Abstract
Objective
The purpose of this study was to determine the effect of 2 concentrations of topical, comfrey-based botanical creams containing a blend of tannic acid and eucalyptus to a eucalyptus reference cream on pain, stiffness, and physical functioning in those with primary osteoarthritis of the knee.
Methods
Forty-three male and female subjects (45-83 years old) with diagnosed primary osteoarthritis of the knee who met the inclusion criteria were entered into the study. The subjects were randomly assigned to 1 of 3 treatment groups: 10% or 20% comfrey root extract (Symphytum officinale L.) or a placebo cream. Outcomes of pain, stiffness, and functioning were done on the Western Ontario and MacMaster Universities Osteoarthritis Index. Participants applied the cream 3× a day for 6 weeks and were evaluated every 2 weeks during the treatment.
Results
Repeated-measures analyses of variance yielded significant differences in all of the Western Ontario and MacMaster Universities Osteoarthritis Index categories (pain P < .01, stiffness P < .01, daily function P < .01), confirming that the 10% and 20% comfrey-based creams were superior to the reference cream. The active groups each had 2 participants who had temporary and minor adverse reactions of skin rash and itching, which were rapidly resolved by modifying applications.
Conclusion
Both active topical comfrey formulations were effective in relieving pain and stiffness and in improving physical functioning and were superior to placebo in those with primary osteoarthritis of the knee without serious adverse effects.
Key indexing terms: Osteoarthritis, Knee pain, Topical, Therapeutic, Botanicals
Introduction
More than 21% of US adults (46.4 million persons) are affected by arthritis,1 and nearly 27 million of those have clinical osteoarthritis (OA).2 Osteoarthritis is the most common form of arthritis and is a major cause of disability and suffering, particularly in the aging population. Early 20th century pathologists and radiologists observed osteophytes within articulations and referred to the abnormality of the joint as OA.3 Osteoarthritis is now thought to be a collection of similar diseases affecting the joints rather than a single disease.4 The disease most frequently affects the knee and hand5 and is the primary reason for joint replacement surgery.6 It is estimated that, by age 65 years, 80% of the population will have detectable radiographic changes typical of OA and 60% of those with detectable radiographic changes will have pain, whereas 15% to 30% will have mobility problems.7
The most common clinical features of OA include pain, stiffness, swelling, and inflammation. Other signs of OA include crepitus, bony enlargement, deformity, instability, restricted movement, warmth, effusion, synovial thickening, and muscle weakness or wasting.4 Risk factors for OA include advancing age, repetitive motion, family history, obesity, and injury.8 As no cure is currently available for OA, treatment focuses on reducing symptoms. Such treatment includes exercise or orthoses and usually involves analgesic or nonsteroidal anti-inflammatory drugs (NSAIDs). Unfortunately, other than analgesic therapy, most physicians believe that little can be done for the disease. Corrective surgery, which does not include joint replacement, can cost between $8000 and $20 000; and surgery that includes joint replacement can cost as much as $70 000. Those who do not elect to have surgery will have to contend with the continuing cost of pain relief and anti-inflammatory medication. The mean US medical care expenditures for adults with arthritis and other rheumatic conditions in 2003 was $6978, and the total cost was $321.8 billion.9 As the rate of OA is predicted to increase because of the aging baby boomers and extended life expectancy, the number of people suffering adverse effects from analgesic and NSAID use such as kidney and liver disease and ulcers will increase. With the current frequency and severity of adverse effects from analgesics and NSAIDs, suggestions for less toxic treatments of OA are warranted.4,10 Indeed, some of the most prescribed drugs for arthritis have been withdrawn from public consumption by pharmaceutical companies because of severe adverse effects. For example, some COX-2 inhibitors were found to increase edema and blood pressure, thus increasing the risk for stroke and other cardiovascular events.
Natural remedies may reduce dependency on NSAIDs and analgesics and could have an important role in the treatment of OA even if they were only moderately effective.11 For instance, natural agents, such as capsaicin, has been shown to provide relief for OA.12 Earlier clinical studies also suggest that OA improves following consumption of selected vitamins10 and glucosamine/chondroitin.13 However, a recent meta-analysis suggests that glucosamine/chondroitin intervention for treatment of OA of the knee has not been conclusive.14
Comfrey (Symphytum officinale L.), also known as knit bone, has long been advocated in folk medicine for the treatment of wounds, sores, sprains, and bone fractures. In Germany, comfrey has been used in medicine since 1920 for the treatment of musculoskeletal conditions.15 It has been suggested that the efficacy of comfrey is primarily due to its anti-inflammatory, analgesic, granulating promoting, and antiexudative properties.16,17 Comfrey pharmacological components include rosmarinic acid and tannin. Rosmarinic acid is a natural polyphenol antioxidant, and both rosmarinic acid and tannin are considered anti-inflammatory agents. Because of the alkaloid component of comfrey, the safest mode of delivery is by applying a comfrey cream to the skin. The skin is the largest organ in the body, is easily accessible, and allows prolonged periods of applications of formulations for transdermal absorption, making it the likely target for drug delivery techniques. One study comparing a 10% comfrey cream and a 1% reference cream found that those applying the 10% cream for 2 to 3 days showed clinically significant faster reduction of wound size over the reference cream.18 Kuceara et al19 found that a concentration of 10% comfrey cream significantly improved back pain on activity, at rest, and during palpation over a 1% reference cream and concluded that the results confirmed known anti-inflammatory and analgesic effects of topical comfrey. More recently, clinical studies have shown comfrey to be therapeutically beneficial for neuromuscular conditions. For instance, Bleakley et al20 concluded that comfrey root cream decreases pain and improves function in acute ankle sprains. Similarly, another study15 concluded that a cream of comfrey root extract, when administered on acute ankle sprains, was clearly superior (P < .0001) to a placebo in the reduction of pain of edema and in the increase in mobility. In addition, the authors found no adverse effect in the use of the comfrey cream. In comparing an NSAID gel with a comfrey extract cream, 2 separate studies found that the comfrey cream was superior in reducing edema and pain and in improving movement to the NSAID cream.21,22 Again, no adverse effects were reported in either study. In reference to bone, when comfrey was administered orally, radiographic bone density has been shown to increase around titanium implants.23 Specific to OA, a study involving 220 patients with diagnosed knee OA for an average of 6.5 years, the patients were treated with daily applications of comfrey cream or a placebo. The results yielded significant reduction in pain and increases in mobility and quality of life in the comfrey cream users when compared with the placebo users.24 The authors concluded that comfrey root extract cream was “well suited for treatment of OA of the knee.” In another study using a 2-week observational period, patients receive 1 to 3 comfrey treatments per day. More than 66% of the patients were able to reduce or even discontinue their intake of NSAIDs and other specific concomitant medication with the comfrey treatment.25
Evidence links oxygen free radicals to tissue damage in virtually all diseases, particularly chronic inflammatory diseases such as OA; and they serve as signaling messengers in the development of inflammation and osteoclastogenesis common in the pathogenesis of arthritis.26-28 Antioxidants provide protection against the damaging effects of oxygen free radicals. Regan et al27 compared injured knees with OA knees and found significantly less antioxidants in OA joint fluid, thereby concluding that the decline in antioxidants in the joint fluid accelerates the damaging oxidant effect on extracellular matrix in the cartilage. Based on the detrimental effects of oxidative free radicals, it has been suggested that substances high in antioxidants can reduce or eliminate tissue damage present in arthritis29 and have a therapeutic effect on collagen-induced arthritis.30
Tannic acid (TA), an antioxidant, contains antimutagenic and anticarcinogenic properties that exhibit oxygen free radical trapping activity. Levanon and Stein31 suggested that the ability of TA to augment glycosaminoglycan binding to collagen most possibly contributes to the structural reinforcement of synovial articulating surfaces. One study confirmed that the anti-inflammatory and antinociceptive properties of Satureja khuzistanica (Lamiaceae), a native medicinal plant of Iran, were comparable to those of indomethacin and morphine and suggested that tannin might be responsible for the anti-inflammatory and antinociceptive activities.32 Furthermore, phlorotannin-rich extracts have shown significant antioxidant radical scavenging activity, showing strong OA therapeutic benefits through in vitro experiments.33
The purpose of the present study was to compare the effectiveness of 2 concentrations (10% and 20%) of a unique blend of comfrey root extract (S officinale L.), TA, and eucalyptus to a reference cream containing eucalyptus only on primary knee OA pain, stiffness, and physical functioning. To our knowledge, the combination of comfrey and TA in the treatment of OA is unique and has yet to be investigated. Similar previous studies have used reference creams in pseudoplacebo comparisons.18,19 For the present study, the placebo/reference cream was eucalyptus oil, which has been shown to be absorbed readily through the skin.34
Eucalyptus oil contains α-pinene and 1,8-cineole and demonstrates strong radical scavenging activity as an antioxidant.35
Methods
Subjects
The study was a multicenter, randomized, double-blind, placebo-controlled clinical trial approved by the Oklahoma State University Institutional Review Board. Participants were recruited through newspaper advertisements and from the patient base of the chiropractic practices involved in the study and gave consent to participate. Based on the recommendations by Altman,36 subjects had to meet the following specific criteria to be eligible for participation in the study:
-
•
Had primary, symptomatic, unilateral, or bilateral knee OA. If the patient had bilateral knee OA, the most painful knee was used for the study.
-
•Had met the criteria for knee OA of the American College of Rheumatology37
-
1.Moderate/medium degree of pain in the knee for most days of the previous month
-
2.Four of the following additional criteria:
-
•Age more than 25 years
-
•Morning stiffness for less than 30 minutes
-
•Crepitus on active or passive movement of the knee
-
•Tenderness on pressure of the periarticular tissue or muscular insertions of the knee at the admission examination
-
•No palpable warmth of the knee
-
•
-
1.
-
•
Had at least a 3-month history of knee OA
-
•
Had taken medication for pain control for at least 1 month
-
•
Had moderate to severe pain of at least 4 on a 0 to 10 numerical rating scale
-
•
Participants had to agree to not take any pain medication for knee pain during the treatment phase of the study.
Subjects with the following were not eligible for participation in the study:
-
•
Any known or suspected allergies or sensitivities to the ingredients in the test formulations
-
•
In the clinician's opinion, be considered an inappropriate candidate for the study because of concomitant medical or psychiatric conditions
-
•
Secondary forms of OA as well as primary inflammatory joint diseases such as rheumatoid arthritis
-
•
Administration of intraarticular drugs or “chondroprotective” agents within the prior 3 months
-
•
Engaged in disability-related litigation related to the knee
-
•
Pain in the knee from other pathologies
-
•
Prior knee joint replacement surgery
-
•
Open lesions over the knee
Seventy-three prospective subjects were initially screened for eligibility to participate in the study. Of these, 30 participants were excluded because they failed to meet the established criteria (Fig 1).
Fig 1.
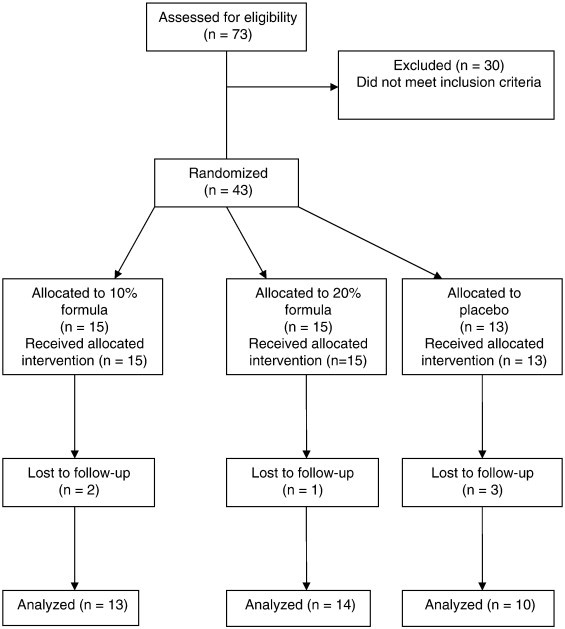
Flow of participants through the study.
Interventions
For the experimental cream used in this study, we used 2 concentrations of 4Jointz (4Jointz, PO Box 5218, Brisbane 4001, Australia). This product is a comfrey (S officinale L.)-based extract blended with TA, eucalyptus oil, and other nonactive additives. The extracting comfrey solvent was 50% ethanol and 50% water. The 2 concentrations of the comfrey extract (10% and 20% strength by volume) ointments were compared with a pseudoplacebo of eucalyptus similar in color, texture, and smell to the experimental ointments. The ointments and placebo were applied topically thrice daily to the knee.
Procedure
Following an oral briefing of the participants' requirements and completion of the consent form, subjects completed a demographic questionnaire, a medical history questionnaire, and a Western Ontario and MacMaster Universities (WOMAC) Osteoarthritis Index Version VA301. Subjects were also examined by a primary health care professional. This examination centered on the affected joint and included a thorough assessment of the condition. Following completion of the questionnaires and examination, researchers determined if the volunteer met the requirements for the study.
Randomized treatments were assigned to the participants in strictly ascending order after enrollment. Directions were given to the participants by the researchers on the proper application of the cream and completion of the measuring instruments. Creams were to be applied by gentle circular motions for 3 to 5 minutes over the affected area 3×/d. The amount used was approximately 9.5 mg/d, one third of which was applied at each application. This dosage was sufficient to envelop the entire knee joint with a thin layer of cream to be massaged in. An information sheet explaining application of the cream was given to all participants.
Any subject taking medication for knee OA underwent a 1-week washout period used to eliminate possible data contamination. Fourteen of the patients were subject to washout. Patients were also given a form to record medication use during the study. Following baseline assessments for pain, stiffness, and daily function, subjects were given one of the experimental creams or placebo with application directions. The duration of the study was approximately 6 weeks actual treatment with clinic visits every 2 weeks, totaling 4 times over a 6-week period. During each visit to the clinic, the subjects returned the empty tubes, completed the WOMAC Osteoarthritis Index Version VA3.1, and were asked about adverse events and compliance to the regime. Furthermore, research assistants contacted the subjects in the middle of each week to ask about and to encourage compliance.
Outcomes
The primary outcome measure was the pain subscale of the WOMAC Osteoarthritis Index Version VA3.1. The WOMAC instrument is designed to measure joint pain, stiffness, and discomfort in selected daily activities or tasks and uses a visual analog scheme involving a 100-mm line with polar extremes at each end. For each item on the questionnaire, the participants place a mark along the line to indicate their degree of pain/discomfort. Five questions are in reference to pain, 2 in reference to stiffness, and 17 regarding daily function. The WOMAC scales have been determined to be internally consistent with Cronbach α coefficients of .83, .87, and .96 and with test-retest reliability intraclass correlation coefficients of 0.74, 0.58, and 0.92.38
Randomization
The randomization scheme was done using the Web site Randomization.com (http://www.randomization.com). Participants were assigned to their group a priori. By random, double-blind design, subjects received a 6-week supply of 1 of the 3 creams. The study creams were packaged in tubes according to the assigned treatment and numbered consecutively. Participants were allocated to the next consecutively numbered tube by the clinician at each study center who also enrolled each of the participants. The random allocation sequence was concealed until the end of treatment.
The participants, those administering the treatments, and those assessing the outcomes were masked to group assignment. Success of blinding was determined by asking the participating clinicians if they were able to determine group assignment. None of the clinicians was able to delineate group assignment. As the color, texture, and smell of the different formulations were very similar, the participants were unable to determine to which group they had been assigned.
Statistical analysis
One-way analyses of variance (ANOVAs) were used to compare the groups at baseline on the demographic variables. Repeated-measures ANOVAs were used to determine differences in group mean values for pain, stiffness, and function as measured by the WOMAC subscales. Newman-Keuls post hoc tests were used to determine the specific combinations of significance. Two-way ANOVAs were used to assess baseline group differences in pain, stiffness, and activity. An α level of .01 was used to determine significant difference between groups.
Results
Of the 43 eligible subjects, 6 were lost to follow-up because of (a) withdrawal from the study (n =2), (b) relocation to another city (n =1), (c) or failure to maintain compliance in application of cream (n =3), thus leaving 37 completed data sets (Fig 1). The characteristics of the groups at baseline are shown in Table 1. Participants' ages ranged from 45 to 83 years, and there was no significant difference between the groups (P > .01). Body mass index ranged from 20.9 to 50.2, and there was no significant difference between the groups (P > .01). Results of baseline group comparisons of the WOMAC scales were as follows: pain P = .83, stiffness P = .28, and activity P = .64, thus indicating initial statistical differences among the groups.
Table 1.
Baseline mean values and standard deviations for group characteristics
| Variable (M, SD) | 20% | 10% | Place | P |
|---|---|---|---|---|
| Age (y) | 66 (10.5) | 60 (8.9) | 65 (9.5) | .87 |
| BMI | 28.7 (5.8) | 29.3 (7.6) | 31.9 (7.5) | .84 |
| Female (%) | 69 | 75 | 50 | .36 |
| Pain | 276.5 (91.9) | 237.8 (100.7) | 219.4 (108.9) | .83 |
| Stiffness | 111.6 (55.7) | 121.3 (44.1) | 97.6 (44.9) | .28 |
| Function | 1009.7 (365.3) | 1002.3 (275.8) | 775.6 (354.7) | .64 |
M, Mean; BMI, body mass index.
On the primary outcome of change in the WOMAC pain subscale, the 10% formula demonstrated a 50.3% change, the 20% formula a 52.1% change, and the placebo a 24.3% change (Fig 2) from pretest to 6-week end point. A repeated-measures ANOVA and subsequent Newman-Keuls post hoc analysis resulted in a significant (P < .01) difference between pretest and all of the 2-week mean values for both the 10% and 20% creams and a significant (P < .01) change from the second week to the sixth week and from the fourth week to the sixth week for the 10% concentration. Although the placebo registered an improvement in pain, the level of improvement was not statistically significance (P > .01).
Fig 2.
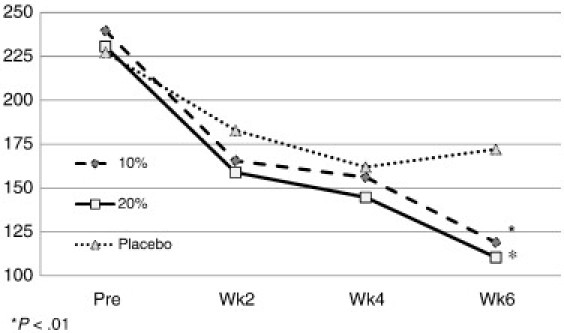
Pre and post WOMAC score mean values for pain by group.
On the secondary outcome of change in the WOMAC stiffness score, the 10% and 20% formulae yielded a 44.1% and 56.9% change, respectively, whereas the placebo registered a change of 24.1% (Fig 3), from pretest to 6-week end point. The repeated-measures ANOVA and subsequent Newman-Keuls post hoc test resulted in a significant difference between pretest and all of the 2-week mean values for both the 10% formula and the 20% formula. The placebo yielded an improvement in stiffness, but the level of improvement was not statistically significance (P > .01).
Fig 3.
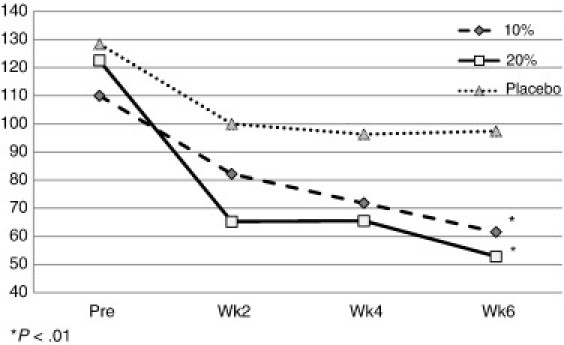
Pre and post WOMAC score mean values for stiffness by group.
On the WOMAC function subscale, the 10% formula resulted in a 52.1% change, the 20% formula a 53.9% change, and the placebo a 22.9% change (Fig 4) from pretest to 6-week end point. The repeated-measures ANOVA and subsequent Newman-Keuls post hoc test resulted in a significant difference between pretest and all of the 2-week mean values for the 20% formula. The 10% formula demonstrated significant improvements between baseline and weeks 4 and 6, but not for week 2. The placebo yielded an improvement in stiffness, but the level of improvement failed to reach significance (P > .01).
Fig 4.
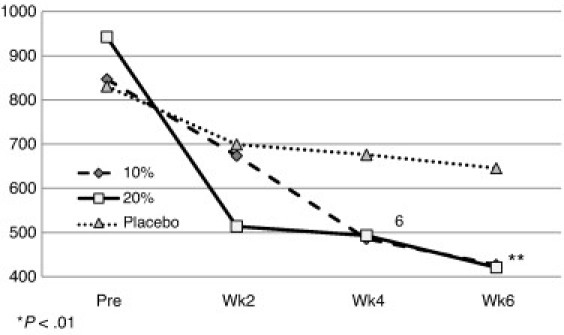
Pre and post WOMAC score mean values for function by group.
Both the 10% and 20% formulae were significantly superior to placebo in reducing pain and stiffness and improving daily activities in those with primary OA of the knee. The 20% formula produced a slightly greater reduction in pain and stiffness and a slight improvement in function than the 10% formula; however, differences were not significant. There were no important adverse effects reported by any participant in any group. Of minor consequences, 2 subjects in both the 10% and 20% formula groups and none of the participants in the placebo group developed adverse effects. The minor and temporary adverse effects consisted of itching and rash of the skin in the area that the cream was applied; however, the effects resolved rapidly before the end of treatment. No participant withdrew from the study because of adverse effects.
Discussion
In addition to synthetic drug therapies, natural herbal and botanical agents have been investigated as an alternative treatment of OA. Botanicals such as stinging nettle,39 devil's claw, turmeric, and ginger40 have yielded positive results, whereas avocado soybean fraction, rose hip, and seed powder showed moderate positive results and Boswellia serrata gum resin registered poor outcomes.41 Previous studies using comfrey have all suggested that the herb is effective in treating edema15,22 and pain associated with OA. Previous studies have found a 46%42,43 and a 54.7%24 reduction in OA knee pain following a regimen of comfrey cream use compared with a 10.7% improvement in a placebo group.24 For the present study, the 10% and 20% creams reduced pain by 50.3% and 52.1% and stiffness by 44.1% and 56.9%, and improved function by 49.5% and 55.3%, respectively. The placebo yielded reductions of only 19.6% for pain and 22.1% for stiffness, and improvement of 16.3% for function. The initial positive placebo responses began to level off and/or deteriorate over time, whereas the 10% and 20% formulae continued to produce positive results. Initial pain improvement and subsequent leveling off were also observed in 2 similar comfrey extract studies.15,21 Indeed, the placebo effect in pain studies can be significant and has been labeled a nuisance.44 For example, one study found a 49% placebo improvement in back pain,45 whereas another found a 57% improvement in bone metastasis pain46, prompting some to suggest that a placebo is effective in treating symptoms of OA.47,48 However, placebo results tend to be unsustainable, unpredictable, and ephemeral depending on the situation. In determining the minimal clinically important improvement of pain and function in patients with knee OA, Tubach et al suggested minimal clinically important improvement for absolute changes to be −19.9 mm (−40.8%) for pain and −9.1 (−26.0%) for the WOMAC function subscale score.49
Studies using comfrey have reported reductions in joint pain after 7 days24 and 12 days42,43 and reductions in ankle pain and swelling in as little as 4 days.15 Initial improvements (observation in the first 2 weeks) in the current study demonstrated pain improvement by 30.9% for the 10% cream, 31.3% for the 20% cream, and 19.6% for the placebo; stiffness improvement for the 10% and 20% comfrey cream and placebo by 19.6%, 46.8%, and 22.1%, respectively; and function improvement of 20.5% (10% cream), 45.5% (20% cream), and16.3% (placebo).
With the efficacy in the topical formulae used in the current study and with less adverse effects than have been found with NSAIDs,50 the results of the current study are encouraging and warrant a closer look at the safe and effective means for treating the discomfort of OA via topical botanical agents. In conclusion, the 10% and 20% formulae were both effective in relieving pain and stiffness and improving daily function within the first 2 weeks of treatment; and improvement continued at each 2-week assessment period for the duration of the study. For the 3 variables addressed, the placebo yielded initial positive, but nonsignificant, responses.
Limitations
It warrants mentioning that factors that influence drug absorption via transdermal delivery across the skin include differences in skin characteristics on different parts of the body,51 age, sex, body composition, and blood supply.52
As is the case with most pilot studies, small samples reduce the statistical power of the data. Further studies with larger sample sizes; with longer observations periods; and with age, sex, and body composition comparisons are encouraged. Another recommendation for further study of the efficacy of this botanical agent is to strictly screen patients on the severity of ailment. For instance, include only those who score in the 50th percentile on each of the WOMAC subscale items. The 50th percentile using the VAS method includes only those scoring 50 or more in each category. It is unrealistic to believe that those with pain and stiffness below this level will reflect a noteworthy change because the room for change is minimal.
Conclusion
Both active topical comfrey formulations were effective in relieving pain and stiffness and in improving physical functioning and were superior to placebo in those with primary OA of the knee without serious adverse effects.
Funding sources and potential conflicts of interest
No funding sources or conflicts of interest were reported for this study.
References
- 1.Helmick C., Felson D., Lawrence R., Gabriel S., Hirsch R., Kwoh C. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis And Rheumatism. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence R., Felson D., Helmick C., Arnold L., Choi H., Deyo R. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheum. 2008;58(1):26–30. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieppe P. Osteoarthritis: time to shift the paradigm. Brit Med J. 1999;1999(218):1299–1300. doi: 10.1136/bmj.318.7194.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creamer P., Hochberg M.C. Osteoarthritis. Lancet. 1997;350:503–509. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 5.Berman B.M., Singh B.B., Lao L., Langenberg P., Li H., Hadhazy V. Randomized trial of acupuncture as an adjunctive therapy in osteoarthritis of the knee. J Rheumatol. 1999;38:346–354. doi: 10.1093/rheumatology/38.4.346. [DOI] [PubMed] [Google Scholar]
- 6.Summers M.N., Haley W.E., Reveille J.O., Alarcon G.S. Radiographic assessment and psychological variables as predictors of pain and functional impairment in osteoarthritis of the knee and hip. Arthritis Rheum. 1988;31:204–209. doi: 10.1002/art.1780310208. [DOI] [PubMed] [Google Scholar]
- 7.Bradley J.D., Brandt K.D., Katz B.P., Kalasinski L.A., Ryan S.I. Comparison of an anti-inflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engl J Med. 1991;325:87–91. doi: 10.1056/NEJM199107113250203. [DOI] [PubMed] [Google Scholar]
- 8.MacFarlane D.G., Bucklan-Wright J.C., Emery P., Fogelman I., Clark B., Lynch J. Comparison of clinical, radionuclide, and radiographic features of osteoarthritis of the hands. Annal Rheum Dis. 1991;50:623–626. doi: 10.1136/ard.50.9.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelin E., Murphy L., Cisternas M., Foreman A., Pasta D., Helmick C. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56(5):1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAlindon T.E., Felson D.T., Zhang Y., Hannan M.T., Alabadi P., Weissman B. Relation of dietary intake on serum levels of vitamin D to progression in the Framingham Study. Ann Int Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 11.McAlindon T.E., LaValley M.P., Gulin J.P., Felson D.T. Glucosamine and chondroitin for treatment of osteoarthritis. A systematic quality assessment and meta-analysis. J Am Med Assoc. 2000;283:1460–1475. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 12.Katz J., Shah T. Persistent pain in the older adult: what should we do now in light of the 2009 American geriatrics society clinical practice guideline? Pol Arch Med Wewn. 2009;119(12):795–800. [PubMed] [Google Scholar]
- 13.Panicker S., Borgia J., Fhied C., Mikecz K., Oegema T. Oral glucosamine modulates the response of the liver and lymphocytes of the mesenteric lymph nodes in a papain-induced model of joint damage and repair. Osteoarhr Cartil. 2009;17(8):1014–1021. doi: 10.1016/j.joca.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Samson D., Grant M., Ratko T., Bonnell C., Ziegler K., Aronson N. Treatment of primary and secondary osteoarthritis of the knee. Evid Rep Technol Assess. 2007;(157):1–157. [PMC free article] [PubMed] [Google Scholar]
- 15.Koll R, Buhr M, Dieter R, Pabst H, Predel H, Petrowicz O, et al. Efficacy and tolerance of a comfrey root extract (Extr. Rad. Symphyti) in the treatment of ankle distorsions: results of a multicenter, randomized, placebo-controlled, double-blind study. Phytomedicine 2004;11(6):470-7. [DOI] [PubMed]
- 16.Kommission E. Monographie Symphyti radix (Beinwellwurzel) Bundesanzeiger. 1990:318. [Google Scholar]
- 17.Schmidtke-Schrezenmeier G., Reck R., Gerster G. Behandlung der nichtaktivierten Gonarthrose. Besserung durch ein Phytotherapeutikum. Therapiewoche. 1992;42:1322–1325. [Google Scholar]
- 18.Barna M., Kucera A., Hladícova M., Kucera M. Wound healing effects of a Symphytum herb extract cream (Symphytum x uplandicum NYMAN): results of a randomized, controlled double-blind study. Wien Med Wochenschr. 2007;157(21-22):569–574. doi: 10.1007/s10354-007-0474-y. [DOI] [PubMed] [Google Scholar]
- 19.Kucera M., Barna M., Horàcek O., Kàlal J., Kucera A., Hladìkova M. Topical Symphytum herb concentrate cream against myalgia: a randomized controlled double-blind clinical study. Adv Ther. 2005;22(6):681–692. doi: 10.1007/BF02849961. [DOI] [PubMed] [Google Scholar]
- 20.Bleakley C., McDonough S., MacAuley D. Some conservative strategies are effective when added to controlled mobilisation with external support after acute ankle sprain: a systematic review. Aust J Physiother. 2008;54(1):7–20. doi: 10.1016/s0004-9514(08)70061-8. [DOI] [PubMed] [Google Scholar]
- 21.D'Anchise R., Bulitta M., Giannetti B. Comfrey extract ointment in comparison to diclofenac gel in the treatment of acute unilateral ankle sprains (distortions) Arzneimittelforschung. 2007;57(11):712–716. doi: 10.1055/s-0031-1296672. [DOI] [PubMed] [Google Scholar]
- 22.Predel H., Giannetti B., Koll R., Bulitta M., Staiger C. Efficacy of a comfrey root extract ointment in comparison to a diclofenac gel in the treatment of ankle distortions: results of an observer-blind, randomized, multicenter study. Phytomedicine. 2005;12(10):707–714. doi: 10.1016/j.phymed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Sakakura C., Neto R., Bellucci M., Wenzel A., Scaf G., Marcantonio E. Influence of homeopathic treatment with comfrey on bone density around titanium implants: a digital subtraction radiography study in rats. Clin Oral Implants Res. 2008;19(6):624–628. doi: 10.1111/j.1600-0501.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 24.Grube B., Grünwald J., Krug L., Staiger C. Efficacy of a comfrey root (Symphyti offic. adix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine. 2007;14(1):2–10. doi: 10.1016/j.phymed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Koll R., Klingenburg S. Therapeutic characteristance and tolerance of topical comfrey preparations. Results of an observational study of patients. FortschrMed Orig. 2002;120(1):1–9. [PubMed] [Google Scholar]
- 26.Yudoh K., Nguyen V., Nakamura H., Hongo-Masuko K., Kato T., Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7(2):R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regan E., Bowler R., Crapo J. Joint fluid antioxidants are decreased in osteoarthritic joint compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr Cartil. 2008;16(4):515–521. doi: 10.1016/j.joca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Roman-Blas J., Contreras-Blasco M., Largo R., Alvarez-Soria M., Castañeda S., Herrero-Beaumont G. Differential effects of the antioxidant n-acetylcysteine on the production of catabolic mediators in IL-1beta–stimulated human osteoarthritic synoviocytes and chondrocytes. Eur J Pharma. 2009;623(1-3):125–131. doi: 10.1016/j.ejphar.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Zampieron E., Kamhi E. Alternative treatment for arthritis. J Indian Rheumatol Assoc. 2004;12:139–142. [Google Scholar]
- 30.Cho M., Heo Y., Park M., Oh H., Park J., Woo Y. Grape seed proanthocyanidin extract (GSPE) attenuates collagen-induced arthritis. Immunol Lett. 2009;124(2):102–110. doi: 10.1016/j.imlet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Levanon D., Stein H. Quantitative analysis of chondroitin sulphate retention by tannic acid during preparation of specimens for electron microscopy. Histochemic J. 1995;27(6):457–465. [PubMed] [Google Scholar]
- 32.Amanlou M., Dadkhah F., Salehnia A., Farsam H., Dehpour A. An anti-inflammatory and anti-nociceptive effects of hydroalcoholic extract of Satureja khuzistanica Jamzad extract. J Pharm Pharm Sci. 2005;8(1):102–106. [PubMed] [Google Scholar]
- 33.Shin H., Hwang H., Kang K., Lee B. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharm Res. 2006;29(2):165–171. doi: 10.1007/BF02974279. [DOI] [PubMed] [Google Scholar]
- 34.Weyers W., Brodbeck R. Skin absorption of volatile oils. Pharmacokinetics. Pharmazie Unserer Zeit. 1989;18:82–86. doi: 10.1002/pauz.19890180304. [DOI] [PubMed] [Google Scholar]
- 35.Singh H.P., MIttal S., Kaur S., Batish D.R., Kohil R.K. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. Am J Phys Med Rehabil. 1991;70(1):29–33. doi: 10.1021/jf9012407. [DOI] [PubMed] [Google Scholar]
- 36.Altman R.D. Criteria for classification of clinical osteoarthritis. J Rheumatol. 1991;18:10–12. [PubMed] [Google Scholar]
- 37.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1989;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 38.Roos E.M., Klässbo M., Lohmander L.S. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28(4):210–215. doi: 10.1080/03009749950155562. [DOI] [PubMed] [Google Scholar]
- 39.Rayburn K., Fleischbein E., Song J., Allen B., Kundert M., Leiter C. Stinging nettle cream for osteoarthritis. Alt Ther Health Med. 2009;15:60–61. [PubMed] [Google Scholar]
- 40.Gregory P.J., Sperry M., Wilson A.F. Dietary supplements for osteoarthritis. Am Fam Physician. 2008;77:177–184. [PubMed] [Google Scholar]
- 41.Chrubasik J.E., Roufogalis B.D., Chrubasik S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytotherapy Res. 2007;7:675–683. doi: 10.1002/ptr.2142. [DOI] [PubMed] [Google Scholar]
- 42.Tschaikin Extrakt aus Symphytum officinale. Wirksamkeit und veträglichkeit bie topisher anwendung. Naturheilpraxis. 2004;57:576–578. [Google Scholar]
- 43.Klingenburg S. Wärmendes Topikum mit Symphytum officinale. Erfahrungsheilkunde. 2004;6:350–354. [Google Scholar]
- 44.Doherty M., Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage. 2009;17:1255–1262. doi: 10.1016/j.joca.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Schwetschenau P., Ramirez A., Johnston J., Wiggs C., Martins A. Double-blind evaluation of intradiscal chymopapain for herniated lumbar discs. Early results. J Neurosurgery. 1976;45(6):622–627. doi: 10.3171/jns.1976.45.6.0622. [DOI] [PubMed] [Google Scholar]
- 46.Boureau F., Leizorovicz A., Caulin F. The placebo effect in bone metastatic pain. Presse Médicale (Paris, France: 1983) 1988;17(21):1063–1066. [PubMed] [Google Scholar]
- 47.Zhang W., Robertson J., Jones A.C., Dieppe P.A., Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 48.Grace D., Rogers J., Skeith K., Anderson K. Topical diclofenac versus placebo: a double blind, randomized clinical trial in patients with osteoarthritis of the knee. J Rheumatol. 1999;26:2659–2663. [PubMed] [Google Scholar]
- 49.Tubach F., Ravaud P., Baron G., Falissard B., Logeart I., Bellamy N. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bookman A.A.M., Williams S.A., Shainhouse J.Z. Effect of topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trial. Can Med Assoc J. 2004;171:333–338. doi: 10.1503/cmaj.1031793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnusson B.M., Walters K.A., Roberts M.S. Veterinary drug delivery: potential for skin penetration enhancement. Adv Drug Deliv Rev. 2001;50:205–227. doi: 10.1016/s0169-409x(01)00158-2. [DOI] [PubMed] [Google Scholar]
- 52.Morganti P., Ruocco E., Wolf R., Ruocco V. Percutaneous absorption and delivery systems. Clin Derm. 2001;19:489–501. doi: 10.1016/s0738-081x(01)00183-3. [DOI] [PubMed] [Google Scholar]


