Abstract
Tolerance to otherwise lethal cerebral ischemia in vivo or to oxygen-glucose deprivation (OGD) in vitro can be induced by prior transient exposure to N-methyl-d-aspartic acid (NMDA): preconditioning in this manner activates extrasynaptic and synaptic NMDA receptors and can require bringing neurons to the “brink of death.” We considered if this stressful requirement could be minimized by the stimulation of primarily synaptic NMDA receptors. Subjecting cultured cortical neurons to prolonged elevations in electrical activity induced tolerance to OGD. Specifically, exposing cultures to a K+-channel blocker, 4-aminopyridine (20–2500 μm), and a GABAA receptor antagonist, bicuculline (50 μm) (4-AP/bic), for 1–2 days resulted in potent tolerance to normally lethal OGD applied up to 3 days later. Preconditioning induced phosphorylation of ERK1/2 and CREB which, along with Ca2+ spiking and OGD tolerance, was eliminated by tetrodotoxin. Antagonists of NMDA receptors or l-type voltage-gated Ca2+ channels (L-VGCCs) applied during preconditioning decreased Ca2+ spiking, phosphorylation of ERK1/2 and CREB, and OGD tolerance more effectively when combined, particularly at the lowest 4-AP concentration. Inhibiting ERK1/2 or Ca2+/calmodulin-dependent protein kinases (CaMKs) also reduced Ca2+ spiking and OGD tolerance. Preconditioning resulted in altered neuronal excitability for up to 3 days following 4-AP/bic washout, based on field potential recordings obtained from neurons cultured on 64-channel multielectrode arrays. Taken together, the data are consistent with action potential-driven co-activation of primarily synaptic NMDA receptors and L-VGCCs, resulting in parallel phosphorylation of ERK1/2 and CREB and involvement of CaMKs, culminating in a potent, prolonged but reversible, OGD-tolerant phenotype.
It is well established that the brain can be prepared to withstand an ischemic insult by a process known as preconditioning (1). Preconditioning can be achieved by subjecting the brain to transient ischemia in vivo or cultured neurons to oxygen and glucose deprivation (OGD)2 in vitro, resulting in delayed, potent, and reversible tolerance to otherwise lethal ischemia/OGD. Neurons can also be preconditioned by exposure to numerous environmental or chemical stimuli that generate “cross-tolerance” to ischemia (2). A key role for activation of the NMDA type of glutamate receptor during preconditioning by ischemia or OGD is well established (3–10), although agreement is not complete (11, 12). Moreover, transient non-lethal exposure to high dose glutamate or NMDA is sufficient on its own to also induce potent cross-tolerance to ischemia (13) or to OGD (1, 6, 14). To date, preconditioning achieved either by transient ischemia OGD or NMDA can require bringing neurons to the “brink of death” (1, 14, 15), a trait shared with some other preconditioning paradigms (2). Induction of a state resistant to ischemia or OGD by brink of death preconditioning may be a “byproduct” of neurons responding to the intense stressor, a state that persists only until normal baseline homeostasis is restored.
Can NMDA receptors be engaged in preconditioning neurons against ischemia without approaching the threshold required for death? Synaptic plasticity describes a process whereby the strength of synapses, as well as the efficiency of communication between synapses, is modified by normal (as well as pathological) activity. Neuronal fate can differ depending on the location of NMDA receptors on neurons, with survival or demise linked with synaptic or extrasynaptic NMDA receptor activation, respectively. Perhaps synaptic NMDA receptor activation associated with synaptic plasticity may circumvent the brink of death requirement of preconditioning, a constraint that may be more associated with activation of extrasynaptic NMDA receptors (14). Here we investigate if increasing synaptic input is sufficient to induce tolerance to OGD in neurons.
Elevations in electrical activity, achieved by exposing neurons to a GABAA receptor antagonist, bicuculline, sometimes combined with a K+ channel blocker, 4-aminopyridine (4-AP/bic treatment), or by treatment with low concentrations of NMDA, induce tolerance in neuronal culture to apoptosis induced by serum deprivation or by exposure to chemical agents, or to excitotoxicity induced in apoptotic and non-apoptotic manners, or oxidative stress (16–22). Activation of synaptic NMDA receptor-mediated activation and phosphorylation of CREB (pCREB) is implicated in neuroprotection elicited by electrical activity. Likewise, activation of synaptic NMDA receptors induces prolonged phosphorylation and activation of extracellular signal-regulated kinases, ERK1/2, which are members of the mitogen-activated protein kinase family, (23–25), although the role of ERK1/2 in neuroprotection induced by electrical activity has not yet been examined. In contrast, extrasynaptic NMDA receptor activation can induce an ERK1/2 and CREB phosphorylation “shut-off” pathway.
We considered if 4-AP/bic treatment of cultured cortical neurons induced a synaptic NMDA receptor-mediated signaling pathway, which culminated in tolerance to OGD, thereby accomplishing preconditioning in a manner more aligned with neuronal signaling associated with synaptic plasticity, rather than with a brink of death stress.
EXPERIMENTAL PROCEDURES
Materials—Tissue culture dishes and plates were purchased from either Du Pont-Invitrogen (Burlington, Ontario, Canada) or VWR Canlab (Mississauga, Ontario, Canada). Glass coverslips were purchased from Bellco Glass, Inc. (Vineland, NJ). Fetal bovine serum was bought from Gemini Bio (Woodland, CA). Minimal essential medium was obtained from Wisent Canadian Laboratories (St-Bruno, Quebec, Canada). Horse serum was acquired from HyClone Laboratories (Logan, UT). Fluo-4-AM was bought from Molecular Probes (Eugene, OR). H89 was bought from Axxora (San Diego, CA). 4-AP, 5,7-dichlorothiokynurenic acid, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801), KN-62, and KN-93 were purchased from Tocris Bioscience (Ellisville, MO). Bicuculline, 8-cyclopenthyltheophylline, glybenclamide, memantine, nifedipine, N-methyl-d-aspartate (NMDA), propidium iodide (PI), tetrodotoxin (TTX), U0126, KT 5720, tolbutamide, PKA inhibitory fragment 14–22 (myristoylated), staurosporine, a protease mixture inhibitor, and all other reagents were purchased from Sigma. The serum-free protein block and Antibody Diluting Buffer were purchased from DakoCytomation (Mississauga, Ontario, Canada).
Preparation of Cultures—Cultures of E18 rat cortical neurons were prepared as described previously, with experiments performed on cultures grown 14–18 days in vitro (26).
Experimental Treatment of Cultures—For preconditioning experiments, each 12-well plate was subjected to 4 conditions in culture medium: 4-AP/bic (preconditioned), control wash, 4-AP/bic plus antagonist/inhibitor, or antagonist/inhibitor alone, followed by washing and replacement with stored culture medium. OGD was performed as previously described (26) by placing 12-well plates containing cortical cultures exposed to a basic salt solution (which had the following composition (n mm): 140 NaCl, 5 KCl, 2 CaCl2, 20 HEPES, 0.03 glycine, pH 7.4) in a 37 °C incubator housed in an anaerobic glovebox (Forma Scientific, Marjetta, OH).
Assessment of Neuronal Injury—Neuronal injury was assessed 24 h following treatments as described previously using PI, with fluorescence quantitated using a Cytofluor 2350 fluorescence platereader (Millipore Corp., Bedford, MA) (26). Briefly, cultures were exposed to basic salt solution containing 3 mm glucose with 33 μg/ml PI for 30 min at 22 °C, followed by measurement of fluorescence intensities from four locations within each well (Ex = 520 ± 20 nm; Em = 645 ± 20 nm). For normalization purposes, PI was added to sister cultures that were untreated (0% PI-uptake) or exposed to 100 μm NMDA for 15 min to kill all neurons (100% PI-uptake).
Intracellular Ca2+ Spiking—To monitor intracellular Ca2+ spiking, fluorescence images were acquired every 2 s for 120 s from cultures previously loaded with 4.5 μm Fluo-4-AM (26). Excitation was delivered through a Lambda DG-5 shutter instrument (Sutter Instrument Co, Novato, CA) housing a Zeiss Axiovert 200 inverted microscope (Ex 480 ± 15 nm; Em 535 ± 20 nm) equipped with a Fluar 40×/1.3 numerical aperture oil immersion objective, with images captured using a low light-sensitive Retiga Exi camera (QImaging, Burnaby, BC, Canada). Due to variability between coverslips in 4-AP/bic-induced Ca2+-spiking profiles, each coverslip served as its own internal control. A minimum of two sets of 120-s runs was acquired for 4-AP/bic alone, and then for 4-AP/bic with a drug, with a different region of interest chosen for each spike train. For each drug evaluated, images were acquired from a minimum of two coverslips from a minimum of two different platings. Plots of Ca2+ spike trains represent average intensities calculated from 12–15 neuronal soma.
Immunoblotting—The effect of preconditioning on levels of CREB (phosphorylated and non-phosphorylated), ERK1/2 (phosphorylated), or actin was determined by immunoblotting. Cultures were washed with basic salt solution and exposed to radioimmune precipitation assay buffer (50 mm Tris, 150 mm NaCl, 2 mm EDTA, 0.1% SDS, 1% deoxycholate, 1% Triton X-100, phenylmethylsulfonyl fluoride, and protease mixture inhibitor), manually homogenized over ice, vortexed briefly and stored at –80 °C. Upon re-thawing, samples were vortexed for 30 s and centrifuged at 5000 × g for 5 min at 4 °C. The protein concentration of supernatants was determined using a Bio-Rad DC protein assay kit. Protein homogenates were loaded (15 μg) onto 10% SDS-PAGE gels and subsequently transferred to polyvinylidene difluoride membranes (NEN Life Science Products, Boston, MA). Membranes were blocked with 5% skim milk-TBST (200 mm Tris, 140 mm NaCl, 1% Tween-20 (v/v), pH 7.60) for 1 h at room temperature, and then for 4 h at 4 °C. Immunoblotting was performed by overnight incubation (4 °C) with either anti-phospho-Ser133 CREB (1:2000 in 5% milk-TBST, rabbit polyclonal, Upstate (Millipore), Lake Placid, NY) or anti-phospho-ERK1/2 (1:1000 in 5% milk-TBST, mouse monoclonal (E10), Cell Signaling Technology, Beverly, MA). Membranes were washed 3 × for 5 min each and placed into the appropriate horseradish peroxidase-linked anti-mouse/rabbit secondary antibody (1:5000 for anti-pCREB or CREB and 1:2000 for anti-pERK1/2 in 5% milk-TBST, goat anti-rabbit, Amersham Biosciences). After each phosphor-antibody was examined, membranes were subjected to a stripping buffer (62 mm Tris, 69 mm SDS, 0.8% β-mercaptoethanol) for 30 min at 55 °C with constant agitation before being reprobed with anti-CREB (1:1000, rabbit polyclonal, Santa Cruz Biotechnology Inc., Santa Cruz, CA) or anti-actin (1:5000, rabbit polyclonal, Sigma-Aldrich). Membranes were treated with an ECL solution (Amersham Biosciences, Baie d'Urfè, PQ, Canada) and exposed to imaging film (Kodak). Images acquired within the linear range of exposures were quantified by densitometric analyses of background-subtracted bands using Northern Eclipse software (Empix Imaging Inc., Mississauga, Ontario, Canada). Immunoblots are representative of three to five experiments from a minimum of three different platings, with each experiment comprised of two to three wells/condition.
Immunofluorescence—Immunofluorescence was performed on cultures to detect cellular pCREB immunoreactivity. Cultures were fixed in 4% paraformaldehyde for 10 min, exposed to 0.25% Triton X-100 in phosphate-buffered saline for 10 min, and, to block nonspecific staining, cultures were exposed to serum-free protein block (DakoCytomation) for 1 h at room temperature. Cultures were then treated with anti-phospho-Ser133 CREB (1:300 in Antibody Diluting Buffer) and anti-MAP2 (1:300 in Antibody Diluting Buffer, mouse monoclonal, Sigma) for 1 h at room temperature, followed by treatment with secondary antibodies (Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 568 goat anti-mouse, Molecular Probes, Eugene, OR) for 1 h at room temperature protected from light. Omitting the primary antibody exposure served as a negative control. Coverslips were mounted on slides using Vectashield Hard Set Mounting Medium for Fluorescence (Vector, Burlingame, CA). Fluorescence imaging was performed using an LSM-410 Zeiss inverted laser scanning microscope (Carl Zeiss, Thornwood, NY).
MEA Acquisition and Analysis—Prior to initial use, a multielectrode dish (MEA, Alpha MED Sciences, Tokyo, Japan) was washed with deionized water, sterilized with 70% ethanol and UV radiation, and coated overnight with 0.1% polyethyleneimine in 25 mm borate buffer. Cells were dispensed into a silicon tube placed on an MEA to allow coverage of the 64 electrodes but not the perimeter reference electrodes; this tubing was removed 3 h later after cells had adhered to the MEA. Each electrode had a 20-μm diameter, an inter-electrode distance of 100 μm, an impedance of <22 kΩ (1 kHz, 50 mV applied sinusoidal wave), and an average RMS noise of 4.07 μV. For a recording session, the MEA was removed from the standard incubator and placed into a 34 °C incubator, allowed to recover for 15–30 min, and connected to a MED64 recording system (Alpha MED Sciences), and 60 s of activity was acquired at 20 kHz for each of 64 channels using MEA software.
Analyses were performed using Matlab. Data were pre-processed by down-sampling the original resolution of 20 kHz to 1 kHz by taking the mean across samples within each bin time (other techniques such as taking the maximum value provided similar qualitative results). Confidence lines were computed by determining the mean and standard deviation of each channel over the 60-s window, followed by subtracting the standard deviation (S.D.) from the mean of each channel: Confidence-Linec = Meanc–(3 × S.D.c), where c indexes the channels. The same procedure was applied for every experimental condition, except the S.D. used to compute the confidence line was always calculated for the baseline condition. Peaks in each channel were identified by scanning through the 60-s time window and determining when the amplitude crossed the confidence line (i.e. went from a value above the confidence line to a value below it). The temporal distributions of peaks were obtained by computing the temporal interval between peaks, and averaging across all channels for a given condition. The distributions of amplitude were obtained by computing the difference between the peak amplitude and the confidence line: PeakAmplitudec,t = Amplitudec,t–ConfidenceLinec, where t indexes the timing of a peak, and Amplitudec,t is the raw amplitude of a channel c at peak t. Each MEA was analyzed separately by performing a Student's t test (α = 0.05, with Bonferroni correction for multiple comparisons: 5 conditions × 5 cultures, α = 0.05/25 = 0.002). For each MEA, the average timing and amplitude of peaks for each individual channel was determined, followed by comparisons aimed at determining if there were significant differences in the timing and amplitude of peaks between the baseline and other conditions.
Statistical Analyses—Data are presented as the mean ± S.E. Statistical comparisons were made by analysis of variance (ANOVA) or the Student's t test. When significant differences were observed, the Bonferroni test was employed for multiple comparisons. Statistical significance was inferred at p < 0.05, unless a Bonferroni correction was applied (as noted above), in which case p < 0.002.
RESULTS
4-AP/bic Preconditioning Induces OGD Tolerance—Neuronal glutamatergic synaptic activity can be enhanced by 4-aminopyridine (4-AP), which prolongs the duration of neuronal action potentials, and by bicuculline (bic), a GABAA receptor antagonist, which induces network disinhibition, resulting in action potential-dependent intracellular Ca2+ spiking and synaptic release of neurotransmitters such as glutamate (27). To determine if excitatory activity elevated in this manner induced tolerance to otherwise lethal OGD, rat cortical neuron cultures were exposed to 4-AP (2.5 mm) and bicuculline (50 μm) (4-AP/bic preconditioning) for 48 h, followed by washout and a 24-h recovery period, and exposed to 65–80 min OGD. In the absence of preconditioning, OGD resulted in destruction of neuronal morphology and substantial PI uptake and fluorescence; in contrast, OGD did not have these effects on preconditioned cultures, because neuronal morphology was preserved and PI fluorescence was negligible (Fig. 1, A–H). Bicuculline treatment alone was not as effective as 4-AP/bic in providing OGD tolerance (data not shown).
FIGURE 1.
A–H, preconditioning rat cortical neurons with 4-aminopyridine/bicuculline induces tolerance to otherwise lethal OGD. Neurons were subjected to sham wash or to preconditioning by 2.5 mm 4-AP plus 50 μm bicuculline (4-AP/bic) for 48 h, followed by a 24-h recovery period, then subjected to control wash or 65–80 min OGD, followed by assessment of neurotoxicity by measurement of the % PI-uptake 24 h later. Same-field phase contrast and PI fluorescence image pairs were acquired from the following conditions: A and B, sham wash; C and D, 4-P/bic preconditioning, followed by OGD; E and F, OGD; or G and H, 100% neuronal kill achieved by subjecting sister cultures to 100 μm NMDA for 15 min. I, preconditioning increases neuronal activity. Neuronal intracellular Ca2+ spiking in Fluo-4-loaded neurons 0.5–2.5 h following application of sham wash, 50 μm bicuculline (bic), 20 μm 4-aminopyridine plus 50 μm bicuculline (4-AP/bic), or to 4AP/bic applied with a Na+ channel blocker, tetrodotoxin (TTX, 1 μm). J–P, a wide range of 4-AP/bic concentrations and duration of exposure, as well as the duration of the recovery interval between preconditioning and OGD, result in neuroprotection. Cultures were subjected to sham wash or to preconditioning with 50 μm bicuculline combined with 4-AP (20, 300, or 2500 μm) for 4–48 h, followed by washout and recovery for 0–96 h, subsequently exposed to 65–80 min OGD, followed by measurement of % PI-uptake 24 h later (n = 12–18). Preconditioning resulted in a significant suppression of % PI-uptake following OGD (p < 0.05; calculated using an ANOVA), except for the shortest 4-AP/bic duration investigated (4 h; Fig. 1P) or for the longest interval between preconditioning and OGD investigated (96 h; Fig. 1J).
To confirm Ca2+ spiking by preconditioning, neuronal intracellular Ca2+ levels were monitored in Fluo-4-loaded cultures. The frequency and amplitude of action-potential-dependent Ca2+ spikes increased above background levels upon exposure to bicuculline, and was further increased with the co-addition of 4-AP (Fig. 1I). Hence, preconditioning by bicuculline alone might have failed due to insufficient elevation of electrical activity. Blocking Na+ channels with TTX eliminated Ca2+ spiking induced by 4-AP/bic (Fig. 1I), confirming a required role for electrically driven Ca2+ spiking (16).
The range of conditions able to provide OGD tolerance was determined by varying the concentration of 4-AP, and the durations of exposure to 4-AP/bic and recovery period following 4-AP/bic washout (Fig. 1, J–P). Concentrations of 4-AP ranging from 20 μm (“low”) to 2500 μm (“high”) in 4-AP/bic treatments all provided robust OGD tolerance, with 24–48 h representing an optimum length of treatment. The optimum recovery period following 4-AP/bic washout was 0–48 h; a 72-h interval resulted in some loss of OGD tolerance for low 4-AP/bic preconditioning, whereas a 96-h interval resulted in no protection against OGD for any 4-AP concentration. Arguing against protection by any 4-AP/bic remaining after washout, subjecting neurons to 4-AP/bic during OGD was not neuroprotective (data not shown). Exposing neurons to 4-AP/bic for only 4 h did not induce OGD tolerance (Fig. 1P), suggesting the requirement for a minimum duration of exposure. The ability of 4-AP to induce OGD tolerance at concentrations two orders of magnitude lower than a lethal concentration (∼5 mm; data not shown) suggests that preconditioning did not threaten neuronal survival.
Because excessive activation of glutamate or NMDA receptors (excitotoxicity) underlies neuronal death induced by OGD, we attempted to confirm tolerance to lethal glutamate or NMDA receptor activation by 4-AP/bic preconditioning (20, 28), but were not successful (supplemental Fig. S1, A and B, respectively). In view of this discrepancy, we evaluated if apoptotic tolerance could be confirmed. Tolerance to staurosporine during 4-AP/bic treatment (acute) was not observed (supplemental Fig. S2, A and B), but tolerance to this apoptotic agent added after 4-AP/bic washout (delayed) was verified (supplemental Fig. S2C) (16, 20). Overall, the different neurotoxicity profiles might be attributed to different neuron culture systems (hippocampals elsewhere versus corticals).
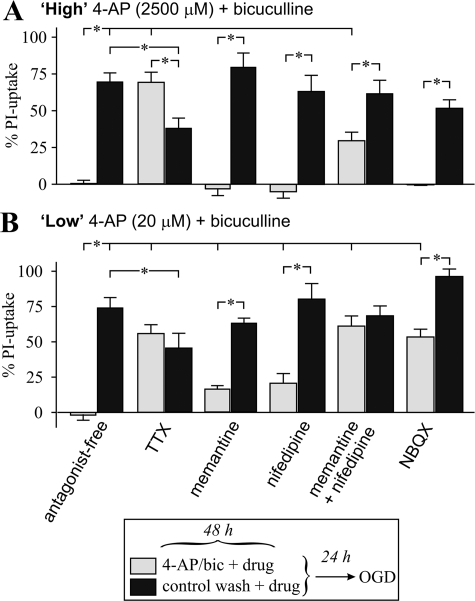
Receptor Dependence of Preconditioning—Receptors activated during preconditioning were identified by evaluating whether including a receptor antagonist during preconditioning suppressed OGD tolerance; both the highest (2500 μm, Fig. 2A) and lowest (20 μm, Fig. 2B) 4-AP concentrations were evaluated, because additional receptors can be activated at millimolar 4-AP concentrations (29). A Na+ channel blocker, TTX, reversed OGD tolerance produced by both high and low 4-AP/bic (although TTX was moderately protective on its own for an unknown reason). Thus, action potential-dependent neurotransmitter release was required for development of OGD tolerance.
FIGURE 2.
Identification of receptors involved in high or low 4-AP/bic preconditioning. Cortical neuron cultures were subjected to (A) high 4-AP/bic preconditioning (2.5 mm 4-AP with 50 μm bicuculline) (light bars) or control wash (dark bars) in the absence (labeled antagonist-free) or presence of a receptor antagonist for 48 h, followed by a 24-h recovery and then exposure to 65–80 min OGD, with assessment of the % PI-uptake performed 24 h later. B, cultures were treated as described in A, except preconditioning was accomplished using low 4-AP/bic (20 μm 4-AP with 50 μm bicuculline). Antagonists examined were a Na+ channel blocker, tetrodotoxin (1 μm TTX; n = 18); an NMDA receptor antagonist, memantine (12.5 μm; n = 9–15); an l-type voltage-gated Ca2+ channel antagonist, nifedipine (5 μm; n = 9); memantine plus nifedipine (n = 12); and an AMPA receptor antagonist, NBQX (10 μm; n = 12). Brackets denote a significant difference (p < 0.05) between indicated conditions using an ANOVA.
Protection against OGD was significantly suppressed by the presence of memantine (12.5 μm) during exposure to low (Fig. 2B) but not high (Fig. 2A) 4-AP/bic treatment. Memantine may not fully block rapid activation of the NMDA receptor during an action potential (30), so we examined a higher affinity antagonist, MK-801. However, in a control experiment, exposing cultures to MK-801 alone induced tolerance to subsequent OGD (supplemental Fig. S3), possibly due to the presence of residual antagonist and/or induction of a preconditioning stress response (31). This protective effect likely accounted for why MK-801 did not reverse 4-AP/bic induced tolerance to OGD. Summarizing, a role for NMDA receptors was established using memantine.
Blocking l-type voltage-gated Ca2+ channel (L-VGCCs) with nifedipine or AMPA receptors with NBQX during low 4-AP/bic preconditioning partially suppressed OGD tolerance (Fig. 2B), but neither of these antagonists had any effect on high 4-AP/bic preconditioning (Fig. 2A). Combining memantine with nifedipine more effectively suppressed OGD tolerance than by either antagonist alone for both high (Fig. 2A) or low (Fig. 2B) 4-AP/bic preconditioning, suggesting that co-activation of NMDA receptors and L-VGCCs contributes to the generation of OGD tolerance.
Preconditioning accomplished by inducing epileptiform activity by kainic acid administration in vivo induces activation of adenosine A1 receptors and KATP channels (32). To determine if parallels existed, we examined whether these receptors contributed to 4-AP/bic preconditioning. Blocking adenosine A1 receptors with 8-cyclopenthyltheophylline, or KATP channels with glybenclamide or tolbutamide, during low 4-AP/bic preconditioning did not reverse OGD tolerance (supplemental Fig. S3). Hence, key differences exist between these forms of preconditioning.
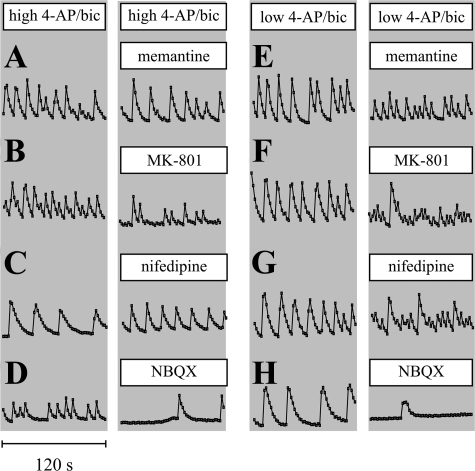
Receptor Dependence of Ca2+ Spiking—To determine if suppression of Ca2+ spiking and OGD tolerance by antagonists was correlated, single-cell Ca2+ fluorescence images were acquired from Fluo-4-loaded cortical cultures. High (Fig. 3, A–D) or low (Fig. 3, E–H) 4-AP/bic treatment induced Ca2+ spiking. As was expected based on the key role for AMPA receptor-mediated post-synaptic depolarization during an action potential, NBQX markedly suppressed Ca2+ spiking frequencies during both high and low 4-AP/bic treatments (although this finding contrasts with results published elsewhere (18)). Memantine or nifedipine diminished Ca2+ spiking amplitudes to a greater degree during low compared with high 4-AP/bic treatment. MK-801 suppressed Ca2+ spiking amplitudes more effectively than memantine, likely due to the long off-rate of MK-801 compared with memantine from the NMDA receptor. Memantine, nifedipine and MK-801 each increased the frequency of Ca2+ spiking, particularly during low 4-AP/bic exposure; Hardingham et al. (28) have also reported that blocking L-VGCCs with nimodipine increased the burst frequency of neuronal firing in an undetermined manner. Taken together, the ability of receptor antagonists to suppress Ca2+ spiking amplitudes correlated with their ability to suppress OGD tolerance at different 4-AP concentrations.
FIGURE 3.
Identification of receptors involved in Ca2+ spiking. To monitor neuronal Ca2+ spiking, cultures were loaded with 4.5 μm Fluo-4-AM, treated with 4-AP/bic in the absence or presence of a receptor antagonist for 0.5–2.5 h, and fluorescence images were acquired for 120 s. Cultures were exposed to high 4-AP/bic (A–D) or low 4-AP/bic (E–H) before and after treatment with memantine (12.5 μm)(A and E), MK-801 (2.5 μm)(B and F), nifedipine (5 μm)(C and G), and NBQX (10 μm)(D and H). Ca2+ spike trains represent average intensities calculated from 12–15 neuronal soma.
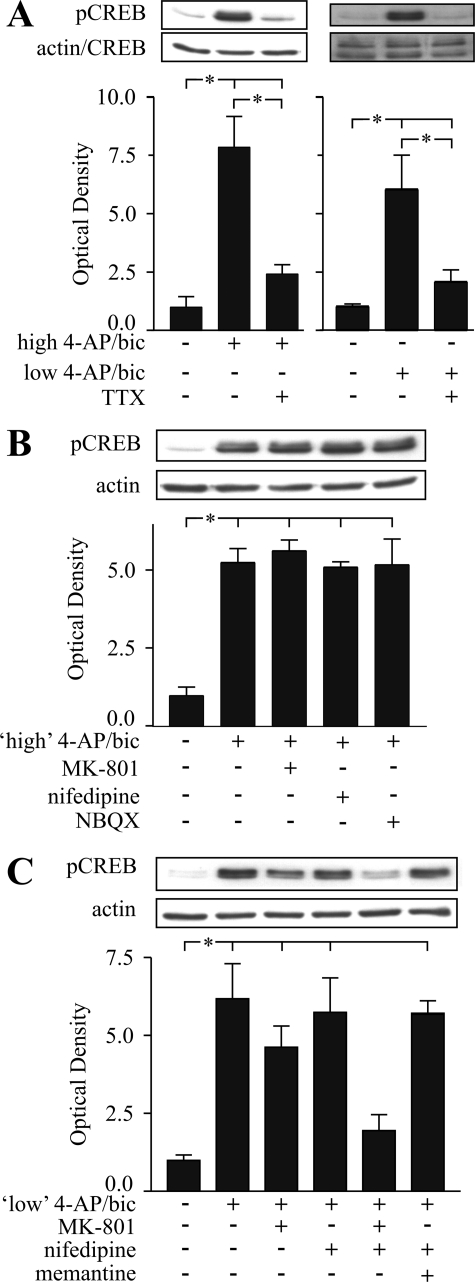
Receptor Dependence of CREB Phosphorylation during Preconditioning—Activation of synaptic NMDA receptors can result in phosphorylation and activation of CREB, which can be inhibited via a “shut-off pathway” if extrasynaptic NMDA receptors are activated (16) or with prolonged network disinhibition (33). Preconditioning by low or high 4-AP/bic treatment induced a sustained and constant elevation in pCREB levels in neurons, while CREB levels were invariant (control wash did not result in any change in pCREB or CREB levels (supplemental Fig. S4, A, C, and D)) consistent with a minimal role, if any, for activation of extrasynaptic NMDA receptors or signaling able to result in CREB de-phosphorylation. Interestingly, levels of pCREB were depressed for 24 h following washout of high, but not low, 4-AP/bic, measured relative to control washed cultures (supplemental Fig. S4A). In contrast, treatment with high 4-AP/bic for only 4 h (supplemental Fig. S4B) or low 4-AP/bic for 48 h (supplemental Fig. S4C) did not result in depressed pCREB levels following washout. Taken together, a threshold dose and duration of exposure to 4-AP/bic appeared required to produce dampened pCREB levels during recovery, although this depression was not required for induction of an OGD-tolerant state (at least by low 4-AP/bic treatment).
To determine the effect of receptor antagonists on pCREB elevations, antagonists were included during high or low 4-AP/bic application. TTX eliminated pCREB elevations caused by high or low 4-AP/bic treatment (Fig. 4A), confirming that electrical activity underlies increased CREB phosphorylation. During high 4-AP/bic treatment, MK-801, nifedipine or NBQX did not significantly reduce pCREB elevations (Fig. 4B). During low 4-AP/bic exposure, memantine and nifedipine alone or in combination had no effect, MK-801 partially suppressed pCREB rises, and MK-801 combined with nifedipine eliminated pCREB elevations (Fig. 4C). Taken together, co-activation of NMDA receptors, likely primarily synaptic in origin, and L-VGCCs during preconditioning induced a sustained phosphorylation of CREB, which could be prevented only when both receptor types were effectively inhibited.
FIGURE 4.
Identification of receptors involved in CREB phosphorylation during preconditioning. Representative immunoblots and densitometric analyses showing expression of pCREB, and CREB or actin, from protein samples collected under the following conditions: A, 48-h exposure to high or low 4-AP/bic ± aNa+ channel blocker, TTX (1 μm); B, 6-h exposure to high 4-AP/bic ± MK-801 (2.5 μm), nifedipine (5 μm), or NBQX (10 μm); C, 6-h exposure to low 4-AP/bic ± MK-801, nifedipine, nifedipine plus MK-801, or nifedipine plus memantine. Immunoblots representing n = 3–5 experiments were subjected to densitometric analysis, with values presented as the ratio of intensities of pCREB:CREB or pCREB:actin bands. Brackets denote a significant difference (p < 0.05) between indicated conditions using an ANOVA.
Role of ERK1/2 in Preconditioning—Activation of synaptic NMDA receptors can result in prolonged phosphorylation and activation of ERK1/2, which can be prevented via a shut-off pathway if extrasynaptic NMDA receptors are activated (23–25), paralleling the opposing effect of synaptic and extrasynaptic NMDA receptors documented for CREB phosphorylation (28). ERK1/2 can be phosphorylated (pERK1/2) via activation of L-VGCCs, NMDA, or AMPA receptors (34–38), potentially leading to phosphorylation (RSK) of CREB via intermediate kinases such as ribosomal S6 kinase (36). As far as we are aware, the role of ERK1/2 was not previously investigated in this model of preconditioning. An examination of the role of pERK1/2 was merited. Inhibiting MEK1/2, which is upstream of ERK1/2, with U0126 during low 4-AP/bic preconditioning partially suppressed OGD tolerance (Fig. 5A), eliminated pERK1/2 elevations as expected (data not shown), and partially suppressed pCREB elevations (Fig. 5C), suggesting that a MEK/ERKs/ribosomal S6 kinase pathway contributed to CREB phosphorylation and OGD tolerance. However, U0126 decreased the Ca2+ spiking frequency (Fig. 5B), potentially contributing to the suppression of OGD tolerance by U0126. In contrast, U0126 did not alter 4-AP/bic-induced Ca2+ spiking elsewhere (18), but a MEK 1 inhibitor, PD 98059, reduced the bicuculline-induced Ca2+ spiking frequency (39), so how inhibition of MAP kinase modulates Ca2+ spiking requires further scrutiny.
FIGURE 5.
Role of ERK1/2 phosphorylation in preconditioning. A, cortical neuron cultures were subjected to 48-h low 4-AP/bic preconditioning (light bars) or to control wash (dark bars), in the absence (untreated) or presence of a pERK1/2 inhibitor, U0126 (20 μm; n = 12), followed by a 24-h recovery and then exposure to 65–80 min OGD, and measurement of the % PI-uptake 24 h later. B, to monitor neuronal Ca2+ spiking, cultures were loaded with 4.5 μm Fluo-4-AM, treated with low 4-AP/bic in the absence or presence of U0126 for 0.5–2.5 h, and fluorescence images were acquired for 120 s. C, representative pCREB and CREB immunoblots and densitometric analyses of pCREB:CREB ratios from n = 4 experiments following a 48-h treatment with control wash or low 4-AP/bic in the presence or absence of U0126 (20 μm). D, representative pERK1/2 and actin immunoblots and densitometric analyses of pERK1/2: actin ratios from n = 4 experiments for protein samples collected following a 6-h treatment with control wash or low 4-AP/bic in the presence or absence of TTX (1 μm), memantine (12.5 μm), MK-801 (2.5 μm), or NBQX alone (10 μm). E, representative pERK1/2 and actin immunoblots from n = 4 experiments and densitometric analysis of pERK1/2:actin ratios following a 6-h treatment with control wash or low 4-AP/bic ± MK-801 (2.5 μm), nifedipine (5 μm), MK-801 plus nifedipine, or nifedipine plus memantine. Brackets denote a significant difference (p < 0.05) between indicated conditions using an ANOVA.
To identify receptors involved in inducing pERK1/2 rises, antagonists were added during low 4-AP/bic treatments. TTX eliminated pERK1/2 increases (Fig. 5D), indicating coupling of synaptic activity and ERK1/2 phosphorylation. pERK1/2 elevations were not affected by memantine, MK-801, NBQX (in a control experiment, this surprising lack of effect of NBQX was not due to an ability of NBQX alone to phosphorylate ERK1/2; Fig. 5D), nifedipine (Fig. 5E), or nifedipine combined with memantine (Fig. 5E), but were decreased when nifedipine was combined with MK-801 (Fig. 5E). Hence, parallel activation of NMDA receptors, likely primarily synaptic in origin, with L-VGCCs resulted in elevated pERK1/2 levels, which could be prevented only when both receptors were strongly blocked, mirroring the trend observed for OGD tolerance and pCREB elevations.
Role of PKA and CaMKs in Preconditioning—CREB DNA-binding activity can be enhanced following phosphorylation of Ser-133 by PKA (40). Ca2+-induced nuclear translocation of ERK1/2 and subsequent phosphorylation of CREB via other protein kinases can be initiated by PKA (41), and a neuronal cAMP/PKA-ERK-pCREB signaling pathway has been previously identified (42). We determined if inhibiting PKA blocked phosphorylation of ERK1/2 and CREB and prevented generation of OGD tolerance. Inhibiting PKA using H89 during low 4-AP/bic preconditioning significantly reduced OGD tolerance (supplemental Fig. S3). H89 can exert unrelated effects (43); inhibiting PKA with KT 5720 or PKA inhibitory fragment 14–22 did not suppress OGD tolerance (supplemental Fig. S3), so data based on H89 could not be confirmed, suggesting no role for PKA.
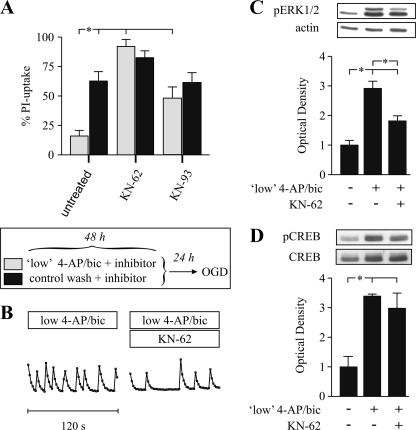
Nuclear CaM kinases such as CaMKIV are sufficient to induce CREB-dependent gene transcription (44). Inhibiting the activation of CaMKs such as CaMKII, CaMKIV, and CaMKV with KN-62 (5 μm) or KN-93 (10 μm) during low 4-AP/bic preconditioning abolished OGD tolerance (Fig. 6A). KN-62 also reduced the frequency of Ca2+ spiking (Fig. 6B) and significantly suppressed the pERK1/2 elevation (Fig. 6C), but not the pCREB rise (Fig. 6D). Thus, CaMKs activation contributes to 4-AP/bic-induced Ca2+ spiking and OGD tolerance independently of CREB phosphorylation.
FIGURE 6.
Role for CaMKs in preconditioning. A, cortical neuron cultures were subjected to 48-h low 4-AP/bic preconditioning (20 μm 4-AP with 50 μm bicuculline) (light bars) or to control wash (dark bars), in the absence (untreated) or presence of a CaMK inhibitor, KN-62 (5 μm; n = 12) or KN-93 (10 μm; n = 12), followed by 65–80 min OGD 24 h later and measurement of the % PI-uptake 24 h later. B, to monitor neuronal Ca2+ spiking, cultures were loaded with 4.5 μm Fluo-4-AM, treated with low 4-AP/bic in the absence or presence of KN-62 for 0.5–2.5 h, and fluorescence images were acquired for 120 s. C, representative pERK1/2 and actin immunoblots and densitometric analysis of pERK1/2:actin ratios from n = 5 experiments for protein samples collected following a 6-h treatment with control wash or low 4-AP/bic in the presence or absence of KN-62 (5 μm). D, representative pCREB and CREB immunoblots and densitometric analyses from n = 3 experiments of pCREB: CREB ratios for protein samples collected following a 6-h treatment with control wash or low 4-AP/bic in the presence or absence of KN-62 (5 μm). Brackets denote a significant difference (p < 0.05) between indicated conditions using an ANOVA.
Discrepancies exist in cellular signaling with previous reports. Compared with the current study, pCREB levels were more (18) or less (45) effectively suppressed by U0126 or KN-62, and inhibiting CaMK resulted in a more effective prevention of ERK1/2 phosphorylation (46). Different culture or experimental conditions may exist, highlighting the importance of establishing relevant signaling within each laboratory.
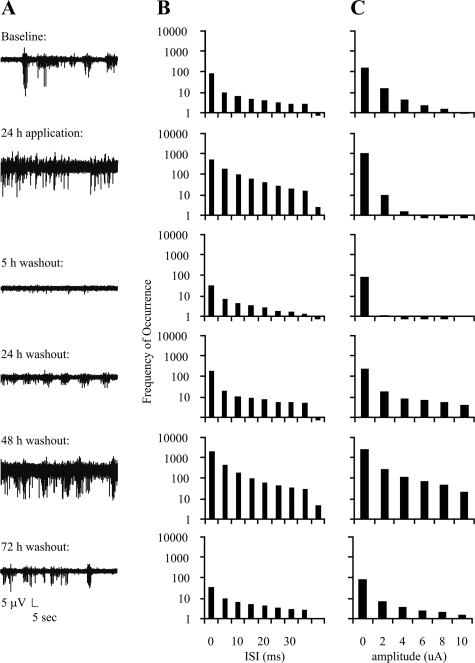
Effect of Preconditioning on Electrical Activity—Exposing neurons to bicuculline for a short duration (minutes) may produce lasting modifications in neuronal network activity (39, 47), raising the question of whether preconditioning in the present study resulted in prolonged alterations in neuronal activity. Following culturing of neurons on 64-channel MEAs, amplitudes and timing of peaks were evaluated for six conditions: baseline, 24-h time point of a 48-h exposure to low 4-AP/bic, and 5, 24, 48 and 72 h following 4-AP/bic washout. Neurons displayed a high level of network-driven synaptic activity expected from high density cultures (48) (representative traces are shown in Fig. 7A; five MEAs comprising three different platings were analyzed). Relative to baseline conditions, statistically significant changes (p < 0.002) in activity were observed at various time points. A substantial decrease in inter-peak intervals across all channels was observed 5-h post-washout, followed by an increased 48-h post-washout (Fig. 7B). These changes were accompanied by a significant decrease in peak amplitude 5-h post-washout, and a significant increase in 48-h post-washout (Fig. 7C). Despite these changes, all cultures returned to baseline levels of timing and amplitude after 72-h washout. Two types of control experiments were performed, in which activity was monitored for the same time points as above for a 48-h exchange of media (three MEAs comprising two different platings) or 0.2% DMSO vehicle (six MEAs comprising four different platings were examined). Using similar analyses and levels of statistical confidence over identical time points, no significant differences in either media- or DMSO-exchanged conditions were observed (data not shown), demonstrating that the fluctuations observed with 4AP-bic were not due to spontaneous changes in cultures or to vehicle. Hence, although not toxic, the stimulation protocol used to precondition induces long term changes in neuronal excitability. Cultures exhibited altered activity for the entire time (72 h after 4-AP/bic washout) that neurons existed in a tolerant state to OGD.
FIGURE 7.
Effect of 4-AP/bic treatment on electrical activity monitored with 64-channel MEAs. A, representative MEA recordings of electrical activity monitored for 60 s from a representative multielectrode during baseline conditions, 24 h during low 4-AP/bic preconditioning, and 5, 24, 28, and 72 h post-washout; B and C, histogram summaries plotting frequency of occurrence versus inter-spike interval (ISI) and peak amplitudes, respectively (five MEAs comprising three different platings were analyzed).
DISCUSSION
Activity-dependent Generation of OGD Tolerance—A prolonged elevation of electrical activity induced potent resistance to OGD tolerance. Consistent with a preconditioning-based mechanism, treatment of cultures with 4-AP/bic before but not during OGD induced a protracted, yet ultimately reversible, state of tolerance. OGD adds to a growing list of neuronal insults that elevated electrical activity may be effective against, with OGD perhaps most relevant to cerebral ischemia. The ability of electrical activity to combat insults that are ischemic, apoptotic, or oxidative stress-based also suggests potential merit against traumatic brain injury, Alzheimer disease, or other neurodegenerative conditions, which may be comprised of either one or a mixture of these and other neurodegenerative components. To date, electrical stimulation of the spinal cord induced tolerance to spinal cord injury (49), and 4-AP has also been used in vivo to precondition against kainic acid-induced excitotoxicity in vivo (50). The requirement for a prolonged and marked elevation in electrical activity may account for why a much shorter duration of bicuculline exposure was not successful in generating OGD tolerance (51). Some restriction in general applicability of activity-induced neuroprotection may exist, though, based on our inability to confirm previous reports of tolerance to NMDA/glutamate toxicity or acute tolerance to staurosporine.
Modifications in network activity observed following preconditioning correlated with the period during which neurons existed in an OGD-tolerant state (up to 72 h). The subdued electrical activity noted within 24 h after preconditioning correlates with the period of CREB hypo-phosphorylation. An important question is whether the depressed network activity resulted from synaptic scaling, which is a form of homeostatic plasticity in which synaptic connections are “tuned” in response to variable activity levels. Chronic exposure to bicuculline can result in overuse hyposensitivity by scaling synaptic strength downward (opposite changes can occur as neurons exhibit “disuse hypersensitivity” after activity blockade) (52, 53). Interestingly, a reduction in the frequency of Ca2+ spiking observed following OGD-preconditioning was important for development of tolerance to OGD (54).
The NMDA Receptor in Preconditioning—Action potential-mediated presynaptic release of neurotransmitters underlies preconditioning, because TTX reversed Ca2+ spiking and OGD tolerance. Ca2+ spiking via concomitant activation of NMDA receptors and L-VGCCs was required for development of OGD tolerance. These data are consistent with participation of primarily synaptic NMDA receptors. Phosphorylation of ERK1/2 and CREB was sustained during preconditioning; if extrasynaptic NMDA receptors had been activated to an appreciable degree, a “phosphorylation shut-off” pathway would have been activated (16, 24, 55, 56). Remarkably, the prolonged Ca2+ spiking did not cause neuronal demise.
Nonetheless, a less exclusive role for the NMDA receptor appears to exist for electrical activity induced by 4-AP/bic, particularly at higher 4-AP concentrations. Most studies consider the effect of MK-801 on bicuculline alone. MK-801 did not eliminate 4-AP/bic-induced elevations in pERK1/2 (Fig. 5E; confirming results elsewhere (57)) or pCREB (Fig. 4, B and C) or Ca2+ spiking (Fig. 3, B and F), whereas MK-801 eliminated Ca2+ spiking and pCREB elevations induced by bicuculline treatment alone (16). Tolerance to staurosporine induced by 4-AP/bic treatment has been attributed to synaptic NMDA receptor activation, because combining MK-801 with 4-AP/bic applied prior to staurosporine exposure resulted in toxicity equivalent to that caused by staurosporine alone (16). However, in a control experiment, preincubation of MK-801 alone before staurosporine exacerbated toxicity (16). Hence, the ability of MK-801 to reverse 4-AP/bic-induced tolerance to staurosporine may have been simply due to MK-801 exacerbating staurosporine-induced toxicity, rather than MK-801 acting against the preconditioning mechanism activated by 4-AP/bic treatment. Other possible modes of action are that 4-AP (micromolar) blocks several delayed rectifier currents, and higher concentrations can block additional channels (IC50 of 0.7 mm to inhibit IA currents) and pumps (IC50 of 1.2 mm to inhibit the Na+/K+ATPase-pump) in cortical neurons, and 1 mm 4-AP facilitates Ca2+ influx through voltage-sensitive channels (29, 58, 59), as well as cytosolic and nuclear Ca2+ loading (28).
The current findings bridge a key gap between the preconditioning and synaptic plasticity fields, based on the demonstration that a near-death experience achieved via NMDA receptor activation is not required for 4-AP/bic preconditioning. Both extrasynaptic and synaptic NMDA receptors are likely activated in preconditioning induced by transient exogenous NMDA. Preconditioning induced by synaptic (benign) or extrasynaptic (near-death) NMDA receptor activation may induce tolerance to OGD via different mechanisms. Low levels of NMDA induce electrical activity (19), which may mimic salient aspects of 4-AP/bic preconditioning, but higher levels of NMDA required for brink of death preconditioning do not induce electrical activity. Preconditioning by exogenous NMDA induces tolerance to lethal NMDA (14, 60) but not to staurosporine (6). Chronic exposure of neurons to bicuculline to elevate synaptic NMDA receptor activity substantially induces changes in levels of many signaling proteins within the postsynaptic density (61). Synaptic and extrasynaptic NMDA receptor activation induce quite distinct gene-expression programs (62). Further comparison of signaling activated by synaptic and extrasynaptic NMDA receptors in preconditioning is required, so considering extrasynaptic NMDA receptors solely in a neurotoxic context (55) may not be warranted.
The Pleiotropic Nature of Preconditioning by Electrical Activity—Tolerance to apoptosis reported elsewhere or to OGD display similar features: superior tolerance when adding 4-AP to bicuculline, a wide range of appropriate 4-AP concentrations, a necessity for prolonged stimulatory periods, protection observed after termination of activity and a sensitivity to TTX and to NMDA receptor blockade (16–18). Thus, a threshold level of synaptic activity must be exceeded to generate tolerance, although this threshold is higher for OGD tolerance. Gene expression is known to be regulated by the frequency of Ca2+ spiking (63). These similarities raise the possibility of tolerance induction to a wide variety of stressors via a common convergence point. At present, it remains paradoxical as to how electrical activity induces tolerance to these very different insults. Apoptosis (staurosporine) and necrosis (OGD) are considered to reside at opposite ends of a Ca2+-centric neurotoxicity continuum, with Ca2+-mediated hypofunction and hyperfunction underlying apoptosis and necrosis, respectively (64).
Supplementary Material
Acknowledgments
Assistance with Ca2+ fluorescence imaging from Dr. R. Monette is gratefully acknowledged.
This work was supported by the Heart & Stroke Foundation of Ontario (to Paul Morley) and the Canadian Stroke Network (to P. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Footnotes
The abbreviations used are: OGD, oxygen-glucose deprivation; 4-AP/bic, 4-aminopyridine/bicuculline; CaMK, Ca2+/calmodulin-dependent protein kinase; 8-CPT, 8-cyclopenthyltheophylline; L-VGCCs, L-type voltage-gated Ca2+ channels; pERK1/2, ERK1/2 phosphorylation; CREB, cAMP-response element-binding protein; pCREB, CREB phosphorylation; PKA, protein kinase A; PI, propidium iodide; TTX, tetrodotoxin; MEA, multielectrode array; NMDA, N-methyl-d-aspartic acid; GABAA, γ-aminobutyric acid, type A; ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase/ERK kinase; MK-801, (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate; ANOVA, analysis of variance.
References
- 1.Kirino, T. (2002) J. Cereb. Blood Flow Metab. 22 1283–1296 [DOI] [PubMed] [Google Scholar]
- 2.Tauskela, J. S., Gendron, T., and Morley, P. (2004) in: Cerebral Ischemic Tolerance (Schaller, B., ed) pp. 45–94, Nova Science Publishers, Inc., Hauppauge
- 3.Kato, H., Liu, Y., Araki, T., and Kogure, K. (1992) Neurosci. Lett. 139 118–121 [DOI] [PubMed] [Google Scholar]
- 4.Bond, A., Lodge, D., Hicks, C. A., Ward, M. A., and O'Neill, M. J. (1999) Eur. J. Pharmacol. 380 91–99 [DOI] [PubMed] [Google Scholar]
- 5.Mabuchi, T., Kitagawa, K., Kuwabara, K., Takasawa, K., Ohtsuki, T., Xia, Z., Storm, D., Yanagihara, T., Hori, M., and Matsumoto, M. (2001) J. Neurosci. 21 9204–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabb, M. C., and Choi, D. W. (1999) J. Neurosci. 19 1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauskela, J. S., Chakravarthy, B. R., Murray, C. L., Wang, Y., Comas, T., Hogan, M., Hakim, A., and Morley, P. (1999) Brain Res. 827 143–151 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Zulueta, M., Feldman, A. B., Klesse, L. J., Kalb, R. G., Dillman, J. F., Parada, L. F., Dawson, T. M., and Dawson, V. L. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenov, D. G., Samoilov, M. O., and Lazarewicz, J. W. (2002) Neurosignals 11 329–335 [DOI] [PubMed] [Google Scholar]
- 10.Raval, A. P., Dave, K. R., Mochly-Rosen, D., Sick, T. J., and Perez-Pinzon, M. A. (2003) J. Neurosci. 23 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duszczyk, M., Gadamski, R., Ziembowicz, A., Danysz, W., and Lazarewicz, J. W. (2005) Neurotox. Res. 7 283–292 [DOI] [PubMed] [Google Scholar]
- 12.Wrang, M. L., and Diemer, N. H. (2004) Neuroreport 15 1151–1155 [DOI] [PubMed] [Google Scholar]
- 13.Miao, B., Yin, X. H., Pei, D. S., Zhang, Q. G., and Zhang, G. Y. (2005) J. Biol. Chem. 280 21693–21699 [DOI] [PubMed] [Google Scholar]
- 14.Tauskela, J. S., Comas, T., Hewitt, K., Monette, R., Paris, J., Hogan, M., and Morley, P. (2001) Neuroscience 107 571–584 [DOI] [PubMed] [Google Scholar]
- 15.Dirnagl, U., Simon, R. P., and Hallenbeck, J. M. (2003) Trends Neurosci. 26 248–254 [DOI] [PubMed] [Google Scholar]
- 16.Hardingham, G. E., Fukunaga, Y., and Bading, H. (2002) Nat. Neurosci. 5 405–414 [DOI] [PubMed] [Google Scholar]
- 17.Papadia, S., Stevenson, P., Hardingham, N. R., Bading, H., and Hardingham, G. E. (2005) J. Neurosci. 25 4279–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, B., Butcher, G. Q., Hoyt, K. R., Impey, S., and Obrietan, K. (2005) J. Neurosci. 25 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano, F. X., Papadia, S., Hofmann, F., Hardingham, N. R., Bading, H., and Hardingham, G. E. (2006) J. Neurosci. 26 4509–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengtson, C. P., Dick, O., and Bading, H. (2008) BMC. Neurosci. 9 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martel, M. A., Wyllie, D. J., and Hardingham, G. E. (2008) Neuroscience,in press [DOI] [PMC free article] [PubMed]
- 22.Papadia, S., Soriano, F. X., Leveille, F., Martel, M. A., Dakin, K. A., Hansen, H. H., Kaindl, A., Sifringer, M., Fowler, J., Stefovska, V., McKenzie, G., Craigon, M., Corriveau, R., Ghazal, P., Horsburgh, K., Yankner, B. A., Wyllie, D. J., Ikonomidou, C., and Hardingham, G. E. (2008) Nat. Neurosci. 11 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton, G., and Chandler, L. J. (2002) J. Neurochem. 82 1097–1105 [DOI] [PubMed] [Google Scholar]
- 24.Ivanov, A., Pellegrino, C., Rama, S., Dumalska, I., Salyha, Y., Ben Ari, Y., and Medina, I. (2006) J. Physiol. 572 789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulholland, P. J., Luong, N. T., Woodward, J. J., and Chandler, L. J. (2008) Neuroscience 151 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauskela, J. S., Brunette, E., O'Reilly, N., Mealing, G., Comas, T., Gendron, T. F., Monette, R., and Morley, P. (2005) FASEB J. 19 1734–1736 [DOI] [PubMed] [Google Scholar]
- 27.Tapia, R., Medina-Ceja, L., and Pena, F. (1999) Neurochem. Int. 34 23–31 [DOI] [PubMed] [Google Scholar]
- 28.Hardingham, G. E., Arnold, F. J., and Bading, H. (2001) Nat. Neurosci. 4 261–267 [DOI] [PubMed] [Google Scholar]
- 29.Rogawski, M. A., and Barker, J. L. (1983) Brain Res. 280 180–185 [DOI] [PubMed] [Google Scholar]
- 30.Chen, H. S., Pellegrini, J. W., Aggarwal, S. K., Lei, S. Z., Warach, S., Jensen, F. E., and Lipton, S. A. (1992) J. Neurosci. 12 4427–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay, R., Chakravarthy, B., Hewitt, K., Tauskela, J., Morley, P., Atkinson, T., and Durkin, J. P. (2000) J. Neurosci. 20 7183–7192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plamondon, H., Blondeau, N., Heurteaux, C., and Lazdunski, M. (1999) J. Cereb. Blood Flow Metab. 19 1296–1308 [DOI] [PubMed] [Google Scholar]
- 33.Nishimura, M., Owens, J., and Swann, J. W. (2008) Neurobiol. Dis. 29 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron, C., Benes, C., Van Tan, H., Fagard, R., and Roisin, M. P. (1996) J. Neurochem. 66 1005–1010 [DOI] [PubMed] [Google Scholar]
- 35.Dolmetsch, R. E., Pajvani, U., Fife, K., Spotts, J. M., and Greenberg, M. E. (2001) Science 294 333–339 [DOI] [PubMed] [Google Scholar]
- 36.Thomas, G. M., and Huganir, R. L. (2004) Nat. Rev. Neurosci. 5 173–183 [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. Q., Tang, Q., Parelkar, N. K., Liu, Z., Samdani, S., Choe, E. S., Yang, L., and Mao, L. (2004) Mol. Neurobiol. 29 1–14 [DOI] [PubMed] [Google Scholar]
- 38.Mao, L., Tang, Q., Samdani, S., Liu, Z., and Wang, J. Q. (2004) Eur. J. Neurosci. 19 1207–1216 [DOI] [PubMed] [Google Scholar]
- 39.Arnold, F. J., Hofmann, F., Bengtson, C. P., Wittmann, M., Vanhoutte, P., and Bading, H. (2005) J. Physiol 564 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols, M., Weih, F., Schmid, W., DeVack, C., Kowenz-Leutz, E., Luckow, B., Boshart, M., and Schutz, G. (1992) EMBO J. 11 3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Impey, S., Obrietan, K., Wong, S. T., Poser, S., Yano, S., Wayman, G., Deloulme, J. C., Chan, G., and Storm, D. R. (1998) Neuron 21 869–883 [DOI] [PubMed] [Google Scholar]
- 42.Zanassi, P., Paolillo, M., Feliciello, A., Avvedimento, E. V., Gallo, V., and Schinelli, S. (2001) J. Biol. Chem. 276 11487–11495 [DOI] [PubMed] [Google Scholar]
- 43.Marunaka, Y., and Niisato, N. (2003) Biochem. Pharmacol. 66 1083–1089 [DOI] [PubMed] [Google Scholar]
- 44.Chawla, S., Hardingham, G. E., Quinn, D. R., and Bading, H. (1998) Science 281 1505–1509 [DOI] [PubMed] [Google Scholar]
- 45.Hardingham, G. E., Arnold, F. J., and Bading, H. (2001) Nat. Neurosci. 4 565–566 [DOI] [PubMed] [Google Scholar]
- 46.Lenz, G., and Avruch, J. (2005) J. Biol. Chem. 280 38121–38124 [DOI] [PubMed] [Google Scholar]
- 47.Debanne, D., Thompson, S. M., and Gahwiler, B. H. (2006) Epilepsia 47 247–256 [DOI] [PubMed] [Google Scholar]
- 48.Wagenaar, D. A., Pine, J., and Potter, S. M. (2006) BMC. Neurosci. 7 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiki, M., Kobayashi, H., Inoue, R., and Goda, M. (2004) J. Neurotrauma 21 459–470 [DOI] [PubMed] [Google Scholar]
- 50.Ogita, K., Okuda, H., Watanabe, M., Nagashima, R., Sugiyama, C., and Yoneda, Y. (2005) Neuropharmacology 48 810–821 [DOI] [PubMed] [Google Scholar]
- 51.Lange-Asschenfeldt, C., Raval, A. P., and Perez-Pinzon, M. A. (2005) Neurosci. Lett. 384 87–92 [DOI] [PubMed] [Google Scholar]
- 52.Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C., and Nelson, S. B. (1998) Nature 391 892–896 [DOI] [PubMed] [Google Scholar]
- 53.Turrigiano, G. G., and Nelson, S. B. (2004) Nat. Rev. Neurosci. 5 97–107 [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, M., Kawahara, K., Kosugi, T., Yamada, T., and Mioka, T. (2007) Neurochem. Res. 32 988–1001 [DOI] [PubMed] [Google Scholar]
- 55.Hardingham, G. E., and Bading, H. (2003) Trends Neurosci. 26 81–89 [DOI] [PubMed] [Google Scholar]
- 56.Vanhoutte, P., and Bading, H. (2003) Curr. Opin. Neurobiol. 13 366–371 [DOI] [PubMed] [Google Scholar]
- 57.Kalita, K., Kharebava, G., Zheng, J. J., and Hetman, M. (2006) J. Neurosci. 26 10020–10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathie, A., Wooltorton, J. R., and Watkins, C. S. (1998) Gen. Pharmacol. 30 13–24 [DOI] [PubMed] [Google Scholar]
- 59.Wang, X. Q., Xiao, A. Y., Yang, A., LaRose, L., Wei, L., and Yu, S. P. (2003) J. Pharmacol. Exp. Ther. 305 502–506 [DOI] [PubMed] [Google Scholar]
- 60.Pivovarova, N. B., Stanika, R. I., Watts, C. A., Brantner, C. A., Smith, C. L., and Andrews, S. B. (2008) J. Neurochem. 104 1686–1699 [DOI] [PubMed] [Google Scholar]
- 61.Ehlers, M. D. (2003) Nat. Neurosci. 6 231–242 [DOI] [PubMed] [Google Scholar]
- 62.Zhang, S. J., Steijaert, M. N., Lau, D., Schutz, G., Delucinge-Vivier, C., Descombes, P., and Bading, H. (2007) Neuron 53 549–562 [DOI] [PubMed] [Google Scholar]
- 63.Dolmetsch, R. E., Xu, K., and Lewis, R. S. (1998) Nature 392 933–936 [DOI] [PubMed] [Google Scholar]
- 64.Zipfel, G. J., Babcock, D. J., Lee, J. M., and Choi, D. W. (2000) J. Neurotrauma 17 857–869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.