Abstract
Herbivores have been hypothesized to adapt locally to variation in plant defences and such adaptation could facilitate novel associations in the context of biological invasions. Here, we show that in the native range of the viburnum leaf beetle (VLB, Pyrrhalta viburni), two populations of geographically isolated hosts—Viburnum opulus and Viburnum tinus—have divergent defences against VLB oviposition: negative versus positive density-dependent egg-crushing wound responses, respectively. Populations of beetles coexisting with each host show an adaptive behavioural response: aggregative versus non-aggregative oviposition on V. opulus and V. tinus, respectively. In parallel, we show that in North America, where VLB is invasive, defences of three novel hosts are negatively density-dependent, and beetles' oviposition behaviour is aggregative. Thus, local adaptation to plant defences has the potential to facilitate the invasion of herbivores onto novel hosts.
Keywords: insect oviposition strategy, invasion ecology, adaptive deme hypothesis, Chrysomelidae, plant–insect interactions, plant defence theory
1. Introduction
Because plants vary tremendously in their defensive arsenal among species [1] and populations [2], herbivores may encounter geographically heterogeneous mosaics of plant defences and their offence traits are predicted to exhibit local adaptation [3–6]. Despite this widespread notion, relatively little data exist on local adaptation of insects to specific defence traits [7,8].
Adaptation to plant defences may be particularly important in the context of biological invasions [9,10]. Novel host associations occur rapidly when immigrant herbivores become established in new environments. Invasion success might then depend, at least in part, on past coevolutionary interactions [11,12]. For example, a herbivore is more likely to successfully adopt new hosts whose chemical defences resemble those deployed by its coevolved hosts [13–15]. Reciprocally, plants that are evolutionarily naive to the threat posed by specific herbivores are less likely to have defences against them, creating ‘defence-free space’ favouring the herbivore and invasion success [9,16]. In other words, the degree of relatedness between past and new interaction partners is expected to influence the success of an invader and its impact on the new environment [17].
In this study, we investigated the relationships between oviposition behaviour of a specialized herbivore, the viburnum leaf beetle (VLB) Pyrrhalta viburni (Paykull), and plant defences (an egg-crushing wound response) of hosts in its native and introduced range. In a field study, we first documented divergent plant defences in two geographically isolated populations of two native Eurasian hosts, and tested whether oviposition behaviour of local VLB populations was adaptive using a reciprocal transplant experiment. In parallel, we investigated the defences of three novel hosts in the introduced range and the oviposition behaviour of an invasive VLB population. Because plant defences have been hypothesized to play a major role in regulating populations of VLB [18], the extent to which beetle populations are locally adapted to defences may be critical in understanding the success of the VLB invasion.
2. Material and methods
(a). Natural history and background
Pyrrhalta viburni (Coleoptera: Chrysomelidae) is a specialist herbivore of Viburnum spp. (Adoxaceae, Dipsacales) that is native to Eurasia. VLB is invasive in North America, where it has become a serious pest on several North America native Viburnum spp. [19,20]. In its native range, VLB has three main host plants: (i) Viburnum opulus L. (ii) and Viburnum lantana L., which co-occur in areas of Eurasia with temperate and continental climates, and (iii) Viburnum tinus L., which occurs exclusively in the Mediterranean.
Pyrrhalta viburni is a univoltine species that overwinters as eggs and oviposits during the summer months (July–September). Eggs are laid in round cavities (1–2 mm diameter, 1 mm deep) excavated by the females in the terminal twigs of Viburnum shrubs. Each cavity contains an average of eight eggs [21] and is closed with a cap made of a brown secretion produced by the females (2–4 mm length; figure 1a). In North America, VLB oviposition is typically aggregative, a behaviour characterized by a preference for twigs already infested with egg masses for oviposition, and the positioning of newly laid egg masses adjacent to the existing ones [18]. The main benefit of this behaviour is thought to be overcoming a specific type of plant defence: the production of wound tissue crushing or expelling eggs outside the egg cavity in the weeks following oviposition (figure 1b). Egg survivorship was seven times lower on twigs expressing such wound response, and it was negatively correlated with intensity of infestation (number of egg masses per twig) on several Viburnum hosts including V. opulus [18,22]. Twigs heavily infested with egg masses often die, circumventing their ability to produce wound tissue. As a result of aggregative oviposition, VLB egg masses are often found in aligned clusters along twigs of infested shrubs in natural settings (figure 1). However, in the Mediterranean, egg masses are typically more scattered and less clustered on shrubs of the local host-plant V. tinus (G. A. Desurmont 2006 and 2007, personal observation).
Figure 1.
(a) Clusters of viburnum leaf beetle (VLB) egg masses along a Viburnum opulus twig. Eggs are laid inside cavities excavated in the twigs and hidden under the protective ‘egg cap’ secretion. (b) Wound tissue produced by a V. opulus twig encasing four VLB egg masses. Here, egg caps have all fallen off and no viable eggs are visible. Photos by Kent Loeffler.
(b). Insect and plant material, and sites location
The study of local adaptation of VLB to different hosts in its native range was conducted in July–August 2010 at two sites in Europe: the first site (hereafter Tinus site) was a shaded forest understory habitat located in Southern France (Montferrier-sur-Lez, Mediterranean climate) with abundant V. tinus shrubs. The second site (hereafter Opulus site) was an open meadow habitat located in the northern part of France (Willems, temperate climate), an area where V. opulus is the dominant Viburnum species. Shrubs used in both sites were unmanaged and naturally infested with local breeding populations of VLB. Oviposition choice-tests related to the Tinus population were conducted at the USDA ARS European Biological Control Laboratory (EBCL, Montferrier-sur-Lez) under ambient laboratory conditions (approx. 20°C and 15 L : 9 D light regime), and choice-tests of the Opulus population were conducted at a local facility under similar ambient conditions.
The study of oviposition behaviour of VLB in its introduced range was conducted with VLB adults collected in the Ithaca area (NY, USA), where breeding populations have naturally occurred since the early 2000s, and plant material was collected in a local unmanaged common garden of Viburnum spp. Oviposition choice-tests with North American beetles were conducted on both European species (V. opulus and V. tinus) as well as two native North American species (V. rafinesquianum and V. dentatum). Assays were conducted in growth chambers (22°C and 16 L : 8 D light regime) in August 2007 for V. opulus, and in August 2008 for the other species. We will refer to the beetles present and collected at the Tinus, Opulus and Ithaca site as, respectively, Tinus, Opulus and North American beetles in the rest of this paper. Adult colonies of VLB from all sites were kept in plastic containers with a vented lid, and beetles were provided with fresh twigs of their coexisting host-plant for Tinus and Opulus beetles, and with V. opulus and V. dentatum twigs for North American beetles.
(c). Observational studies of density-dependent plant defence
To assess the defences of populations of European and North American Viburnum species in response to natural levels of beetle oviposition, terminal twigs of large, mature and unmanaged Viburnum shrubs were randomly collected at the end of the growing season and carried to the laboratory. No more than 20 twigs were collected from a same shrub. For each twig, the following parameters were recorded: presence of egg masses, total number of egg masses intact, total number of egg masses encased in wound tissue, percentage wound response (i.e. egg masses encased in wound tissue/(egg masses intact + egg masses encased in wound tissue) × 100) and twig vitality (i.e. alive or dead). Twig vitality was gauged by observing the presence of green tissue under the bark or buds of the twig: if a twig possessed green tissue at both ends, then it was considered alive, and if a twig infested with egg masses possessed green tissue on the portion basal to the egg masses but no green tissue on the distal portion, then it was considered killed by VLB oviposition. Per cent twig mortality was then calculated across different twig infestations (such as 1–2, 3–4, 5–6, 7–8, 9–10 egg masses per twig). Categories of infestation with less than three data points were excluded from the analyses.
Density-dependent plant defence was assessed for populations of five Viburnum species: V. tinus (Tinus site, 2006), V. opulus (Opulus site, 2010), V. dentatum (Ithaca, 2005), V. rafinesquianum (Ithaca, 2005) and V. trilobum (Allegheny National Forest, PA, USA, 2008). For V. tinus, 272 total twigs were collected from 19 shrubs; V. opulus, 116 twigs from six shrubs; V. dentatum, 731 twigs from 37 shrubs; V. rafinesquianum, 129 twigs from 13 shrubs; and V. trilobum, 510 twigs from 25 shrubs.
(d). Oviposition preference experiments
Oviposition choice-tests were conducted as follows: two mated VLB females were placed in a plastic cylindrical container (9.5 × 21 cm) with a screened lid. Each container comprised a pair of Viburnum twigs of the tested host-plant kept in floral water tubes, and a pair of leaves as a food source. Each pair of twigs was homogeneous in length (12 cm) and diameter to the nearest half millimetre (from 2 to 4 mm, depending on the pair). One twig was intact (i.e. not previously infested), whereas the other was experimentally infested with 1–12 egg masses laid by one to four females from the same population in the laboratory during the 48 h preceding the trials. The number of new egg masses on all twigs was recorded after 24 and 48 h. In our analysis of oviposition preferences (see below), only the first egg masses recorded for each replicate were used. In oviposition choice-tests conducted with Opulus and Tinus beetles, 20 replicates were used for each of the four reciprocal pairings (i.e. Opulus population–V. opulus, Opulus population–V. tinus, Tinus population–V. opulus, Tinus population–V. tinus). In choice-tests conducted with North American beetles, 20 replicates were used for V. opulus and V. tinus, 10 replicates for V. dentatum and 14 replicates for V. rafinesquianum. Each VLB female was used only once in the experiments.
(e). Statistical analysis
The relationship between infestation density (number of egg masses per twig) and level of wound response and twig mortality were estimated for each host-plant species using linear and nonlinear regression models (JMP 8, SAS Institute Inc.). Shrub (i.e. the specific plant from which twigs were collected) was added as a block factor, and data were log-transformed if needed to meet the assumptions of the model.
Oviposition preference data from the choice-tests were analysed by sign tests on the difference between egg masses laid on the infested and non-infested twig for each replicate (Statistix v. 9.0; Analytical software, 2008); replicates where equal numbers of egg masses (including 0) were laid on both twigs were excluded from the sign tests. Differences in female fecundity were analysed by comparing the total number of egg masses laid on both twigs after 48 h (ANOVA, Statistix v. 9.0; Analytical software 2008).
3. Results
(a). Density-dependent plant defence
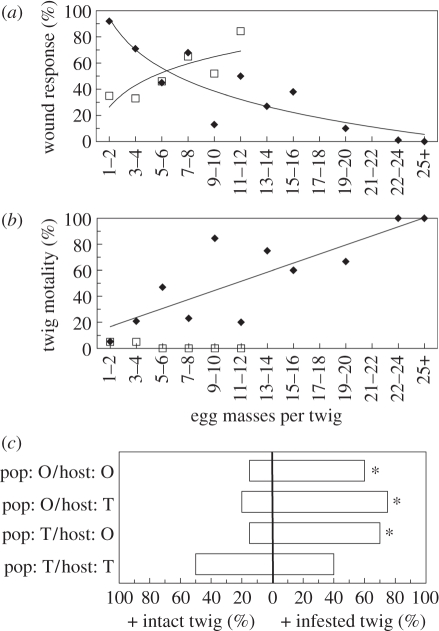
In Europe, at the Opulus site, the average infestation density (number of egg masses per twig) was 8.18 ± 0.6 (mean ± s.e.), ranging from 1 to 33. Infestation density was negatively correlated with V. opulus twig wound response (F1,115 = 51.9, p < 0.001, r2 = 0.31; figure 2a), and positively correlated with twig mortality (F1,9 = 17.4, p < 0.01, r2 = 0.65; figure 2b). At the Tinus site, however, the average infestation density was about half: 4.67 ± 0.36 egg masses per twig, ranging from 1 to 22, and infestation density was positively correlated with V. tinus twig wound response (F1,129 = 5.4, p = 0.02, r2 = 0.23; figure 2a). Twig mortality was negligible (3.81% overall) on V. tinus, independent of infestation (figure 2b).
Figure 2.
Plant defences and beetle oviposition behaviour in Europe. (a) Plant defences against viburnum leaf beetle (VLB) oviposition expressed as percentage wound response (i.e. number of egg masses encased in wound tissue divided by total number of egg masses laid per twig) depending on the extent of infestation (number of egg masses per twig) for V. tinus (squares) and V. opulus (diamonds; mean ± s.e.). (b) Twig mortality depending on the extent of infestation. Lines indicate significant relationships from linear or nonlinear regression (p < 0.05). Raw untransformed data are shown with best-fit curves. (c) Oviposition choice-tests between previously infested and intact (i.e. non-infested) twigs for each VLB population per host-plant pairing (pop, population; host, host-plant; O, V. opulus; T, V. tinus). Horizontal bars represent the percentage of replicates in which females laid more egg masses on the infested twig (+ infested twig) or on the intact twig (+ intact twig; 20 replicates per pairing). In 11% of the replicates, no egg masses or equal numbers of egg masses were laid. Asterisks indicate p < 0.05 (sign-test).
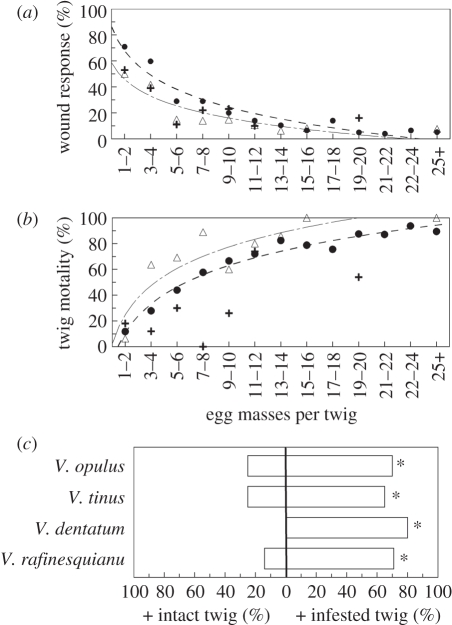
In North America, infestation density was negatively correlated with twig wound response for all three novel host species: F1,499 = 248.5, p < 0.0001, r2 = 0.34 for V. dentatum; F1,108 = 23.9, p < 0.0001, r2 = 0.20 for V. rafinesquianum; and F1,186 = 17.62, p < 0.0001, r2 = 0.13 for V. trilobum, respectively (figure 4a). Twig mortality was positively correlated with the number of egg masses per twig for V. dentatum (F1,12 = 315.3, p < 0.0001, r2 = 0.96) and V. rafinesquianum (F1,8 = 33.0, p < 0.0001, r2 = 0.80), but not for V. trilobum (F1,5 = 0.8, p = 0.42, r2 < 0.01; figure 4b). Mean infestation densities were 13.0 ± 0.4 (range 1–60), 10.8 ± 0.9 (1–45) and 5.3 ± 0.4 (1–22), for V. dentatum, V. rafinesquianum and V. trilobum, respectively.
Figure 4.
Plant defences and beetle oviposition behaviour in North America. (a) Plant defences against viburnum leaf beetle (VLB) oviposition expressed as percentage wound response (i.e. number of egg masses encased in wound tissue divided by total number of egg masses laid per twig) depending on the extent of infestation (number of egg masses per twig) for V. dentatum (circles), V. rafinesquianum (triangles) and V. trilobum (plus symbols; mean ± s.e.). (b) Twig mortality depending on the extent of infestation. Lines indicate significant relationships from nonlinear regression (p < 0.05). Raw untransformed data are shown with best-fit curves. (c) Oviposition choice-tests between previously infested and intact (i.e. non-infested) twigs with an invasive North American population of VLB on four host-plants: V. opulus, V. tinus, V. dentatum and V. rafinesquianum. Horizontal bars represent the percentage of replicates in which females laid more egg masses on the infested twig (+ infested twig) or on the intact twig (+ intact twig; 10–20 replicates per host-plant). In 11% of the total replicates, no egg masses or equal numbers of egg masses were laid. Asterisks indicate p < 0.05 (sign-test).
(b). Oviposition preference experiments
In Europe, VLB females from the Opulus population preferentially chose infested twigs for oviposition when presented either V. opulus twigs (p = 0.03) or V. tinus twigs (p = 0.02; figure 2c). Females from the Tinus population also preferentially chose infested twigs for oviposition when presented with V. opulus twigs (p = 0.01), but not when presented with V. tinus twigs (p = 0.81; figure 2c). Total fecundity after 48 h (i.e. number of eggs deposited on both twigs) was similar on V. opulus and V. tinus (F1,38 < 0.001, p = 0.99) for females from the Opulus population, but was 40 per cent higher on V. tinus than on V. opulus for females of the Tinus population (F1,35 = 5.8, p = 0.02; figure 3).
Figure 3.
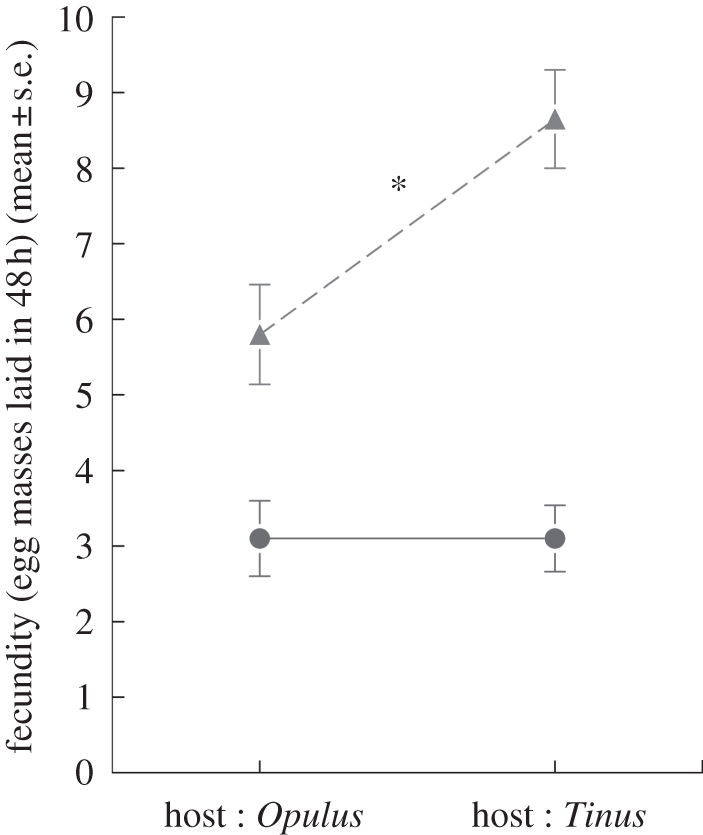
Total realized fecundity over 48 h of females from VLB populations coexisting with Viburnum opulus and V. tinus on both host-plants (mean ± s.e.). Asterisk indicates p < 0.05, one-way ANOVA. Filled circle, population Opulus; filled triangle, population Tinus.
In North America, beetles preferentially chose infested twigs for oviposition when presented with twigs of the two European and two North American Viburnum species tested (all ps < 0.05; figure 4c).
4. Discussion
Because plants defences are potent agents of natural selection and vary between species and populations, populations of herbivores are predicted to adapt to the local mosaics of defences that they experience. This local adaptation to plant defences may either facilitate or impede associations with novel hosts, thus impacting herbivores' potential invasion success in novel environments. Our study shows that, in Europe, populations of two native hosts of the leaf beetle VLB are differentially defended. Although the mode of defence exhibited by both hosts was similar (i.e. production of wound tissue expelling or crushing the egg masses), they showed countervailing responses to infestation density (negatively density-dependent for V. opulus, positively density-dependent on V. tinus; figure 2a). Owing to the rarity of heavily infested twigs (greater than 15 egg masses) at the Tinus site, it is unclear if the V. tinus wound response would be even more strong (or would be overwhelmed) at the high levels of infestation observed at the Opulus site. Nonetheless, the oviposition strategies of Opulus and Tinus beetles are well-matched to the defence patterns of the populations of their local hosts: females coexisting with V. tinus show aggregative oviposition on V. opulus, but not on V. tinus; females coexisting with V. opulus aggregate on both host plants (figure 2c).
The results of our behavioural experiments were matched by our field observations of egg mass distribution for each host: twigs with greater than 10 egg masses were less abundant at the Tinus site (9.9% of the infested twigs) than at the Opulus, Rafinesquianum and Dentatum sites (25.9, 45.0 and 47.7%, respectively; electronic supplementary material, figure S1), suggesting that Tinus beetles do aggregate less than beetles coexisting with the other hosts. However, because of potential differences in beetle abundance between the sampling sites, a direct statistical comparison of egg mass distribution between sites was not possible. In addition, the realized 2-day fecundity of females from the Tinus population was higher on V. tinus than on V. opulus (figure 3). We note that this may not reflect the overall fitness impact, but more likely a reduced motivation to oviposit on the novel host. Both of these results support the notion that beetles from the Tinus population are adapted to their local host; alternatively, beetles from the Opulus population behave similarly and show similar 2-day fecundity on both hosts, apparently making maladaptive decisions on the host with which they do not coexist (i.e. aggregative oviposition on V. tinus). However, because only one population of beetles and plants was used for each native host, the scope of our conclusions is limited to these specific populations. Repeating the evaluation of host defences and beetles' oviposition strategy with additional populations would be necessary to generalize our results.
How do plant defences of European Viburnum spp. compare with their North American congeners? The three New World Viburnum spp. tested (V. dentatum, V. rafinesquianum and V. trilobum) showed the same pattern as V. opulus: a decrease in wound response in response to increased infestation density (figures 2b and 4b). This result is consistent with additional field observations on naive North American and Asian hosts [22] and implies that aggregative oviposition should be beneficial in these three Viburnum spp. Beetle populations behaviourally adapted to V. opulus would then be ‘pre-adapted’ to overcome the defences of these hosts in case of a novel association. The behaviour of VLB in its introduced range matches these assumptions: beetles from the North American population showed aggregative oviposition on all the hosts they were exposed to (V. opulus, V. tinus, V. dentatum and V. rafinesquianum; figure 4c) similar to Opulus beetles (figure 2c). In addition to similarities in defensive capacities, V. opulus and North American Viburnum spp. showed a comparable increase in twig mortality as infestation density increases (with the exception of V. trilobum; figures 2b and 4b), suggesting that killing infested twigs may be the mechanistic basis of overcoming plant defences in these species.
Across 16 Viburnum spp. whose defences have been evaluated, enhanced defensive response to increased oviposition appears to be uniquely displayed by V. tinus [22], and is associated with increased tolerance to oviposition (i.e. negligible twig mortality caused by oviposition; figure 2b). These characteristics could reflect an adaptation of V. tinus to the beetle: VLB is able to impose serious damage to V. tinus shrubs under natural conditions (G. A. Desurmont 2010, personal observation), which may have driven selection towards beetle resistance. Moreover, density-dependent increases in this wound response are reminiscent of induction of defences in response to herbivore attack [23,24]. On the other hand, the exceptional defensive capacity of V. tinus may also originate from the specific environmental conditions inherent to the Tinus site. Although there is evidence that the defensive patterns observed are consistent between gardens for V. opulus [18,22] and V. tinus (G. A. Desurmont 2005, unpublished data), repeating studies of these plant defences in common gardens would distinguish between the genetic basis and the influence of environmental factors on the wound-response capacity of the hosts. In addition, testing the behaviour of Tinus and Opulus beetles on plants from both hosts grown in reciprocal common gardens would help determine whether environmental conditions may influence oviposition behaviour via modification of host traits.
VLB females from the Tinus site revert to aggregative oviposition on V. opulus (figure 2c), indicating that this population exhibits behavioural plasticity, unlike the Opulus population. Although this plasticity appears adaptive (females make the ‘smart choice’ on both hosts), Tinus beetles do not currently encounter V. opulus in their environment. Theory predicts that canalization of traits should be favoured over plasticity by selection in invariable environments, especially if plasticity itself is costly [25]. Therefore, behavioural plasticity of the Tinus beetles may suggest that the pattern of defences observed in the V. tinus population (positively density-dependent) is not invariable and could be population-specific or depend on environmental conditions, which could have favoured the evolution of behavioural plasticity. In addition, it is possible that oviposition preferences of Tinus beetles are based on twig traits common to V. tinus and V. opulus. In North America, VLB females show remarkable plasticity in their oviposition preferences at the twig level: diameter [18], vigour [26], presence of wound tissue and density of previous infestation [27] are all traits influencing twig selection for oviposition. Tinus beetles may thus be able to accurately gauge plant defences of twigs of the novel host (V. opulus) on the basis of traits indicative of wound response common to the coexisting and novel host.
Temporary gregariousness, group-living and differential reproductive investment have been previously described as adaptations of herbivores to plant defences for a number of systems [28–31]. Differences in gregariousness/reproductive strategy resulting from adaptations to plant defences may contribute to genetic divergence and potentially diversification. In Southeast Asia, differences in oviposition preferences have been observed within the complex of Pyrrhalta spp. feeding on Viburnum spp. [32,33]. Pyrrhalta humeralis and Pyrrhalta esakii share similar Viburnum hosts, life histories and mode of oviposition (i.e. deposition of eggs in cavities excavated in the twigs), but P. humeralis lays large egg masses (i.e. eight eggs), often in groups, at the basis of twigs, whereas P. esakii lays isolated, small egg masses (i.e. one to two eggs) at the tip of twigs [34]. Nonetheless, the range of oscillations in oviposition strategies of these beetles and how they may relate to plant defence remains largely unexplored.
Because benefits of gregariousness can be easily negated by density-dependent costs such as intraspecific competition; gregarious behaviours are typically sensitive to environmental variation, which may translate into behavioural plasticity [35,36]. In VLB, plastic oviposition preferences seem to reflect maximization of egg survivorship by reduction of exposure to wound tissue; however, behavioural changes to minimize costs such as intraspecific competition have yet to be documented [27]. It is possible that, because VLB typically does not occur at outbreaking densities in its native range, competition may not have been a significant selective force in the evolution of oviposition behaviour. However, in North America, where outbreaks are frequent and can lead to rapid food shortage for larvae (G. A. Desurmont 2010, personal observation), it is possible that density-dependent costs of gregariousness for larvae may outweigh immediate benefits of overcoming defences on the novel hosts.
In conclusion, because North American beetles and Opulus beetles behave similarly, it is possible that the immigrant population of VLB that established and spread in North America originated from a population that coevolved with a local host exhibiting the same defensive characteristics as V. opulus. Alternatively, given that these beetles locally adapt to host-plant defences in their native range, rapid evolution in the introduced range may also have been possible, leading to a generalized pattern of aggregative oviposition on novel hosts. Indeed, plasticity could have aided in the process [37]. In either case, we advance the concept that adaptation to plant defence may facilitate the invasion of insect herbivores. The aggregative oviposition exhibited by North American beetles probably helped overcome, at least partially, biotic resistance (i.e. defences of the novel hosts) in its introduced environment. In addition to being negatively density-dependent, wound responses of North American Viburnum spp. have been shown to be generally lower than Eurasian spp. [22]. We speculate that the combination of an effective ‘offensive’ behaviour, coupled with a generalized pattern of lower defences in novel hosts, provided a coevolutionary mismatch favouring VLB in its introduced range and facilitating a devastating invasion.
Acknowledgements
We thank Susan Cook-Patton, Sergio Rasmann, James Grover and two anonymous reviewers for comments on the manuscript. This study was supported by grants from the US National Science Foundation (NSF DEB-1118783) and Federal Formula Funds (allocated by the Cornell University Agricultural Experiment Station) to A.A.A. G.A.D. and A.A.A. contributed to the experimental design and data analysis, G.A.D. and F.H. contributed to the data collection and all authors contributed to the writing.
References
- 1.Futuyma D. J., Agrawal A. A. 2009. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl Acad. Sci. USA 106, 18 054–18 061 10.1073/pnas.0904106106 (doi:10.1073/pnas.0904106106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter M. D., Malcolm S. B., Hartley S. E. 1996. Population-level variation in plant secondary chemistry and the population biology of herbivores. Chemoecology 7, 45–56 10.1007/BF01240637 (doi:10.1007/BF01240637) [DOI] [Google Scholar]
- 3.Fox L. R., Morrow P. A. 1981. Specialization species property or local phenomenon? Science 211, 887–893 10.1126/science.211.4485.887 (doi:10.1126/science.211.4485.887) [DOI] [PubMed] [Google Scholar]
- 4.Karban R., Agrawal A. A. 2002. Herbivore offense. Annu. Rev. Ecol. Syst. 33, 641–664 10.1146/annurev.ecolsys.33.010802.150443 (doi:10.1146/annurev.ecolsys.33.010802.150443) [DOI] [Google Scholar]
- 5.Scriber J. M. 2010. Integrating ancient patterns and current dynamics of insect–plant interactions: taxonomic and geographic variation in herbivore specialization. Insect Sci. 17, 471–507 10.1111/j.1744-7917.2010.01357.x (doi:10.1111/j.1744-7917.2010.01357.x) [DOI] [Google Scholar]
- 6.Verhoeven K. J. F., Biere A., Harvey J. A., Van der Putten W. H. 2009. Plant invaders and their novel natural enemies: who is naive? Ecol. Lett. 12, 107–117 10.1111/j.1461-0248.2008.01248.x (doi:10.1111/j.1461-0248.2008.01248.x) [DOI] [PubMed] [Google Scholar]
- 7.Fordyce J. A., Nice C. C. 2004. Geographic variation in clutch size and a realized benefit of aggregative feeding. Evolution 58, 447–450 [PubMed] [Google Scholar]
- 8.Scriber J. M. 2002. Evolution of insect–plant relationships: chemical constraints, coadaptation and concordance of insect/plant traits. Entomol. Exp. Appl. 104, 217–235 10.1046/j.1570-7458.2002.01009.x (doi:10.1046/j.1570-7458.2002.01009.x) [DOI] [Google Scholar]
- 9.Gandhi K., Herms D. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 12, 389–405 10.1007/s10530-009-9627-9 (doi:10.1007/s10530-009-9627-9) [DOI] [Google Scholar]
- 10.Glynn C., Herms D. A. 2004. Local adaptation in pine needle scale (Chionaspis pinifoliae): natal and novel host quality as tests for specialization within and among red and Scots pine. Environ. Entomol. 33, 748–755 10.1603/0046-225X-33.3.748 (doi:10.1603/0046-225X-33.3.748) [DOI] [Google Scholar]
- 11.Lodge D. M. 1993. Biological invasions: lessons for ecology. Trends Ecol. Evol. 8, 133–137 10.1016/0169-5347(93)90025-K (doi:10.1016/0169-5347(93)90025-K) [DOI] [PubMed] [Google Scholar]
- 12.Parker J. D., Burkepile D. E., Hay M. E. 2006. Opposing effects of native and exotic herbivores on plant invasions. Science 311, 1459–1461 10.1126/science.1121407 (doi:10.1126/science.1121407) [DOI] [PubMed] [Google Scholar]
- 13.Callaway R. M., Cipollini D., Barto K., Thelen G. C., Hallett S. G., Prati D., Stinson K., Klironomos J. 2008. Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89, 1043–1055 10.1890/07-0370.1 (doi:10.1890/07-0370.1) [DOI] [PubMed] [Google Scholar]
- 14.Cappuccino N., Arnason J. T. 2006. Novel chemistry of invasive exotic plants. Biol. Lett. 2, 189–193 10.1098/rsbl.2005.0433 (doi:10.1098/rsbl.2005.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W.-M., Feng Y., Ridenour W. M., Thelen G. C., Pollock J. L., Diaconu A., Callaway R. M. 2009. Novel weapons and invasion: biogeographic differences in the competitive effects of Centaurea maculosa and its root exudate (±)-catechin. Oecologia (Berlin) 159, 803–815 10.1007/s00442-008-1234-4 (doi:10.1007/s00442-008-1234-4) [DOI] [PubMed] [Google Scholar]
- 16.Herms D. A., Mattson W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 10.1086/417659 (doi:10.1086/417659) [DOI] [Google Scholar]
- 17.Mitchell C. E., et al. 2006. Biotic interactions and plant invasions. Ecol. Lett. 9, 726–740 10.1111/j.1461-0248.2006.00908.x (doi:10.1111/j.1461-0248.2006.00908.x) [DOI] [PubMed] [Google Scholar]
- 18.Desurmont G. A., Weston P. A. 2011. Aggregative oviposition of viburnum leaf beetle overcomes plant defenses. Ecol. Entomol. 36, 335–343 10.1111/j.1365-2311.2011.01277.x (doi:10.1111/j.1365-2311.2011.01277.x) [DOI] [Google Scholar]
- 19.Majka C. G., LeSage L. 2007. Introduced leaf beetles of the maritime provinces, 3: the viburnum leaf beetle, Pyrrhalta viburni (Paykull) (Coleoptera: Chrysomelidae). Proc. Entomol. Soc. Washington 109, 454–462 [Google Scholar]
- 20.Weston P. A., Desurmont G., Hoebeke E. R. 2007. Viburnum leaf beetle: biology, invasion history in North America, and management options. Am. Entomol. 53, 96–101 [Google Scholar]
- 21.Weston P. A., Diaz M. D., Desurmont G. A. 2008. Ovipositional biology of Viburnum leaf beetle, Pyrrhalta viburni (Coleoptera: Chrysomelidae). Environ. Entomol. 37, 520–524 10.1603/0046-225X(2008)37[520:OBOVLB]2.0.CO;2 (doi:10.1603/0046-225X(2008)37[520:OBOVLB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 22.Desurmont G. A., Donoghue M. J., Clement W. L., Agrawal A. A. 2011. Evolutionary history predicts plant defense against an invasive pest. Proc. Natl Acad. Sci. USA 108, 7070–7074 10.1073/pnas.1102891108 (doi:10.1073/pnas.1102891108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal A. A., Karban R. 2000. Specificity of constitutive and induced resistance: pigment glands influence mites and caterpillars on cotton plants. Entomol. Exp. Appl. 96, 39–49 10.1046/j.1570-7458.2000.00677.x (doi:10.1046/j.1570-7458.2000.00677.x) [DOI] [Google Scholar]
- 24.Karban R., Agrawal A. A., Mangel M. 1997. The benefits of induced defenses against herbivores. Ecology 78, 1351–1355 10.1890/0012-9658(1997)078[1351:TBOIDA]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1351:TBOIDA]2.0.CO;2) [DOI] [Google Scholar]
- 25.Pigliucci M., Murren C. J. 2003. Perspective: genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57, 1455–1464 [DOI] [PubMed] [Google Scholar]
- 26.Desurmont G. A. 2009. Oviposition of Viburnum leaf beetle [Pyrrhalta viburni (Paykull)]: from ecology to biological control of an emerging landscape pest. PhD thesis, Cornell University, Ithaca, USA [Google Scholar]
- 27.Desurmont G. A., Weston P. A. 2010. Stimuli associated with viburnum leaf beetle (Pyrrhalta viburni) aggregative oviposition behavior. Entomol. Exp. Appl. 135, 245–251 10.1111/j.1570-7458.2010.00990.x (doi:10.1111/j.1570-7458.2010.00990.x) [DOI] [Google Scholar]
- 28.Byers J. A. 1991. Pheromones and chemical ecology of locusts. Biol. Rev. Camb. Phil. Soc. 66, 347–378 10.1111/j.1469-185X.1991.tb01146.x (doi:10.1111/j.1469-185X.1991.tb01146.x) [DOI] [Google Scholar]
- 29.Raffa K. F. 2001. Mixed messages across multiple trophic levels: the ecology of bark beetle chemical communication systems. Chemoecology 11, 49–65 10.1007/PL00001833 (doi:10.1007/PL00001833) [DOI] [Google Scholar]
- 30.Fordyce J. A. 2003. Aggregative feeding of pipevine swallowtail larvae enhances host plant suitability. Oecologia 135, 250–257 [DOI] [PubMed] [Google Scholar]
- 31.Fox C. W., Thakar M. S., Mousseau T. A. 1997. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am. Nat. 149, 149–163 10.1086/285983 (doi:10.1086/285983) [DOI] [PubMed] [Google Scholar]
- 32.Park J. Y., Lee J. E. 2004. A taxonomic study on the larvae of the genus Pyrrhalta Joannis (Coleoptera: Chrysomelidae: Galerucinae) from Korea. Entomol. Res. 34, 229–234 10.1111/j.1748-5967.2004.tb00118.x (doi:10.1111/j.1748-5967.2004.tb00118.x) [DOI] [Google Scholar]
- 33.Shinkaji N., Amano H., Nunokawa M., Anbiru T. 1987. Deposition sites of egg-masses of the viburnum leaf beetle, Pyrrhalta-Humeralis (Chen) (Coleoptera, Chrysomelidae), on the host tree, Viburnum-Awabuki K Koch. Jpn. J. App. Entomol. Zool. 31, 391–394 10.1303/jjaez.31.391 (doi:10.1303/jjaez.31.391) [DOI] [Google Scholar]
- 34.Satoh A. 2002. Within-plant distribution of the eggs and larvae of two congeneric Chrysomelid beetles on the same host plant (behavior and ecology). Entomol. Sci. 5, 171–177 [Google Scholar]
- 35.Wood D. L. 1982. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 27, 411–446 10.1146/annurev.en.27.010182.002211 (doi:10.1146/annurev.en.27.010182.002211) [DOI] [Google Scholar]
- 36.Reed R. D. 2003. Gregarious oviposition and clutch size adjustment by a heliconius butterfly. Biotropica 35, 555–559 [Google Scholar]
- 37.Nylin S., Wahlberg N. 2008. Does plasticity drive speciation? Host-plant shifts and diversification in nymphaline butterflies (Lepidoptera: Nymphalidae) during the tertiary. Biol. J. Lin. Soc. 94, 115–130 10.1111/j.1095-8312.2008.00964.x (doi:10.1111/j.1095-8312.2008.00964.x) [DOI] [Google Scholar]