Abstract
Murine protein serine/threonine kinase 38 (MPK38) is a member of the AMP-activated protein kinase-related serine/threonine kinase family that plays an important role in various cellular processes, including cell cycle, signaling pathways, and self-renewal of stem cells. Here we demonstrate a functional association between MPK38 and apoptosis signal-regulating kinase 1 (ASK1). The physical association between MPK38 and ASK1 was mediated through their carboxyl-terminal regulatory domains and was increased by H2O2 or tumor necrosis factor α treatment. The use of kinase-dead MPK38 and ASK1 mutants revealed that MPK38-ASK1 complex formation was dependent on the activities of both kinases. Ectopic expression of wild-type MPK38, but not kinase-dead MPK38, stimulated ASK1 activity by Thr838 phosphorylation and enhanced ASK1-mediated signaling to both JNK and p38 kinases. However, the phosphorylation of MKK6 and p38 by MPK38 was not detectable. In addition, MPK38-mediated ASK1 activation was induced through the increased interaction between ASK1 and its substrate MKK3. MPK38 also stimulated H2O2-mediated apoptosis by enhancing the ASK1 activity through Thr838 phosphorylation. These results suggest that MPK38 physically interacts with ASK1 in vivo and acts as a positive upstream regulator of ASK1.
Apoptosis signal-regulating kinase 1 (ASK1)2 is one of the mitogen-activated protein kinase kinase kinases (MAPKKK) that is stimulated in response to various cellular stresses, including reactive oxygen species, tumor necrosis factor α (TNF-α), Fas, ischemia insult, and anti-tumor agents. ASK1 stimulation leads to activation of the c-Jun NH2-terminal kinase (JNK)/p38 signaling cascade by phosphorylating and activating mitogen-activated protein kinase kinases (MAPKK) such as MKK3, -4, -6, and -7 (1–3). Emerging evidence indicates that ASK1 activity is regulated by its interaction with several cellular partners (3–8), including thioredoxin (Trx), glutaredoxin, heat shock protein 72 (Hsp72), 14-3-3, and protein serine/threonine phosphatase 5 (PP5). For example, Trx and glutaredoxin bind to the NH2- and COOH-terminal domains of ASK1, respectively, and inhibit ASK1 kinase activity, and Hsp72 inhibits ASK1 activation through direct interaction. These findings suggest that other ASK1-interacting proteins could be involved in the regulation of ASK1 activity.
Murine protein serine/threonine kinase 38 (MPK38), also known as maternal embryonic leucine zipper kinase (Melk), is a member of the AMP-activated protein kinase-related serine/threonine kinase family (9, 10). MPK38 was originally identified as a murine counterpart for its human homolog, HPK38/hMelk/KIAA175, that may be involved in the proliferation of interleukin-4-induced normal human keratinocytes (9). The importance of MPK38 in oncogenesis is also underscored by the finding that MPK38 expression is increased in tumor-derived progenitor cells as well as in cancers of nondifferentiated cells (11–13). However, the physiological regulation and functions of MPK38 have remained unclear.
To explore a functional link between ASK1 and MPK38 signaling pathways, we investigated the effect of MPK38 on ASK1 and its downstream targets. We demonstrated that MPK38 physically interacts with ASK1. MPK38 phosphorylates Thr838 (corresponding to Thr845 in mice) within the activation loop of human ASK1, which subsequently leads to the stimulation of ASK1 kinase activity. Moreover, this interaction results in the enhancement of JNK-mediated transactivation and H2O2-induced apoptosis.
MATERIALS AND METHODS
Cell Culture, Plasmids, Reagents, and Cell Line Construction—HEK293, 293T, HaCaT, and SK-N-BE(2)C cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen). HA-tagged wild-type and kinase-dead ASK1 were kindly provided by Dr. H. Ichijo (Tokyo Medical and Dental University, Tokyo, Japan). HA-tagged ASK1-K and ASK1-C were a gift from Dr. S. Cho (Korea Research Institute of Bioscience and Biotechnology, Taejon, Korea). The activator protein 1 (AP-1)-Luc reporter, pSuper vector, and c-Fos were the kind gifts from Dr. Y. Yeom (Korea Research Institute of Bioscience and Biotechnology, Taejon, Korea). Glutathione S-transferase (GST)-tagged MKK6(K82A) and p38 were a gift from Dr. E.-J. Choi (Korea University, Seoul, Korea). To generate the kinase-dead MPK38(K40R) construct, site-directed mutagenesis was carried out using the QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). PCR was performed using the full-length MPK38 cDNA cloned into pBluescript KS (Stratagene) as the template in the presence of forward (5′-GAGATGGTAGCTATACGCATCATGGATAAGAAT-3′) and reverse (5′-ATTCTTATCCATGATGCGTATAGCTACCATCTC-3′) primers. The amplified PCR products were cut with ClaI plus NotI and cloned into pEBG vectors to generate the GST-MPK38(K40R) construct. pEBG-MPK38 (wobble) was generated by QuickChange II site-directed mutagenesis kit using KS-MPK38 as the template and the following mutant primers containing alterations in the nucleotide sequence (5′-CAGGCAGACAAUGGAGGAUTT-3′, amino acids 297–303) of MPK38 siRNA, sense 5′-CAGCAGACAAACCATGGAGGATTTA-3′, and antisense 5′-TAAATCCTCCATGGTTTGTCTGCTG-3′ (14). The identities of all the PCR products were confirmed by nucleotide sequencing analysis on both strands. Anti-FLAG (M2), anti-His, and anti-β-actin antibodies were from Sigma; the anti-hemagglutinin (HA), anti-ASK1, anti-MKK3, anti-ATF2, and anti-poly(ADP-ribose) polymerase (PARP) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); the anti-phospho-MKK3/6(S189/207), anti-p38, anti-phospho-p38(T180/Y182), anti-phospho-ATF2(T71), and anti-phospho-ASK1 (T845) antibodies were from Cell Signaling Technology (Danvers, MA); and the anti-caspase-3 antibody was from Calbiochem. Anti-MPK38 and anti-GST antibodies were described previously (15, 16). Isopropyl β-d-thiogalactopyranoside, ionomycin, thapsigargin, N-acetyl-l-cysteine (Nac), dithiothreitol (DTT), aprotinin, puromycin, and phenylmethylsulfonyl fluoride were from Sigma. TNF-α was purchased from R & D Systems. Polyvinylidene difluoride membrane was from Millipore Corp. (Bedford, MA). [γ-32P]ATP was from PerkinElmer Life Sciences. To prepare HaCaT cells stably expressing MPK38-specific shRNA (MPK38(KD)), double-stranded oligonucleotide (forward, 5′-GATCCCCCAGGCAGACAATGGAGGATTTCAAGAGAATCCTCCATTGTCTGCCTGTTTTTGGAAA-3′; reverse, 5′-AGCTTTTCCAAAAACAGGCAGACAATGGAGGATTCTCTTGAAATCCTCCATTGTCTGCCTGGGG-3′; MPK38 sequence underlined) was cloned into the pSuper vector. MPK38(KD) cells were screened in the presence of 1.5 μg/ml puromycin until all control parental HaCaT cells died.
Construction of ASK1(K709R) and ASK1-N Mutants—ASK1(K709R) mutants were generated by PCR as described previously (17). In brief, HA-ASK1(K709R) was used as the template for amplification with either the ASK1 forward 5′-GCGTCGACATGAGCACGGAGGCGGAC-3′ (SalI site underlined) or reverse 5′-GCGCGGCCTCAAGTCTGTTTGTTTCG-3′ (NotI site underlined) primer, in conjunction with one of the following mutant primers containing alterations in the nucleotide sequence of ASK1(K709R): for T838A, sense 5′-TGTACTGAAGCATTTACTGGT-3′, antisense 5′-ACCAGTAAATGCTTCAGTACA-3′; for S83A, sense 5′-CGGGGCAGCGCAGTTGGCGGG-3′, antisense 5′-CCCGCCAACTGCGCT GCCCCG-3′; for S967A, sense 5′-AGGAGTATAGCATTGCCGGTA-3′, antisense 5′-TACCGGCAATGCTATACTCCT-3′; and for S1034A, sense 5′-GCTCCTCCTGCACCTGAAGAA-3′, antisense 5′-TTCTTCAGGTGCAGGAGGAGC-3′. The reaction parameters were 4 min at 94 °C, 1 cycle; 30 s at 94 °C, 1 min at 50–60 °C, 3 min at 72 °C, 18 cycles; 7 min at 72 °C, 1 cycle. The amplified PCR products were separated by agarose gel electrophoresis, excised from the gel, and the PCR mixtures were subjected to a second round of PCR amplification in the absence of primers using the following parameters: 4 min at 94 °C, 1 cycle; 30 s at 94 °C, 1 min at 40 °C, 3 min at 72 °C, 6 cycles; 7 min at 72 °C, 1 cycle. A third round of PCR amplification was then performed using ASK1 forward and reverse primers and the following parameters: 4 min at 94 °C, 1 cycle; 30 s at 94 °C, 1 min at 62 °C, 3 min at 72 °C, 18 cycles; 7 min at 72 °C, 1 cycle. To generate GST-tagged fusion proteins of ASK1(K709R) mutants, amplified PCR products were digested with SalI and NotI and ligated into pGEX4T-3 (Amersham Biosciences). To generate the HA-ASK1-N construct, PCR was performed using HA-ASK1(WT) as the template. The following primers were used: forward primer (5′-GCGTCGACATGAGCACGGAGGCGGACGAGGGC-3′) containing a SalI site (underlined), reverse primer (5′-GCGTCGACCTCAAAAAACTTTTTACAATGAAG-3′) containing a SalI site (underlined). The amplified PCR products were cut with SalI and cloned into a pcDNA-HA vector using XhoI site to generate the HA-ASK1-N.
In Vivo and in Vitro Binding Assay—Each plasmid DNA utilized in the study was transfected into HEK293, 293T, or HaCaT cells using WelFect-Ex™ Plus (WelGENE, Daegu, Korea), according to the manufacturer's instructions. In vivo binding assays were performed as described previously (18). For native PAGE to determine the in vitro binding between MPK38 and ASK1, the procedure was the same as that of denaturing SDS-PAGE, except that solutions did not contain SDS or β-mercaptoethanol, and samples were not boiled prior to loading (18).
In Vitro Kinase Assay—In vitro kinase assays were performed as described previously (6). Cells transiently transfected with the indicated expression vectors were harvested and lysed with buffer (20 mm HEPES, pH 7.9, 10 mm EDTA, 0.1 m KCl, and 0.3 m NaCl). The cleared lysates were subjected to immunoprecipitation by incubation for 2 h at 4 °C with the appropriate antibodies. After washing the immunoprecipitate three times with lysis buffer, then twice with each kinase buffer (for ASK1, 20 mm Tris-HCl, pH 7.5, 0.1 mm sodium orthovanadate, 1 mm DTT, and 20 mm MgCl2; for MKK3/6, 25 mm HEPES, pH 7.4, 0.1 mm sodium orthovanadate, 25 mm sodium β-glycerophosphate, 2 mm DTT, and 25 mm MgCl2; for p38, 50 mm HEPES, pH 7.4, 1 mm sodium orthovanadate, 0.2 mm DTT, 1 mm phenylmethylsulfonyl fluoride, and 1 mm MgCl2), the immunoprecipitates were assayed for the indicated protein kinase activities in the presence of each kinase buffer containing 5 μg of recombinant GST-tagged substrates. The reaction mixtures were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and detected by autoradiography. Recombinant GST-tagged ZPR9, MKK6(K82A), p38, and ATF2 were used as substrates for MPK38, ASK1, MKK3 or MKK6, and p38 mitogen-activated protein kinase (MAPK), respectively. Protein concentration was determined by the Bradford assay.
Assays for MPK38 and JNK Activities—Cells were transiently transfected with GST-tagged MPK38 or JNK, along with indicated expression vectors, and solubilized with lysis buffer (20 mm HEPES, pH 7.9, 10 mm EDTA, 0.1 m KCl, and 0.3 m NaCl). The cleared lysates were precipitated by glutathione-Sepharose beads. The GST precipitates were washed three times with lysis buffer and twice with kinase buffer (for MPK38, 50 mm HEPES, pH 7.4, 1 mm DTT, and 10 mm MgCl2; for JNK, 20 mm HEPES, pH 7.6, 0.1 mm sodium orthovanadate, 25 mm sodium β-glycerophosphate, 2 mm DTT, and 20 mm MgCl2), and then subjected to an in vitro kinase assay using recombinant ZPR9 (19) or c-Jun as a substrate in the presence of 5 μCi of [γ-32P]ATP, followed by SDS-PAGE and autoradiography.
Small Interfering RNA (siRNA) Experiments—The MPK38 siRNAs of the oligonucleotide 1 (5′-CAGGCAGACAAUGGAGGAUTT-3′) targeting a coding region (amino acids 297–303) and oligonucleotide 2 (5′-AACCCAAGGGUAACAAGGATT-3′) targeting a coding region (amino acids 156–162) on MPK38 (GenBank™ accession number NM010790), as well as ASK1 siRNA (5′-GGUAUACAUGAGUGGAAUUTT-3′) (20), were synthesized from SamChully Pharm. Co., Ltd. (Seoul, Korea). The sense and antisense oligonucleotides for each siRNA were mixed and heated at 90 °C for 2 min, and the combined reaction was incubated at 30 °C for 1 h. HEK293 cells grown were plated in 6-well flat-bottomed microplates (Nunc) at a concentration of 2 × 105 cells per well the day before transfection. siRNA oligonucleotides with the indicated concentrations were transfected into cells using WelFect-Ex™ Plus. After 48 h of transfection, immunoblottings were carried out to confirm the down-regulation of target proteins.
Luciferase Reporter Assay—293T cells were transfected according to the WelFect-Ex™ Plus method with the AP-1-Luc reporter plasmid, along with each expression vector as indicated. After 48 h, the cells were harvested, and luciferase activity was monitored with a luciferase assay kit (Promega) following the manufacturer's instructions. Light emission was determined with a Berthold luminometer (Microlumat LB96P). Total DNA concentration was kept constant by supplementing with empty vector DNAs. The values were adjusted with respect to expression levels of a cotransfected β-galactosidase reporter control, and experiments were repeated at least three times.
Assays for Apoptosis—For cell death experiments using the green fluorescent protein (GFP) system (18), HEK293 cells grown on sterile coverslips were transfected with pEGFP (a GFP-encoding expression vector) together with expression vectors as indicated. After 24 h of transfection, the cells were washed with phosphate-buffered saline and then incubated for 40 h in serum-free medium. The cells were fixed with ice-cold 100% methanol, washed three times with phosphate-buffered saline, and then stained with 4′,6′-diamidino-2-phenylindole dihydrochloride. The 4′,6′-diamidino-2-phenylindole dihydrochloride-stained nuclei of GFP-positive cells were analyzed for apoptotic morphology by fluorescence microscopy. The percentage of apoptotic cells was calculated as the number of GFP-positive cells with apoptotic nuclei divided by the total number of GFP-positive cells. Terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed according to the manufacturer's instructions (Roche Applied Science). Cells exposed to 1 mm H2O2 for 9 h were used as a positive control.
RESULTS
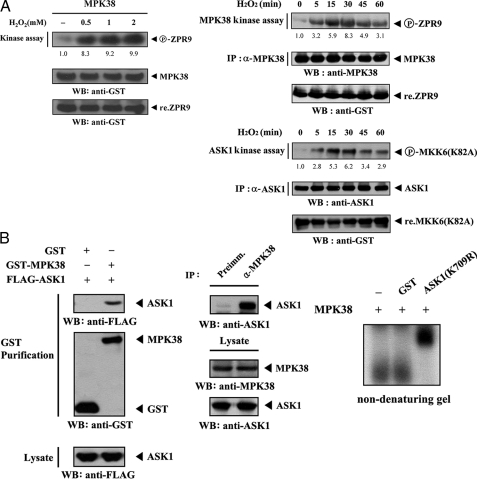
Direct Interaction of MPK38 and ASK1 in Vivo and in Vitro—We initially performed in vitro kinase assays using 293T cells to identify MPK38-specific stimuli that modulate MPK38 kinase activity. Among the various stimuli tested, MPK38 precipitate from cell lysates treated with H2O2 at concentrations of more than 0.5 mm significantly induced MPK38 kinase activity compared with untreated MPK38 precipitate (Fig. 1A, left). We next analyzed the kinetics of MPK38 and ASK1 kinase activity in H2O2-stimulated 293T cells. The stimulation of MPK38 and ASK1 kinase activity was detected at 5 min after treatment with 2 mm H2O2, peaked at 30 min, and decreased thereafter (Fig. 1A, right). These results suggested that cross-talk between MPK38 and ASK1 signaling pathways may exist as H2O2 is well known to activate ASK1.
FIGURE 1.
Direct interaction of MPK38 with ASK1 in vivo and in vitro. A, regulation of MPK38 and ASK1 kinase activity by H2O2. 293T cells transiently transfected with wild-type GST-MPK38 were incubated with increasing amounts of H2O2 for 30 min, and MPK38 was purified on glutathione-Sepharose beads. The GST precipitates were subjected to an in vitro kinase assay using ZPR9 as a substrate, followed by SDS-PAGE and autoradiography (left). For H2O2 time-dependent regulation of MPK38 and ASK1 kinase activity, 293T cells were treated with 2 mm H2O2 for the indicated times. Cell lysates were immunoprecipitated by the indicated antibodies, and the kinase activities of MPK38 and ASK1 were determined by in vitro kinase assays using ZPR9 and MKK6(K82A) as substrates (right). The circled P-ZPR9 and P-MKK6(K82A) indicate the phosphorylated ZPR9 and MKK6(K82A), respectively. The relative level of kinase activity was quantitated by densitometric analyses, and fold increase relative to untreated samples was calculated. IP, immunoprecipitation; WB, Westernblot. B, invivo and invitro association of MPK38 with ASK1. GST alone and GST-MPK38 were cotransfected with FLAG-ASK1 into 293T cells. GST fusion proteins were purified on glutathione-Sepharosebeads (GST Purification), and the amounts of complex formation and FLAG-ASK1 used for the in vivo binding assay were determined by anti-FLAG antibody immunoblot (left). Cell lysates from HEK293 cells were subjected to immunoprecipitation using either rabbit preimmune serum (Preimm.) or anti-MPK38 antibody (α-MPK38), followed by immunoblot analysis using an anti-ASK1 antibody to determine the complex formation between endogenous MPK38 and ASK1 (middle). As a control, the expression levels of MPK38 and ASK1 in the total cell lysate were analyzed by immunoblot using anti-MPK38 and anti-ASK1 antibodies, respectively. For native PAGE (8%) of the MPK38-ASK1 complex (right), purified recombinant MPK38 was autophosphorylated as described under “Materials and Methods.” Autophosphorylated MPK38 (2–3 μg) was incubated with unlabeled recombinant GST or GST-ASK1(K709R) (each 5 μg) at room temperature for 1 h. C, effect of MPK38 and ASK1 kinase activities on MPK38-ASK1 association. HEK293 cells were transiently transfected with the appropriate expression plasmids, and MPK38-ASK1 complex formation was determined by Western blot analysis using anti-HA or anti-FLAG antibody as described in B. D, modulation of MPK38-ASK1(K709R), MPK38(K40R)-ASK1, or MPK38(K40R)-ASK1(K709R) complex formation by H2O2. HEK293 cells transfected with the indicated expression vectors were incubated with or without 2 mm H2O2 for 30 min. The level of MPK38-ASK1 complex was analyzed by immunoblot using an anti-HA antibody. E, H2O2 and TNF-α modulation of MPK38-ASK1 interaction. HEK293 cells transfected with the expression vectors indicated were treated with 2 mm H2O2 for 30 min or 500 ng/ml TNF-α for 30 min. Cell lysates were purified on glutathione-Sepharose beads (GST Purification) and immunoblotted with an anti-FLAG antibody (upper). The endogenous level of MPK38-ASK1 complex in the presence or absence of H2O2 (or TNF-α) was also analyzed by immunoblot using an anti-ASK1 antibody (lower). The relative level of MPK38-ASK1 complex formation was quantitated by densitometric analyses and fold increase relative to untreated samples expressing wild-type MPK38 and ASK1 was calculated (C–E).
To test whether MPK38 is associated with ASK1 in cells, we performed cotransfection experiments using GST-MPK38 and FLAG-ASK1 expression vectors. The interactions between FLAG-tagged ASK1 proteins and GST-MPK38 fusion proteins were analyzed by immunoblotting with an anti-FLAG antibody. ASK1 was detected in the coprecipitate only when coexpressed with GST-MPK38 but not with control GST alone, demonstrating that MPK38 physically interacts with ASK1 (Fig. 1B, left). To confirm the endogenous interaction between MPK38 and ASK1, we carried out coimmunoprecipitation experiments using endogenous MPK38 and ASK1 in HEK293 cells. Immunoprecipitation of endogenous MPK38 by anti-MPK38 antibody and then immunoblotting with an anti-ASK1 antibody led to the clear identification of an interaction between the two endogenous MPK38 and ASK1 proteins (Fig. 1B, middle). We have further determined this association using other cell lines, including NIH 3T3 cells and R1.1 hematopoietic cells highly expressing MPK38 (15), and we confirmed that this association could occur in vivo (data not shown). We also analyzed the in vitro association of purified, recombinant ASK1 with MPK38 using nondenaturing PAGE. Autophosphorylated recombinant MPK38 was incubated with an unlabeled, recombinant kinase-dead form of ASK1 (ASK1(K709R)) or with GST as a nonspecific control. A shift in the mobility of 32P-labeled MPK38 was clearly evident upon incubation in the presence of ASK1(K709R), but it was undetectable when 32P-labeled MPK38 was incubated with GST alone or in the absence of ASK1(K709R), providing additional evidence of a physical association between MPK38 and ASK1 (Fig. 1B, right).
To investigate whether the activity of both kinases was involved in the association between MPK38 and ASK1, we used an in vivo binding assay to examine the effects of the kinase-dead forms of MPK38 and ASK1 on MPK38-ASK1 complex formation. Expression of kinase-dead ASK1 (ASK1(K709R)) or ASK1(T838A), which is defective in ASK1 activation, resulted in a significant reduction of complex formation compared with expression of wild-type ASK1 (Fig. 1C, left and middle). Similar results were also observed with the kinase-dead MPK38 (MPK38(K40R)) in the analysis of the association between MPK38 and ASK1 (Fig. 1C, right). Consistent with this, the interaction was very weakly detected when the kinase-dead forms of both MPK38 and ASK1 were expressed (Fig. 1C, right, 6th lane). In addition, H2O2 treatment did not contribute to the modulation of the association between MPK38 and ASK1 containing kinase-dead MPK38 and/or ASK1 (Fig. 1D, 3rd and 4th lanes versus 5th to 10th lanes), indicating that the kinase activity of both MPK38 and ASK1 is important for H2O2-mediated modulation of MPK38-ASK1 complex formation.
Modulation of MPK38-ASK1 Complex Formation by ASK1 Stimuli—We assessed whether H2O2, a stimulator of ASK1, can influence the MPK38-ASK1 complex formation in HEK293 cells transfected with plasmid vectors expressing GST-MPK38 and FLAG-ASK1. After 48 h of transfection, cells were incubated in media with or without 2 mm H2O2 for 30 min. MPK38 was precipitated and the coprecipitation of ASK1 was determined by anti-FLAG immunoblot. MPK38-ASK1 association was significantly increased by H2O2 treatment compared with untreated control (Fig. 1E, upper left). Similarly, the interaction of MPK38 and ASK1 appears to be increased by other ASK1 stimuli, including TNF-α, endoplasmic reticulum stress (thapsigargin), and calcium overload (ionomycin) (Fig. 1E, upper right, and data not shown). We also determined the effect of ASK1 stimulators on the physical interaction between endogenous MPK38 and ASK1 in HEK293 cells. Exposure of the cells ASK1 stimuli, such as H2O2, TNF-α, thapsigargin, and ionomycin resulted in a considerable increase in endogenous MPK38-ASK1 complex formation (Fig. 1E, lower, and data not shown), suggesting the involvement of MPK38 in the ASK1 signaling pathway.
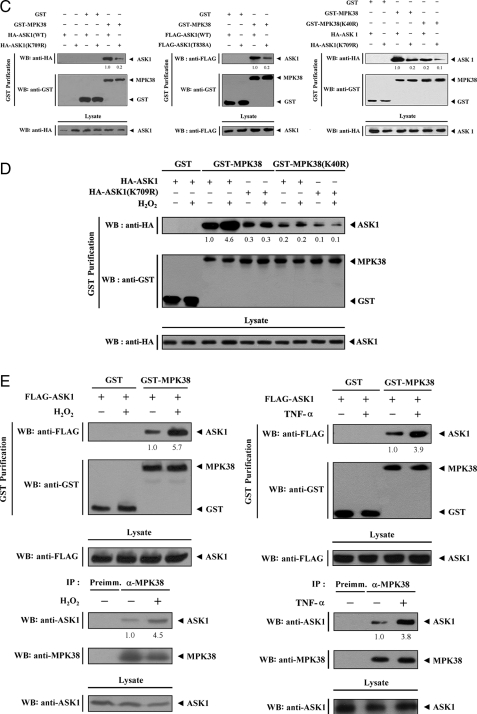
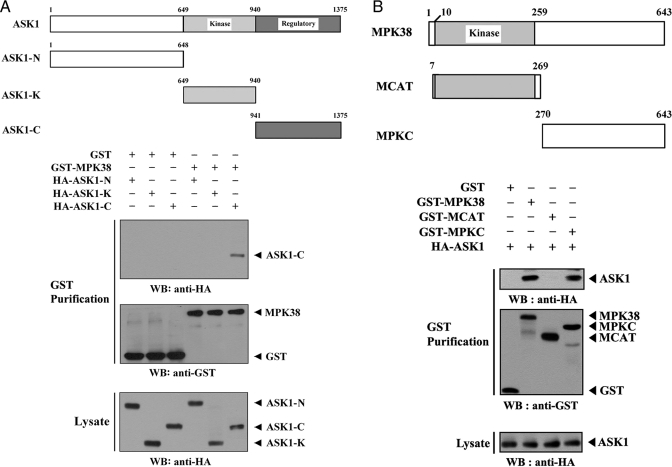
Mapping of the Binding Domain Required for the MPK38-ASK1 Interaction—We performed in vivo binding assays to determine which domain of ASK1 contributes to MPK38 binding. The carboxyl-terminal regulatory domain (ASK1-C, amino acids 941–1375) of ASK1 was found to be responsible for MPK38 binding, whereas the amino-terminal (ASK1-N, amino acids 1–648) and kinase (ASK1-K, amino acids 649–940) domains were unable to bind with MPK38 (Fig. 2A), indicating that ASK1 physically interacts with MPK38 through its carboxyl-terminal regulatory domain.
FIGURE 2.
Mapping of the ASK1 and MPK38 interaction domains. The schematic structures of wild-type and deletion constructs of ASK1 (A) and MPK38 (B) are indicated. Numbers indicate the amino acid residues corresponding to the domain boundaries. 293T cells transiently transfected with the indicated expression vectors were lysed and then precipitated using glutathione-Sepharose beads (GST Purification), and immunoblotted with an anti-HA antibody to determine the level of MPK38-ASK1 complex formation. The same blot was re-probed with an anti-GST antibody to confirm the expression of GST fusion proteins in the coprecipitates, and the expression level of ASK1 in total cell lysates was determined by immunoblot analysis using an anti-HA antibody. These experiments were independently performed at least three times with similar results. WB, Western blot.
To identify the region of MPK38 required for ASK1 binding, we next performed in vivo binding assays with cells transfected with two deletion constructs MCAT, harboring the kinase catalytic domain (amino acids 7–269) of MPK38, and MPKC, comprising the carboxyl-terminal regulatory domain (amino acids 270–643). Wild-type MPK38 and MPKC exhibited binding to ASK1, whereas the MCAT construct was not capable of ASK1 binding (Fig. 2B), indicating that the carboxyl-terminal regulatory domain of MPK38 was responsible for ASK1 binding. Together, these results demonstrate that the physical association between MPK38 and ASK1 is mediated through their carboxyl-terminal regulatory domains.
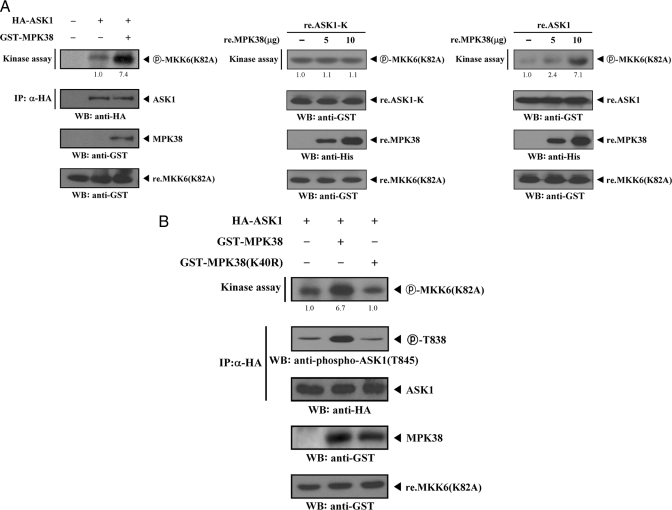
MPK38 Stimulates ASK1 Kinase Activity in a Kinase-dependent Manner—To establish the physiological role for the MPK38-ASK1 interaction, we investigated the effects of this interaction on ASK1 function. To determine whether MPK38 has an effect on ASK1 kinase activity, 293T cells were transiently transfected with ASK1 alone or cotransfected with MPK38. The recombinant MKK6(K82A) protein expressed in Escherichia coli was purified and used as a substrate for the ASK1 kinase assay. ASK1 kinase activity significantly increased when ASK1 was coexpressed with MPK38 (Fig. 3A, left). In a separate experiment with recombinant wild-type ASK1, we also demonstrated that recombinant MPK38 proteins stimulated ASK1 kinase activity in a dose-dependent manner (Fig. 3A, right). However, the stimulatory effect of recombinant MPK38 on ASK1 kinase activity was not observed in the presence of recombinant ASK1-K, which was unable to bind with MPK38 (Fig. 3A, middle).
FIGURE 3.
Enhancement of ASK1 kinase activity by MPK38. A, stimulation of ASK1 kinase activity by MPK38. 293T cells were transiently transfected with HA-ASK1 in the presence or absence of GST-MPK38. Cell lysates were subjected to immunoprecipitation (IP) with an anti-HA antibody, and the HA immunoprecipitates were analyzed for ASK1 kinase activity by an in vitro kinase assay using recombinant MKK6(K82A) as a substrate. The circled P-MKK6(K82A) indicates phosphorylated MKK6(K82A). The same blot was stripped and re-probed with an anti-HA antibody to determine the expression level of immunoprecipitated ASK1 (left, 2nd panel), and the expression level of GST-MPK38 in total cell lysates was examined by immunoblot analysis using an anti-GST antibody. (left, 3rd panel). The presence of equivalent amounts of substrate was verified by immunoblotting with an anti-GST antibody (left, bottom panel). 5 μg of recombinant ASK1-K (re.ASK1-K) or wild-type ASK1 (re.ASK1) proteins were incubated at room temperature for 1 h with the indicated amount of recombinant MPK38 (re.MPK38) in 50 μl of 25 mm HEPES buffer, pH 7.4, and then analyzed for ASK1 kinase activity by an in vitro kinase assay using recombinant MKK6(K82A) as a substrate (middle and right). WB, Western blot. B, requirement of MPK38 activity for MPK38-induced stimulation of ASK1 kinase activity. After 48 h of transfection with the indicated expression vectors, 293T cell lysates were subjected to immunoprecipitation with an anti-HA antibody and then analyzed by an in vitro kinase assay with recombinant MKK6(K82A) substrate (top panel). ASK1 Thr838 phosphorylation in the HA immunoprecipitates was determined by immunoblot analysis using an anti-phospho-ASK1(T845) antibody (2nd panel). The expression levels of immunoprecipitated ASK1 and wild-type and kinase-dead MPK38 in total cell lysates were analyzed using anti-HA and anti-GST antibodies, respectively (3rd and 4th panels). The relative level of ASK1 kinase activity was quantitated by densitometric analyses, and fold increase relative to control expressing ASK1 or ASK1-K alone was calculated. re., recombinant.
To examine whether the activity of MPK38 was involved in the ASK1 activation, we analyzed the effect of the kinase-dead mutant of MPK38 (MPK38(K40R)) on ASK1 kinase activity using an in vitro kinase assay. Coexpression of kinase-dead MPK38 had no effect on the modulation of ASK1 kinase activity compared with expression of wild-type ASK1 alone (Fig. 3B, top panel, 1st lane versus 3rd lane). Similarly, the phosphorylation of Thr838 of ASK1, which correlates with ASK1 activation (21), was not elevated by transfection with kinase-dead MPK38 (Fig. 3B, 2nd panel). The levels of immunoprecipitated ASK1 proteins were analyzed, and similar expression levels were found for the ASK1 construct (Fig. 3B, 3rd panel), indicating that the observed differences in phosphorylated MKK6(K82A) were not because of differences in ASK1 expression levels of HA immunoprecipitates. Taken together, these experiments demonstrate that MPK38 may be a positive regulator of ASK1 activity.
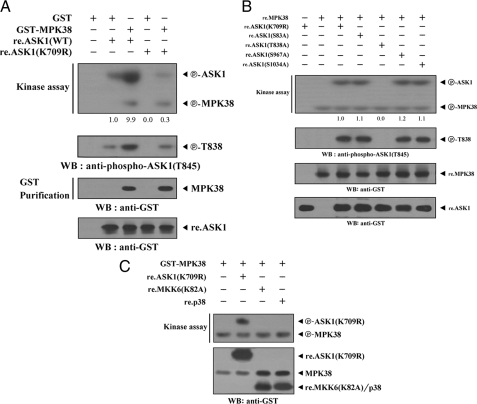
MPK38 Phosphorylates Thr838 within the Activation Loop of ASK1—Given that ASK1 physically interacts with MPK38 (see Figs. 1 and 2), we next determined whether ASK1 can act as a substrate for MPK38. The recombinant nonphosphorylated form of the ASK1(K709R) or wild-type ASK1 protein was expressed in E. coli, purified, and used as substrates for the MPK38 kinase assay. Extracts from 293T cells expressing GST-MPK38 were purified with glutathione-Sepharose beads and incubated with [γ-32P]ATP to allow phosphorylation of the recombinant ASK1(K709R) or wild-type ASK1. In addition to the increase in the phosphorylation of wild-type ASK1, ASK1(K709R) phosphorylation was observed in the presence of MPK38 (Fig. 4A, top panel), indicating that ASK1 may be a substrate for MPK38. A similar result was also observed in the phosphorylation of ASK1 Thr838 (Fig. 4A, 2nd panel).
FIGURE 4.
Phosphorylation of ASK1 by MPK38. A, for an in vitro kinase assay, ∼3–4 μg of recombinant ASK1(K709R) or wild-type ASK1 was mixed with 10 μm ATP, 5 μCi of [γ-32P]-ATP, and 10 mm MgCl2 in 20 μl of kinase buffer (see “Materials and Methods”) and incubated with the precipitated MPK38 for 15 min at 37 °C with frequent gentle mixing (top panel). ASK1 Thr838 phosphorylation was determined by immunoblot analysis using an anti-phospho-ASK1(T845) antibody (2nd panel). The position and expression levels of GST-MPK38, re.ASK1(K709R), and re.ASK1(WT) were monitored by immunoblotting with an anti-GST antibody (3rd and bottom panels). The relative level of kinase activity was quantitated by densitometric analyses, and fold increase relative to control containing recombinant wild-type ASK1 alone was calculated. WB, Western blot. B, identification of MPK38 phosphorylation sites on ASK1. An in vitro kinase assay was performed with 5 μg of recombinant ASK1(K709R) or one of its substitution mutants (ASK1(S83A), ASK1(T838A), ASK1(S967A), or ASK1(S1034A)), as well as recombinant wild-type MPK38 (10 μg), as described in A (top panel). The phosphorylation of ASK1 Thr838 and expression of recombinant MPK38 and ASK1 were determined by immunoblotting with anti-phospho-ASK1(T845) and anti-GST antibodies, respectively (2nd to bottom panels). The relative level of kinase activity was quantitated by densitometric analyses, and fold increase relative to control samples containing ASK1(K709R) was calculated. C, after 48 h of transfection with GST-MPK38, HEK293 cell lysates were subjected to precipitation with glutathione-Sepharose beads (GST Purification) and then analyzed by in vitro kinase assays with recombinant ASK1(K709R), MKK6(K82A), and p38 as substrates. The circled P-ASK1(K709R) and circled P-MPK38 indicate the phosphorylated ASK1(K709R) and autophosphorylated MPK38, respectively. re., recombinant.
As the phosphorylation of Thr838 of human ASK1 (Thr845 in mice) correlates with ASK1 activation (21) and MPK38 stimulates ASK1 kinase activity (Fig. 3), we used an in vitro kinase assay using recombinant MPK38 to examine whether this and other (Ser83, Ser967, and Ser1034) ASK1 phosphorylation sites play a role in MPK38-mediated phosphorylation. Mutation of ASK1(K709R) Thr838 to Ala838 completely abolished MPK38-dependent phosphorylation compared with the control with ASK1(K709R) as a substrate (Fig. 4B, top panel, 3rd lane versus 5th lane), indicating that the Thr838 within the activation loop of ASK1 represents a potential phosphorylation site for MPK38. These results suggest that MPK38 directly phosphorylates ASK1 on Thr838 through physical interaction and activates ASK1.
MPK38 Is a Potential Upstream Kinase of ASK1—To examine whether ASK1 can phosphorylate MPK38 through direct interaction, we conducted an in vitro kinase assay using recombinant nonphosphorylated MPK38 (MPK38(K40R)) and wild-type MPK38 as substrates. ASK1 was unable to phosphorylate recombinant MPK38(K40R) (supplemental Fig. S1). Moreover, the phosphorylation of recombinant wild-type MPK38 was not affected by ASK1 (supplemental Fig. S1, lane 4 versus lane 5). These results suggest that, despite physical binding, MPK38 may not be a substrate for ASK1. Based on this, together with the above results obtained in Fig. 4, A and B, we reasoned that MPK38 may be an upstream kinase that activates ASK1. To examine this hypothesis, MPK38 was purified on glutathione-Sepharose beads using HEK293 cell extracts expressing GST-MPK38. MPK38 kinase activity was determined by in vitro kinase assays using recombinant ASK1(K709R), MKK6(K82A), or p38 as substrates. MPK38 phosphorylated recombinant ASK1(K709R), whereas phosphorylation of recombinant MKK6(K82A) and p38 by MPK38 was not detectable (Fig. 4C). These findings suggest that MPK38 may act as an upstream regulator of the MAPKKK ASK1.
MPK38 Increases the Interaction between ASK1 and Its Substrate MKK3—We next examined whether MPK38 contributes to the interaction between ASK1 and its substrate MKK3. HEK293 cells transfected with vectors expressing FLAG-ASK1 and HA-MKK3 in the presence or absence of MPK38 were subjected to immunoprecipitation using an anti-HA antibody, followed by immunoblot analysis using an anti-FLAG antibody. There was a significant increase in complex formation between ASK1 and MKK3 in cells coexpressing MPK38 compared with expression in the absence of MPK38 (Fig. 5A). In contrast, no difference in complex formation was observed in the presence of N-acetyl-l-cysteine (Nac), a potent antioxidant (Fig. 5B). This indicates that intracellular redox regulation might be involved in MPK38-mediated increase of the association between ASK1 and MKK3. We also observed a similar trend showing the importance of the phosphorylation of Thr838 of ASK1 in MPK38-mediated modulation of the complex formation between ASK1 and MKK3 (Fig. 5C). Consistent with this, knockdown of endogenous MPK38 decreased the complex formation between ASK1 and MKK3, although this reduction was overcome by expressing a wobble mutant of MPK38 (Fig. 5D). These results suggested that ASK1 activation by MPK38 increased the interaction between ASK1 and its substrate.
FIGURE 5.
MPK38-mediated modulation of the association between ASK1 and MKK3. A–C, HEK293 cells were transfected with the indicated combinations of HA-MKK3, FLAG-ASK1, FLAG-ASK1(T838A), GST-MPK38, and GST-MPK38(K40R). Cell lysates were then subjected to immunoprecipitation (IP) with an anti-HA antibody, and the resulting immunoprecipitates were analyzed by immunoblot analysis with an anti-FLAG antibody to determine the association of MKK3 with ASK1 in the presence or absence of 15 mm Nac for 30 min. The expression levels of ASK1 and MPK38 in total cell lysates were analyzed by immunoblot using anti-FLAG and anti-GST antibodies. D, effect of MPK38 knockdown on the association between ASK1 and MKK3 was determined by immunoblotting with the indicated antibodies using HEK293 cells transiently transfected with MPK38 siRNA 1 (left) or HaCaT cells (MPK38(KD)) stably expressing MPK38 shRNA (right). MPK38(KD) cells were transfected with 4 μg of plasmid (MPK38(W)) containing a wobble mutant cDNA encoding MPK38 with three synonymous point mutations within the siRNA target sequence (right). The relative level of ASK1-MKK3 complex formation was quantitated by densitometric analyses, and fold increase relative to control expressing ASK1 and MKK3 in the absence of MPK38 was calculated. WB, Western blot.
Because ASK1 was shown to form inactive complexes with Trx or 14-3-3 (3, 8, 22), and ASK1 homo-oligomerization was important for its kinase activity (21, 23), we also investigated the effect of MPK38 on Trx (or 14-3-3) binding to ASK1 and ASK1 homo-oligomerization. Results indicated that MPK38 stimulated ASK1 activity by dissociating Trx (or 14-3-3) from the ASK1-Trx (or 14-3-3) complex through Thr838 phosphorylation and that the homo-oligomerization of ASK1 was highly dependent on intracellular redox status as well as phosphorylation of Thr838 of ASK1 (supplemental Figs. S2 and S3).
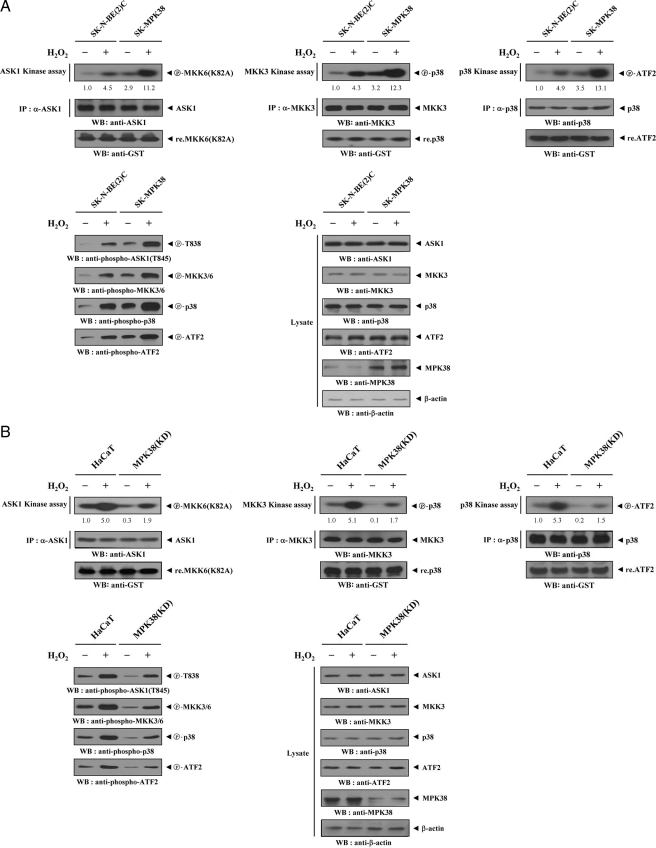
MPK38 Stimulates Signaling Downstream of ASK1—We examined whether MPK38 affects signaling downstream of ASK1, as Hsp72, another interacting partner of ASK1, was previously shown to modulate signaling downstream of ASK1 through its direct interaction with ASK1 (6). Parental SK-N-BE(2)C cells or SK-N-BE(2)C cells stably expressing pCMV-MPK38 (SK-MPK38) were treated with H2O2 and subsequently immunoprecipitated using anti-ASK1, anti-MKK3, and anti-p38 antibodies. Endogenous ASK1, MKK3, and p38 kinase activities were evaluated using in vitro kinase assays. H2O2 sufficiently stimulated the kinase activities of ASK1, MKK3, and p38 in parental SK-N-BE(2)C cells, and these effects were further enhanced by overexpression of MPK38 (Fig. 6A, SK-MPK38, upper panels). A similar result was also observed in immunoblot analysis using anti-phospho-specific antibodies for ASK1 Thr838, MKK3/6, p38, and ATF2 (Fig. 6A, lower left). Consistent with this, knockdown of endogenous MPK38 showed an opposite trend in the modulation of ASK1, MKK3, and p38 kinase activities (Fig. 6B), suggesting that MPK38 stimulates ASK1-mediated signaling to p38 kinase.
FIGURE 6.
Effect of MPK38 on ASK1 downstream signaling. A, MPK38 stimulates ASK1 downstream signaling. Parental SK-N-BE(2)C cells or MPK38 stable transfectants (SK-MPK38) were incubated in the presence or absence of 2 mm H2O2 for 30 min. Cell lysates were subjected to immunoprecipitation (IP) using the indicated antibodies, and the immunoprecipitates were assayed for activities of ASK1, MKK3, or p38 by in vitro kinase assays using the indicated substrates. The expression levels of ASK1, MKK3, and p38 in the immunoprecipitates were analyzed by immunoblot using the indicated antibodies. The presence of equal amounts of each substrate in these assays was confirmed by immunoblotting with an anti-GST antibody (upper, bottom panels). WB, Western blot. B, modulation of ASK1 downstream signaling by knockdown of endogenous MPK38. Parental HaCaT cells or HaCaT cells stably expressing MPK38 shRNA (MPK38(KD)) were treated with 2 mm H2O2 for 30 min. The immunoprecipitates were also subjected to in vitro kinase assays as described in A. ASK1 Thr838, MKK3/6, p38, and ATF2 phosphorylation were determined by immunoblot analysis using the indicated phospho-specific antibodies (A and B, lower, left panels). The expression levels of ASK1, MKK3, p38, ATF2, and MPK38 in total cell lysates were analyzed by immunoblot using the indicated antibodies (A and B, lower, right panels). The relative level of kinase activity was quantitated by densitometric analyses, and fold increase relative to untreated samples in parental cells was calculated.
To determine whether MPK38 targets ASK1 directly, we also performed in vitro kinase assays of ASK1, MKK3, MKK6, and p38 in the presence or absence of recombinant MPK38. HEK293 cells were transiently transfected with HA-ASK1, HA-MKK3, HA-MKK6, or HA-p38 and treated with or without H2O2. The cells were then subjected to immunoprecipitation with an anti-HA antibody. The resulting immunoprecipitates were assayed for kinase activities of ASK1, MKK3, MKK6, or p38 in the presence or absence of recombinant MPK38. MPK38 specifically stimulated ASK kinase activity but had no effect on the kinase activities of MKK3, MKK6, or p38 (supplemental Fig. S4). These results suggest that MPK38 enhances ASK1-mediated signaling through direct stimulation of ASK1.
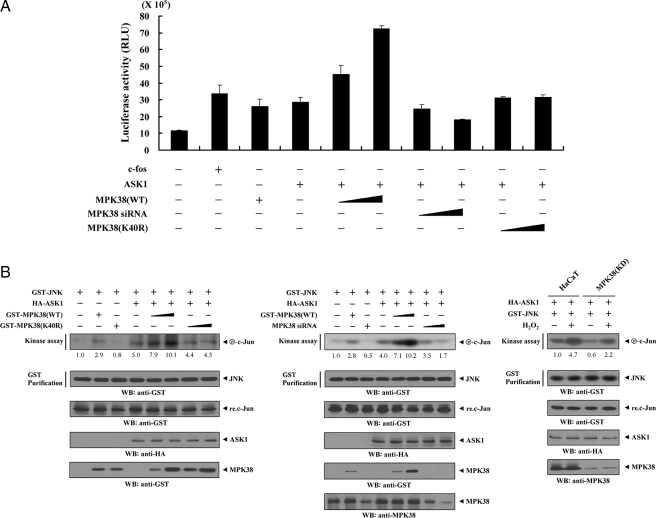
MPK38 Stimulates ASK1-mediated AP-1 Transcriptional Activity in a Kinase-dependent Manner—ASK1 is a MAPKKK involved in the activation of JNK/stress-activated protein kinase and p38 MAPK (2, 24). Because AP-1 is a transcription factor activated by JNK and p38 kinases, we used an AP-1 luciferase reporter to determine whether MPK38 affects ASK1-mediated transactivation. Wild-type MPK38 (MPK38(WT)) significantly increased ASK1-mediated AP-1 transcriptional activity in a dose-dependent manner, whereas kinase-dead MPK38 had no effect (Fig. 7A). We also confirmed the roles of MPK38 in ASK1-mediated transactivation using the MPK38 knockdown system. Transfection of MPK38 siRNA resulted in a significant decrease in ASK1-mediated AP-1 transcriptional activity that was proportional to the amount of MPK38 siRNA transfected (Fig. 7A, 4th lane versus 7th and 8th lanes).
FIGURE 7.
Up-regulation of JNK-mediated transcription by MPK38. A, 293T cells were transfected with 0.2 μg of AP-1 luciferase plasmid and increasing amounts of wild-type (WT) and kinase-dead (K40R) forms of MPK38 (6 and 9 μg) and MPK38 siRNA 1 (50 and 200 nm) as indicated in the presence or absence of c-Fos (0.6 μg). Data shown are means (± S.E.) of three independent experiments. p < 0.05 relative to control; significance calculated by Student's t test. B, enhancement of JNK activity by MPK38. HEK293 cells were cotransfected using GST-JNK (1.5 μg) and HA-ASK1 (2 μg) in the presence or absence of increasing amounts of wild-type (WT) and kinase-dead (K40R) forms of MPK38 (6 and 9 μg) or MPK38 siRNA 1 (100 and 200 nm). An in vitro kinase assay for JNK activity was performed as described under “Materials and Methods.” The amounts of precipitated JNK and the expression level of ASK1 and MPK38 in total cell lysates were determined by immunoblot analysis using anti-GST and anti-HA antibodies. MPK38(KD) cells transfected with GST-JNK and HA-ASK1 were also precipitated using glutathione-Sepharose beads (GST Purification), and the precipitates were subjected to an in vitro kinase assay using c-Jun as a substrate to determine JNK activity (right). The relative level of JNK activity was quantitated by densitometric analyses, and fold increase relative to control HEK293 cells expressing JNK alone or untreated HaCaT cells expressing JNK and ASK1 was calculated. WB, Western blot.
Next, to test the effect of MPK38 on ASK1-mediated JNK activation, HEK293 cells were transfected with vectors expressing GST-JNK and HA-ASK1 in the presence or absence of wild-type and kinase-dead MPK38. JNK activity was evaluated using an in vitro kinase assay with c-Jun as a substrate. As expected, ASK1-mediated JNK activation was markedly increased by wild-type MPK38 in a dose-dependent manner, whereas kinase-dead MPK38 had no effect on ASK1-mediated JNK activation (Fig. 7B, left). To verify whether the knockdown of endogenous MPK38 contributed to altered ASK1-mediated JNK activation, HEK293 cells transfected with GST-JNK and HA-ASK1, together with MPK38-specific siRNA, were purified on glutathione-Sepharose beads, followed by an in vitro kinase assay using c-Jun as a substrate. ASK1-mediated JNK activation was decreased in a dose-dependent manner in MPK38-knockdown cells compared with control cells expressing JNK and ASK1 in the absence of MPK38 siRNA (Fig. 7B, middle, 4th lane versus 7th and 8th lanes). A similar result was also observed in HaCaT cells stably expressing an shRNA targeting MPK38 (MPK38(KD)) (Fig. 7B, right). These results suggest that ASK1 phosphorylation that is mediated via direct interaction with MPK38 increases ASK1-mediated signaling to both JNK and p38 kinases.
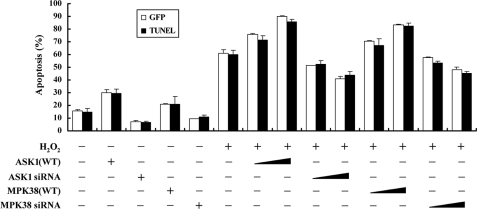
MPK38 Stimulates H2O2-mediated Apoptosis—Because MPK38 interacts with ASK1 (see Figs. 1 and 2) and the ASK1 activation induces apoptotic cell death under various conditions (2, 3), we next examined whether MPK38 could influence H2O2-mediated cell death. Expression of wild-type MPK38 in HEK293 cells resulted in a significant increase in H2O2-induced cell death in a dose-dependent manner, as determined by GFP system and TUNEL staining (Fig. 8). This indicated that MPK38 is involved in H2O2-mediated cell death. We also determined whether the kinase activity of MPK38 was necessary for modulation of H2O2-mediated cell death because only wild-type MPK38 induced ASK1 activity (see Fig. 3B). No difference in the H2O2-mediated cell death was found in the presence of kinase-dead MPK38 compared with the control treated with H2O2 in the absence of MPK38 (supplemental Fig. S5, lane 4 versus lanes 7 and 8), indicating an important role for MPK38 kinase activity in the modulation of H2O2-mediated cell death.
FIGURE 8.
Effect of MPK38 on H2O2-mediated apoptosis. HEK293 cells were transiently transfected with increasing amounts of wild-type MPK38 (0.8 and 1.6 μg) and ASK1 (0.5 and 1 μg) or MPK38 and ASK1 siRNAs (100 and 200 nm) in the presence or absence of H2O2. Apoptotic cell death was determined using the GFP expression system (GFP) or TUNEL. Cells exposed to 1 mm H2O2 for 9 h was used as a positive control. Data shown are means (±S.E.) of three independent experiments. p < 0.05 relative to control; significance calculated by Student's t test.
We also performed knockdown experiments of MPK38 using MPK38 siRNA. Transfection of HEK293 cells with siRNA duplexes targeting MPK38 resulted in a significant decrease of H2O2-mediated apoptosis, proportional to the amount of siRNA used in the transfection (Fig. 8, 6th lane versus 13th and 14th lanes). These data strongly suggest that MPK38 contributes to H2O2-mediated apoptosis by enhancing the ASK1 activity through phosphorylation.
ASK1 has been implicated in caspase-3 activation (25, 26). PARP is known to be as an in vivo substrate of capase-3 (27). Based on this, to investigate whether the stimulation of H2O2-mediated apoptosis by MPK38 is dependent on its activation of caspase-3, leading to the proteolytic cleavage of PARP, we assessed the effects of MPK38 on H2O2-induced caspase-3 activity and PARP cleavage by immunoblotting with anti-caspase-3 and anti-PARP antibodies, respectively. Results indicated that MPK38 stimulated H2O2-mediated apoptosis by up-regulating an in vivo capase-3 activity (supplemental Fig. S6).
DISCUSSION
The mechanism for ASK1 activation in response to apoptotic stimuli has not been fully elucidated, despite the proposed function of ASK1 in mediating multiple cell death pathways. Recent studies have demonstrated that ASK1 activity is positively or negatively regulated by its interacting partners, including tumor necrosis factor receptor-associated factor (28), Daxx (1), JNK/stress-activated protein kinase-associated protein 1 (JSAP1)/JNK-interacting protein 3 (JIP3) (29), thioredoxin (3, 4), glutaredoxin (7), HSP72 (6), Raf-1 (3), Akt/PKB (30), PP5 (5), and 14-3-3 (8). In addition, ASK1 phosphorylation also regulates its activity (5, 21, 30, 31). Akt/PKB binds to and phosphorylates Ser83 of ASK1, resulting in the inhibition of ASK1-mediated apoptosis (30); and 14-3-3 interacts with phosphorylated Ser967 of ASK1 to inhibit ASK1 function (8). Conversely, PP5 dephosphorylates murine Thr845 within the activation loop of ASK1 that is critical for ASK1 activation and thereby inhibits ASK1-mediated apoptosis (5). Although Akt/PKB was recently described as a kinase that is critical for ASK1 Ser83 phosphorylation (30), the question remains which kinases are responsible for ASK1 phosphorylation at multiple sites, including Thr845, Ser967, and Ser1034. To gain insight into the mechanism(s) by which MPK38 positively regulates ASK1 activity, we examined whether the Thr838 (corresponding to Thr845 in mice) plays a key role in MPK38-mediated phosphorylation using an in vitro kinase assay and immunoblot analysis. MPK38 was shown to phosphorylate Thr838 of ASK1, but not Ser83, Ser967, and Ser1034 (Fig. 4B). In addition, the previous finding of Thr845 phosphorylation in kinase-dead ASK1 of H2O2-treated ASK1-null mouse embryonic fibroblasts strongly supports the notion that Thr845 phosphorylation occurs via a putative Thr845 kinase in cells (21). A recent report (32) showing that the ASK1-interacting partner ASK2 activates ASK1 through Thr838 phosphorylation is interesting in regards to the search for this putative kinase.
We examined whether MPK38 functions as an upstream kinase of ASK1 or another MAPKKK, because recent studies showed that ASK1 associated with other MAPKKKs, including TAK1 (33) and Raf-1 (34). These interactions lead to ASK1-mediated inhibition of TAK1-mediated NF-κB signaling as well as Raf-1-mediated inhibition of ASK1 kinase activity. As shown in Fig. 4C, MPK38 was able to phosphorylate GST-ASK1(K709R), but not GST-MKK6(K82A) or GST-p38. Furthermore, neither ASK1 nor TAK1 could phosphorylate GST-MPK38(K40R) in a reciprocal way (see supplemental Fig. S1 and data not shown). These results strongly suggest that MPK38, unlike ASK2 (32), is apparently an upstream kinase of ASK1 but not of other MAPKKKs. In addition, MPK38 and ASK2 are not like each other in terms of their overall structures; MPK38 possesses an amino-terminal kinase domain and a carboxyl-terminal regulatory domain (9), but ASK2 contains a kinase domain in the middle portion with amino- and carboxyl-terminal flanking regions (32). Furthermore, there is only 29% amino acid identity between MPK38 and ASK2, particularly in the kinase domain. Based on this, we speculate that MPK38 and ASK2 work in different ways to regulate ASK1 activity, despite the ability of these two kinases to phosphorylate the same site (Thr838/Thr845) of ASK1. However, we cannot rule out the possibility that ASK1 activation can be also triggered by ASK1 oligomerization, which is independent of direct phosphorylation of Thr845 through putative Thr845 kinases.
Little is known about the biological functions or the molecular mechanism(s) underlying MPK38 activity, despite its implicated involvement in various cellular processes, including cell cycle, spliceosome assembly, carcinogenesis, and self-renewal of stem cells (35). The results presented here suggest that MPK38 stimulates the activation of ASK1-mediated signaling to both JNK and p38 kinases through direct interaction with and phosphorylation of ASK1, and that the MPK38-ASK1 interaction may provide a molecular basis for the proposed several MPK38 functions. Moreover, the role of MPK38 as an inducer of ASK1-mediated signaling may clarify the mechanism(s) underlying MPK38-mediated signaling in carcinogenesis. We have revealed that MPK38 directly binds to and activates ASK1 to stimulate ASK1-mediated signaling through ASK1 phosphorylation at Thr838, and our results suggest that MPK38 is a potential upstream kinase of ASK1. In addition, H2O2-dependent modulation of MPK38 activity may provide an effective way to study the biological role of MPK38 in detail.
Supplementary Material
This work was supported by Korea Science and Engineering Foundation Grant R0A-2007-000-20006-0 and in part by Chungbuk National University Grant 2007. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
Footnotes
The abbreviations used are: ASK1, apoptosis signal-regulating kinase 1; TNF-α, tumor necrosis factor α; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; MAPKKK, MAPK kinase kinase; Trx, thioredoxin; GFP, green fluorescent protein; GST, glutathione S-transferase; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling; HA, hemagglutinin; siRNA, small interfering RNA; shRNA, short hairpin RNA; PARP, poly(ADP-ribose) polymerase; DTT, dithiothreitol; Nac, N-acetyl-l-cysteine.
References
- 1.Chang, H. Y., Nishitoh, H., Yang, X., Ichijo, H., and Baltimore, D. (1998) Science 281 1860–1863 [DOI] [PubMed] [Google Scholar]
- 2.Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K., and Gotoh, Y. (1997) Science 275 90–94 [DOI] [PubMed] [Google Scholar]
- 3.Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono, K., and Ichijo, H. (1998) EMBO J. 17 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, H., Nishitoh, H., Ichijo, H., and Kyriakis, J. M. (2000) Mol. Cell. Biol. 20 2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita, K., Saitoh, M., Tobiume, K., Matsuura, H., Enomoto, S., Nishitoh, H., and Ichijo, H. (2001) EMBO J. 20 6028–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park, H. S., Cho, S. G., Kim, C. K., Hwang, H. S., Noh, K. T., Kim, M. S., Huh, S. H., Kim, M. J., Ryoo, K., Kim, E. K., Kang, W. J., Lee, J. S., Seo, J. S., Ko, Y. G., Kim, S., and Choi, E. J. (2002) Mol. Cell. Biol. 22 7721–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song, J. J., Rhee, J. G., Suntharalingam, M., Walsh, S. A., Spitz, D. R., and Lee, Y. J. (2002) J. Biol. Chem. 277 46566–46575 [DOI] [PubMed] [Google Scholar]
- 8.Zhang, L., Chen, J., and Fu, H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8511–8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil, M., Yang, Y., Lee, Y., Choi, I., and Ha, H. (1997) Gene (Amst.) 195 295–301 [DOI] [PubMed] [Google Scholar]
- 10.Heyer, B. S., Warsowe, J., Solter, D., Knowles, B. B., and Ackerman, S. L. (1997) Mol. Reprod. Dev. 47 148–156 [DOI] [PubMed] [Google Scholar]
- 11.Gray, D., Jubb, A. M., Hogue, D., Dowd, P., Kljavin, N., Yi, S., Bai, W., Frantz, G., Zhang, Z., Koeppen, H., de Sauvage, F. J., and Davis, D. P. (2005) Cancer Res. 65 9751–9761 [DOI] [PubMed] [Google Scholar]
- 12.Hemmati, H. D., Nakano, I., Lazareff, J. A., Masterman-Smith, M., Geschwind, D. H., Bronner-Fraser, M., and Kornblum, H. I. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes, D. R., Yu, J., Shanker, K., Deshpande, N., Varambally, R., Ghosh, D., Barrette, T., Pandey, A., and Chinnaiyan, A. M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9309–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, C., Gale, M., Jr., Keller, B. C., Huang, H., Brown, M. S., Goldstein, J. L., and Ye, J. (2005) Mol. Cell 18 425–434 [DOI] [PubMed] [Google Scholar]
- 15.Yang, Y., Gil, M., Lee, Y., and Ha, H. (1999) Appl. Biochem. Biotechnol. 80 13–22 [DOI] [PubMed] [Google Scholar]
- 16.Jung, H., Kim, T., Chae, H. Z., Kim, K. T., and Ha, H. (2001) J. Biol. Chem. 276 15504–15510 [DOI] [PubMed] [Google Scholar]
- 17.Seong, H.-A., Jung, H., and Ha, H. (2007) J. Biol. Chem. 282 12075–12096 [DOI] [PubMed] [Google Scholar]
- 18.Jung, H., Seong, H.-A., and Ha, H. (2007) J. Biol. Chem. 282 35293–35307 [DOI] [PubMed] [Google Scholar]
- 19.Seong, H.-A., Gil, M., Kim, K.-T., Kim, S. J., and Ha, H. (2002) Biochem. J. 361 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang, M., and Shen, X. (2006) J. Neurochem. 97 234–244 [DOI] [PubMed] [Google Scholar]
- 21.Tobiume, K., Saitoh, M., and Ichijo, H. (2002) J. Cell. Physiol. 191 95–104 [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., Yin, G., Surapisitchat, J., Berk, B. C., and Min, W. (2001) J. Clin. Investig. 107 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotoh, Y., and Cooper, J. A. (1998) J. Biol. Chem. 273 17477–17482 [DOI] [PubMed] [Google Scholar]
- 24.Wang, X. S., Diener, K., Jannuzzi, D., Trollinger, D., Tan, T. H., Lichenstein, H., Zukowski, M., and Yao, Z. (1996) J. Biol. Chem. 271 31607–31611 [DOI] [PubMed] [Google Scholar]
- 25.Hatai, T., Matsuzawa, A., Inoshita, S., Mochida, Y., Kuroda, T., Sakamaki, K., Kuida, K., Yonehara, S., Ichijo, H., and Takeda, K. (2000) J. Biol. Chem. 275 26576–26581 [DOI] [PubMed] [Google Scholar]
- 26.Patel, T., Gores, G. J., and Kaufmann, S. H. (1996) FASEB J. 10 587–597 [DOI] [PubMed] [Google Scholar]
- 27.Chinnaiyan, A. M., Orth, K., O'Rourke, K., Duan, H., Poirier, G. G., and Dixit, V. M. (1996) J. Biol. Chem. 271 4573–4576 [DOI] [PubMed] [Google Scholar]
- 28.Nishitoh, H., Saitoh, M., Mochida, Y., Takeda, K., Nakano, H., Rothe, M., Miyazono, K., and Ichijo, H. (1998) Mol. Cell 2 389–395 [DOI] [PubMed] [Google Scholar]
- 29.Matsuura, H., Nishitoh, H., Takeda, K., Matsuzawa, A., Amagasa, T., Ito, M., Yoshioka, K., and Ichijo, H. (2002) J. Biol. Chem. 277 40703–40709 [DOI] [PubMed] [Google Scholar]
- 30.Kim, A. H., Khursigara, G., Sun, X., Franke, T. F., and Chao, M. V. (2001) Mol. Cell. Biol. 21 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii, K., Goldman, E. H., Park, H. R., Zhang, L., Chen, J., and Fu, H. (2004) Oncogene 23 5099–5104 [DOI] [PubMed] [Google Scholar]
- 32.Takeda, K., Shimozono, R., Noguchi, T., Umeda, T., Morimoto, Y., Naguro, I., Tobiume, K., Saitoh, M., Matsuzawa, A., and Ichijo, H. (2007) J. Biol. Chem. 282 7522–7531 [DOI] [PubMed] [Google Scholar]
- 33.Mochida, Y., Takeda, K., Saitoh, M., Nishitoh, H., Amagasa, T., Ninomiya-Tsuji, J., Matsumoto, K., and Ichijo, H. (2000) J. Biol. Chem. 275 32747–32752 [DOI] [PubMed] [Google Scholar]
- 34.Chen, J., Fujii, K., Zhang, L., Roberts, T., and Fu, H. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beullens, M., Vancauwenbergh, S., Morrice, N., Derua, R., Ceulemans, H., Waelkens, E., and Bollen, M. (2005) J. Biol. Chem. 280 40003–40011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.