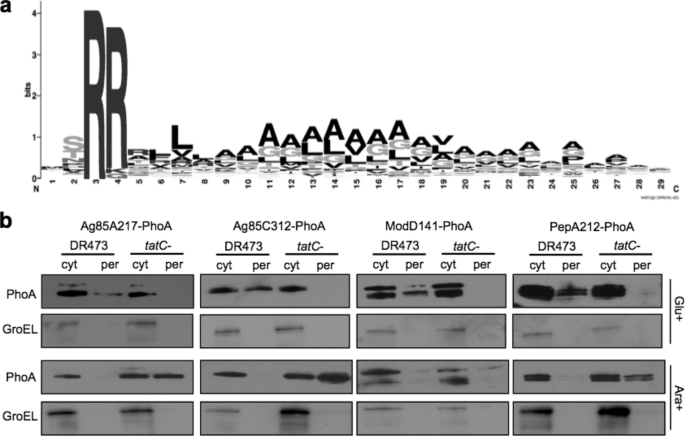
Discovery of Novel M. tuberculosis Tat Export Signals—Based on our ability to detect E. coli export signals, we next sought to determine whether our assay could be used to experimentally verify Tat signal peptides from other bacterial hosts. Due to the important role that protein secretion systems, including the Tat export pathway, play in the virulence of bacterial pathogens (64, 65), we set out to identify Tat-targeting sequences from the genome of the Gram-positive human pathogen M. tuberculosis. Both the M. tuberculosis and M. smegmatis genomes contain open reading frames with homology to components of the Tat export system (TatABC), and the Tat system is apparently essential in M. tuberculosis (66, 67).5 However, to date, no more than five Tat-dependent substrates have been reported for mycobacteria (66, 68, 69) despite the fact that the genomes of M. smegmatis and M. tuberculosis are predicted to encode 49 (69) and, as determined by our own bioinformatic analysis, 61 (supplemental Table S1) putative Tat substrates, respectively. It is noteworthy that several of the M. tuberculosis Tat substrates predicted by our analysis have been experimentally confirmed as authentic Tat substrates, including BlaC, PlcA, PlcB, and Rv2525c (66, 68). Alignment of the entire set of M. tuberculosis substrates and comparison of amino acid frequencies reveals a prototypical Tat motif followed immediately by a moderately hydrophobic alanine-rich h-region (Fig. 7a). Of the 61 predicted Tat substrates in M. tuberculosis, prior proteomic analysis identified four of these, namely Rv0125 (PepA), Rv0129c (Ag85C), Rv1860 (ModD), and Rv3804c (Ag85A), as extracellular proteins (70),6 suggesting that a subset of secreted M. tuberculosis proteins are Tat substrates. We hypothesized that Tat substrates secreted to the culture medium were likely to be virulence factors and thus opted to test these four candidates in our PhoA-based screen. Using the same procedure as outlined earlier, random fusions of the candidate M. tuberculosis genes and the phoA gene were generated by mini-Tn5 phoA insertions. C:ox/P:ox cells were screened, and at least one unique clone encoding an in-frame fusion between an M. tuberculosis targeting signal and the N terminus of mature PhoA was recovered for each of the four candidates. Individually these were Ag85A217-PhoA, Ag85C312-PhoA, ModD141-PhoA, and PepA121-PhoA. Expression of these constructs in C:ox/P:ox tatC- cells confirmed that each was a bona fide Tat substrate (Fig. 7b). Consistent with prior results, export of each was blocked when the cytoplasm was rendered more reducing (Fig. 7b). As described in detail above, each of these fusions was exported in a Tat-independent manner when expressed in C:red/P:ox tatC- cells (Fig. 7b), indicating that this phenomenon was a general feature of Tat export signals and not exclusive to E. coli signals.
FIGURE 7.
Export signals from M. tuberculosis that direct Tat-dependent export of PhoA. a, sequence logo generated for the predicted Tat-dependent substrates found in M. tuberculosis using the HMM analysis. The vertical axis shows information content in bits, and the horizontal axis shows amino acid position. The height of each letter reflects the usage for that amino acid at that position. b, subcellular fractionation of DR473 and DR473 mutant cells deficient in tatC as indicated expressing protein fusions between (from left to right) (i) the first 217 amino acids of Ag85A and PhoA, (ii) the first 312 amino acids of Ag85C and PhoA, (iii) the first 141 amino acids of ModD and PhoA, and (iv) the first 212 amino acids of PepA and PhoA. Cytoplasmic (cyt) and periplasmic (per) samples from cells grown in the presence of glucose (Glu+; top set of blots) or arabinose (Ara+; bottom set of blots) were immunoblotted with monoclonal anti-PhoA followed by polyclonal anti-GroEL.
Finally, to confirm that each of the identified M. tuberculosis Tat signals could faithfully promote Tat export in a mycobacterial host, we tested each in a genetic selection using M. smegmatis cells (68). In this system, the M. tuberculosisβ-lactamase BlaC is used as a Tat-specific reporter based on (i) the fact that it is a native M. tuberculosis Tat substrate and (ii) its ability to confer resistance to β-lactam antibiotics when exported in M. smegmatis. A caveat is that an M. smegmatis strain lacking the BlaC homologue (BlaS) is required to eliminate the native β-lactam antibiotic resistance of M. smegmatis cells. Taken together, antibiotic selection of M. smegmatis blaS- cells expressing fusions between Tat signal peptides and the N terminus of mature BlaC provides a convenient method for verifying mycobacterial Tat substrates. To confirm the Tat dependence of Ag85A, Ag85C, ModD, and PepA in mycobacteria, a fusion of the Tat signal peptide derived from each of the substrates and mature BlaC was expressed in M. smegmatis. Following plating on 75 μg/ml carbenicillin, we observed that mc2155 ΔblaS cells carrying empty plasmid were sensitive to carbenicillin, whereas the same cells expressing a fusion between ssAg85A, ssAg85C, ssModD, or PepA and BlaC exhibited resistance to this level of carbenicillin (Table 3). When these fusions were expressed in Tat-deficient mc2155 ΔblaS ΔtatA cells, sensitivity to carbenicillin was observed (Table 3), confirming that these four proteins were authentic mycobacterial Tat substrates capable of directing Tat-dependent export in M. smegmatis.
TABLE 3.
Export of BlaC fusions in M. smegmatis is dependent on the Tat pathway
|
Strain |
Genotype |
Carbenicillin resistance for mycobacterial
vectora |
|||||
|---|---|---|---|---|---|---|---|
| No plasmid | pVV-BlaC-FH | pVV-ssAg85A-BlaC-FH | pVV-ssAg85C-BlaC-FH | pVV-ssModD-BlaC-FH | pVV-ssPepA-BlaC-FH | ||
| mc2155 | WTb | + | NDc | ND | ND | ND | ND |
| MB692 | ΔtatA | − | − | − | − | − | − |
| PM759 | ΔblaS | − | + | + | + | + | + |
| JM578 | ΔtatA ΔblaS | − | − | − | − | − | − |
Carbenicillin resistance was determined by the presence (+) or absence (−) of single colony growth after 4 days on LB-agar plates supplemented with 75 mg/ml carbenicillin.
WT, wild type.
ND, not determined.
DISCUSSION
In the present study, we have developed an experimental strategy using a Tn5-based transposon probe for the determination of pathway specificity conferred by N-terminal export signals. Facilitated by the creation of a logic table describing PhoA export pathway specificity in DR473 cells and mutant strains derived thereof (see Table 2), we were able to isolate export signals for all of the major modes of transport (Sec, SRP, or Tat) across the inner membrane of E. coli. In our library screening studies, we chose to use permissive screening conditions such that export signals for more than one export pathway were possible. In all cases, the true pathway specificity of each isolated signal was then easily resolved by a simple counter-screen using a different host strain and/or altered growth conditions. However, it should be noted that for the discovery of SRP- and Tat-dependent export signals, more stringent screening in C:ox/P:ox secB-tatC- and C:ox/P:red cells, respectively, could be used to restrict export to a single mode. We expect that our mini-Tn5 phoA strategy could also be used to identify genes encoding inner membrane proteins targeted via the SRP pathway. Indeed, initial screens for SecB-independent export uncovered a number of PhoA fusions to the tetracycline resistance protein TetA (data not shown), a known inner membrane protein with 12 transmembrane spans (71) that was initially encoded in the pBR322 plasmid but removed for this work.
An interesting observation was that for all of the mini-Tn5 phoA libraries made using target genes (e.g. DsbA), only a single unique clone was isolated in each case. Although it is currently unclear why so few unique clones were uncovered, we suspect that this was a result of the relatively small size of the random libraries (∼104 unique conjugation events) and the nonexhaustive screening of each library (only 12 hits were characterized from each library). A further limiting factor is that for a given gene, there are probably only a limited number of truncations that will be capable of efficiently localizing PhoA out of the cytoplasm. Of these, a “privileged” export signal might exist that effectively dominates the results under the conditions tested. We also cannot rule out the possibility that although the mini-Tn5 phoA can in theory insert into many sites in a gene, it may insert into target DNA sequences in a biased manner. Indeed, previous studies have documented that the Tn5 transposon preferentially inserts into regions of DNA termed “hotspots” (72, 73). A final possibility could be due to the generation of siblings during library construction; i.e. cells that carry a plasmid-based transposon insertion but are somehow unable to express the kanamycin resistance protein at a level sufficient to confer resistance to the high level of kanamycin present in the selection step may become nonviable or slow growing. This would cause library diversity to decrease because cells with a higher growth rate in liquid culture will be overrepresented in the library due to the presence of sibling cells.
Despite the lack of diverse hits isolated during our proof-of-concept screens, genome-wide screening for E. coli signals that promoted export in cells grown on arabinose (C:red/P:ox) and separately on glucose (C:ox/P:ox) yielded a total of nine unique clones. This suggests that the approach is indeed a robust method for isolating rare clones from relatively sparse libraries and, in doing so, is able to identify Sec, SRP, and Tat substrates on a global scale and in an unbiased manner. Interestingly, seven of nine of these hits were either single span or multispan integral membrane proteins, which was not entirely unexpected, since ∼25% of the E. coli proteome is predicted to be integral membrane proteins (60). The remaining two hits encoded a SecB-independent substrate (YmgD, isolated under C:red/P:ox conditions) and a Tat-dependent substrate (NarG, isolated under C:ox/P:ox conditions). The NarG clone was of particular interest for a number of reasons. First, isolation of NarG demonstrates the ability of our screening strategy to extend TnphoA-based screening to Tat substrates. This is significant, because Tat substrates are typically excluded from classical TnphoA screening experiments (39) due to the inability of the Tat machinery to export unfolded PhoA from the reducing cytoplasm of wild type bacteria (41). Second, NarG possesses a noncanonical Tat signal peptide in which an Arg-Phe-Arg sequence replaces the typical Arg-Arg motif that is present in the majority of Tat signal peptides characterized to date. Because nearly all bioinformatic algorithms for mining Tat substrates do a poor job of identifying signal peptides that deviate from the canonical primary structure, identification of NarG underscores the power of our approach for the discovery of noncanonical export signals that would otherwise be missed with bioinformatic analysis.
The broad utility of our approach was demonstrated by experimental verification of four secreted proteins from M. tuberculosis carrying putative Tat export signals. Specifically, using our mini-Tn5 phoA strategy, we confirmed that the signal peptides derived from M. tuberculosis Ag85A (antigen 85A), Ag85C (antigen 85C), ModD (Apa; MPT-32), and PepA (a putative serine protease) were each capable of directing Tat-dependent export of PhoA in E. coli. This provides additional evidence that Tat signals from diverse eubacterial species can functionally interact with the E. coli TatABC machinery (3) and suggests that E. coli is an ideal surrogate host for cross-species signal peptide discovery. Moreover, each of these mycobacterial signals was also capable of efficiently exporting M. tuberculosis BlaC in M. smegmatis host cells, indicating that these are indeed bona fide Tat substrates. The significance of this finding is underscored by the fact that secreted mycobacterial antigens are of particular significance in the induction of various immune responses that are responsible for the development of protective immunity or clinical symptoms and complications of disease. For instance, the antigen 85 complex proteins and separately the ModD protein are strongly immunogenic in natural and experimental mycobacterial infections in terms of both induction of antibody synthesis and T-cell-mediated reactions (74, 75). Although not directly demonstrated here, another possible application of our strategy would be to identify export signals on a genome scale via random insertion into the chromosome (74, 75) or via cosmid insertions (76). Interestingly, in the latter case, Braunstein et al. (76) used a genome-wide TnphoA strategy to identify secreted proteins from M. tuberculosis and isolated the Sec-secreted substrate antigen 85B; however, the antigen 85A and 85C components escaped detection, probably due to the fact that, as we now show, each is a Tat substrate.
In addition to the discovery of novel export signals, we observed that Tat-targeted PhoA constructs were exported in a Tat-independent manner when both PhoA was reduced and the essential translocon component TatC was absent from cells. This suggests that initial docking to TatC serves as a key specificity determinant for Tat-specific routing of PhoA, and in its absence, substrates can be rerouted to the Sec pathway, provided they remain compatible (i.e. unfolded, reduced) with the Sec export mechanism. Based on our finding that this rerouting was not observed in strains lacking TatB or TatA/E, these results are entirely consistent with current models of Tat-dependent translocation, suggesting that substrate binding by TatC is an early step in the translocation process that can be decoupled from later export steps, such as assembly of a TatA channel or patch (53, 56, 57).
Supplementary Material
Acknowledgments
We thank Alan Collmer (Cornell University) for various strains and plasmids used in this study, Miriam Braunstein (University of North Carolina) for strains MB692 and JM578, Martin Pavelka (University of Rochester Medical Center) for strain PM759, and Adam Parks (Cornell University) for aid with the conjugational studies. We thank Adam Fisher for helpful discussions of the manuscript.
This work was supported by National Science Foundation Career Award BES 0449080 and a NYSTAR James D. Watson Award (both to M. P. D.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
Footnotes
The abbreviations used are: BCIP, 5-bromo-4-chloro-3-indolyl phosphate; SRP, signal recognition particle; HMM, hidden Markov model; MBP, maltose-binding protein.
M. Marrichi, L. Camacho, D. G. Russell, and M. P. DeLisa, unpublished observations.
L. Camacho, D. G. Russell, M. P. DeLisa, S. Ehrt, and D. Schnappinger, unpublished observations.
S. Fortune and E. Rubin, personal communication.
References
- 1.Bendtsen, J. D., Nielsen, H., von Heijne, G., and Brunak, S. (2004) J. Mol. Biol. 340 783-795 [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., Nielsen, H., Widdick, D., Palmer, T., and Brunak, S. (2005) BMC Bioinformatics 6 167-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronstein, P. A., Marrichi, M., Cartinhour, S., Schneider, D. J., and DeLisa, M. P. (2005) J. Bacteriol. 187 8450-8461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dilks, K., Rose, R. W., Hartmann, E., and Pohlschroder, M. (2003) J. Bacteriol. 185 1478-1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emanuelsson, O., Brunak, S., von Heijne, G., and Nielsen, H. (2007) Nat. Protoc. 2 953-971 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen, H., Engelbrecht, J., Brunak, S., and von Heijne, G. (1997) Protein Eng. 10 1-6 [DOI] [PubMed] [Google Scholar]
- 7.Manoil, C., and Beckwith, J. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 8129-8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey, J., and Manoil, C. (2002) Nat. Biotechnol. 20 839-842 [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez, C., Barondess, J., Manoil, C., and Beckwith, J. (1987) J. Mol. Biol. 195 289-297 [DOI] [PubMed] [Google Scholar]
- 10.Sarthy, A., Michaelis, S., and Beckwith, J. (1981) J. Bacteriol. 145 288-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay, B. B., Starnbach, M. N., Francis, C. L., Stocker, B. A., Chatfield, S., Dougan, G., and Falkow, S. (1988) Mol. Microbiol. 2 757-766 [DOI] [PubMed] [Google Scholar]
- 12.Huang, H. C., Schuurink, R., Denny, T. P., Atkinson, M. M., Baker, C. J., Yucel, I., Hutcheson, S. W., and Collmer, A. (1988) J. Bacteriol. 170 4748-4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson, K. M., and Mekalanos, J. J. (1988) Infect. Immun. 56 2822-2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahme, L. G., Tan, M. W., Le, L., Wong, S. M., Tompkins, R. G., Calderwood, S. B., and Ausubel, F. M. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 13245-13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan, M. W., Rahme, L. G., Sternberg, J. A., Tompkins, R. G., and Ausubel, F. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 2408-2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnenberg, M. S., Calderwood, S. B., Donohue-Rolfe, A., Keusch, G. T., and Kaper, J. B. (1990) Infect. Immun. 58 1565-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber, R. F., and Silverman, P. M. (1988) J. Mol. Biol. 203 467-478 [DOI] [PubMed] [Google Scholar]
- 18.Akiyama, Y., and Ito, K. (1987) EMBO J. 6 3465-3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gott, P., and Boos, W. (1988) Mol. Microbiol. 2 655-663 [DOI] [PubMed] [Google Scholar]
- 20.Herrero, M., de Lorenzo, V., and Neilands, J. B. (1988) J. Bacteriol. 170 56-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoil, C., and Bailey, J. (1997) J. Mol. Biol. 267 250-263 [DOI] [PubMed] [Google Scholar]
- 22.Driessen, A. J., and Nouwen, N. (2008) Annu. Rev. Biochem. 77 643-667 [DOI] [PubMed] [Google Scholar]
- 23.Pugsley, A. P. (1993) Microbiol. Rev. 57 50-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driessen, A. J., Manting, E. H., and van der Does, C. (2001) Nat. Struct. Biol. 8 492-498 [DOI] [PubMed] [Google Scholar]
- 25.Collier, D. N., Bankaitis, V. A., Weiss, J. B., and Bassford, P. J., Jr. (1988) Cell 53 273-283 [DOI] [PubMed] [Google Scholar]
- 26.Weiss, J. B., Ray, P. H., and Bassford, P. J., Jr. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 8978-8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumamoto, C. A., and Beckwith, J. (1985) J. Bacteriol. 163 267-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent, Q. A., de Gier, J. W., von Heijne, G., Kendall, D. A., ten Hagen-Jongman, C. M., Oudega, B., and Luirink, J. (1997) Mol. Microbiol. 25 53-64 [DOI] [PubMed] [Google Scholar]
- 29.Valent, Q. A., Kendall, D. A., High, S., Kusters, R., Oudega, B., and Luirink, J. (1995) EMBO J. 14 5494-5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luirink, J., ten Hagen-Jongman, C. M., van der Weijden, C. C., Oudega, B., High, S., Dobberstein, B., and Kusters, R. (1994) EMBO J. 13 2289-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber, D., Boyd, D., Xia, Y., Olma, M. H., Gerstein, M., and Beckwith, J. (2005) J. Bacteriol. 187 2983-2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schierle, C. F., Berkmen, M., Huber, D., Kumamoto, C., Boyd, D., and Beckwith, J. (2003) J. Bacteriol. 185 5706-5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froderberg, L., Houben, E., Samuelson, J. C., Chen, M., Park, S. K., Phillips, G. J., Dalbey, R., Luirink, J., and De Gier, J. W. (2003) Mol. Microbiol. 47 1015-1027 [DOI] [PubMed] [Google Scholar]
- 34.Giladi, M., Champion, C. I., Haake, D. A., Blanco, D. R., Miller, J. F., Miller, J. N., and Lovett, M. A. (1993) J. Bacteriol. 175 4129-4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauch, K. L., and Beckwith, J. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 1576-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berks, B. C., Sargent, F., and Palmer, T. (2000) Mol. Microbiol. 35 260-274 [DOI] [PubMed] [Google Scholar]
- 37.Settles, A. M., Yonetani, A., Baron, A., Bush, D. R., Cline, K., and Martienssen, R. (1997) Science 278 1467-1470 [DOI] [PubMed] [Google Scholar]
- 38.Weiner, J. H., Bilous, P. T., Shaw, G. M., Lubitz, S. P., Frost, L., Thomas, G. H., Cole, J. A., and Turner, R. J. (1998) Cell 93 93-101 [DOI] [PubMed] [Google Scholar]
- 39.Lewenza, S., Gardy, J. L., Brinkman, F. S., and Hancock, R. E. (2005) Genome Res. 15 321-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruser, T., Yano, T., Brune, D. C., and Daldal, F. (2003) Eur. J. Biochem. 270 1211-1221 [DOI] [PubMed] [Google Scholar]
- 41.DeLisa, M. P., Tullman, D., and Georgiou, G. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 6115-6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher, A. C., Kim, W., and DeLisa, M. P. (2006) Protein Sci. 15 449-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sone, M., Kishigami, S., Yoshihisa, T., and Ito, K. (1997) J. Biol. Chem. 272 6174-6178 [DOI] [PubMed] [Google Scholar]
- 44.Datsenko, K. A., and Wanner, B. L. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6640-6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover, C. K., de la Cruz, V. F., Fuerst, T. R., Burlein, J. E., Benson, L. A., Bennett, L. T., Bansal, G. P., Young, J. F., Lee, M. H., Hatfull, G. F., Snapper, S. B., Barletta, R. G., Jacobs, W. R., Jr., and Bloom, B. R. (1991) Nature 351 456-460 [DOI] [PubMed] [Google Scholar]
- 46.Sargent, F., Bogsch, E. G., Stanley, N. R., Wexler, M., Robinson, C., Berks, B. C., and Palmer, T. (1998) EMBO J. 17 3640-3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen, G., Hayhurst, A., Thomas, J. G., Harvey, B. R., Iverson, B. L., and Georgiou, G. (2001) Nat. Biotechnol. 19 537-542 [DOI] [PubMed] [Google Scholar]
- 48.Bessette, P. H., Aslund, F., Beckwith, J., and Georgiou, G. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 13703-13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardwell, J. C., McGovern, K., and Beckwith, J. (1991) Cell 67 581-589 [DOI] [PubMed] [Google Scholar]
- 50.Kim, J., and Kendall, D. A. (1998) J. Bacteriol. 180 1396-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogsch, E. G., Sargent, F., Stanley, N. R., Berks, B. C., Robinson, C., and Palmer, T. (1998) J. Biol. Chem. 273 18003-18006 [DOI] [PubMed] [Google Scholar]
- 52.Rodrigue, A., Chanal, A., Beck, K., Muller, M., and Wu, L. F. (1999) J. Biol. Chem. 274 13223-13228 [DOI] [PubMed] [Google Scholar]
- 53.Alami, M., Luke, I., Deitermann, S., Eisner, G., Koch, H. G., Brunner, J., and Muller, M. (2003) Mol Cell 12 937-946 [DOI] [PubMed] [Google Scholar]
- 54.Tullman-Ercek, D., DeLisa, M. P., Kawarasaki, Y., Iranpour, P., Ribnicky, B., Palmer, T., and Georgiou, G. (2007) J. Biol. Chem. 282 8309-8316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lorenzo, V., Herrero, M., Jakubzik, U., and Timmis, K. N. (1990) J. Bacteriol. 172 6568-6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cline, K., and Mori, H. (2001) J. Cell Biol. 154 719-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Leeuw, E., Granjon, T., Porcelli, I., Alami, M., Carr, S. B., Muller, M., Sargent, F., Palmer, T., and Berks, B. C. (2002) J. Mol. Biol. 322 1135-1146 [DOI] [PubMed] [Google Scholar]
- 58.Kreutzenbeck, P., Kroger, C., Lausberg, F., Blaudeck, N., Sprenger, G. A., and Freudl, R. (2007) J. Biol. Chem. 282 7903-7911 [DOI] [PubMed] [Google Scholar]
- 59.Strauch, E. M., and Georgiou, G. (2007) J. Mol. Biol. 374 283-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daley, D. O., Rapp, M., Granseth, E., Melen, K., Drew, D., and von Heijne, G. (2005) Science 308 1321-1323 [DOI] [PubMed] [Google Scholar]
- 61.Krogh, A., Larsson, B., von Heijne, G., and Sonnhammer, E. L. (2001) J. Mol. Biol. 305 567-580 [DOI] [PubMed] [Google Scholar]
- 62.Doublet, P., Grangeasse, C., Obadia, B., Vaganay, E., and Cozzone, A. J. (2002) J. Biol. Chem. 277 37339-37348 [DOI] [PubMed] [Google Scholar]
- 63.Chan, C. S., Howell, J. M., Workentine, M. L., and Turner, R. J. (2006) Biochem. Biophys. Res. Commun. 343 244-251 [DOI] [PubMed] [Google Scholar]
- 64.DiGiuseppe Champion, P. A., and Cox, J. S. (2007) Cell Microbiol. 9 1376-1384 [DOI] [PubMed] [Google Scholar]
- 65.Bronstein, P., Marrichi, M., and DeLisa, M. P. (2004) Res. Microbiol. 155 803-810 [DOI] [PubMed] [Google Scholar]
- 66.Saint-Joanis, B., Demangel, C., Jackson, M., Brodin, P., Marsollier, L., Boshoff, H., and Cole, S. T. (2006) J. Bacteriol. 188 6669-6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sassetti, C. M., Boyd, D. H., and Rubin, E. J. (2003) Mol. Microbiol. 48 77-84 [DOI] [PubMed] [Google Scholar]
- 68.McDonough, J. A., Hacker, K. E., Flores, A. R., Pavelka, M. S., Jr., and Braunstein, M. (2005) J. Bacteriol. 187 7667-7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posey, J. E., Shinnick, T. M., and Quinn, F. D. (2006) J. Bacteriol. 188 1332-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho, D., Sung, N., and Collins, M. T. (2006) Proteomics 6 5785-5794 [DOI] [PubMed] [Google Scholar]
- 71.Allard, J. D., and Bertrand, K. P. (1992) J. Biol. Chem. 267 17809-17819 [PubMed] [Google Scholar]
- 72.Berg, D. E., Schmandt, M. A., and Lowe, J. B. (1983) Genetics 105 813-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lodge, J. K., Weston-Hafer, K., and Berg, D. E. (1988) Genetics 120 645-650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huygen, K., Lozes, E., Gilles, B., Drowart, A., Palfliet, K., Jurion, F., Roland, I., Art, M., Dufaux, M., Nyabenda, J., De Bruyn, J., Van Voeren, J.-P., and Deleys, R. (1994) Infect. Immun. 62 363-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar, P., Amara, R. R., Challu, V. K., Chadda, V. K., and Satchidanandam, V. (2003) Infect. Immun. 71 1929-1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braunstein, M., Griffin, T. I., Kriakov, J. I., Friedman, S. T., Grindley, N. D., and Jacobs, W. R., Jr. (2000) J. Bacteriol. 182 2732-2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller, V. L., and Mekalanos, J. J. (1988) J. Bacteriol. 170 2575-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., Datsenko, K. A., Tomita, M., Wanner, B. L., and Mori, H. (2006) Mol. Syst. Biol. 2 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flores, A. R., Parsons, L. M., and Pavelka, M. S., Jr. (2005) Microbiology 151 521-532 [DOI] [PubMed] [Google Scholar]
- 80.Lutz, S., Fast, W., and Benkovic, S. J. (2002) Protein Eng. 15 1025-1030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.