Abstract
Many vertebrates eavesdrop on alarm calls of other species, which is a remarkable ability, given geographical variation in community composition and call diversity within and among species. We used micro-geographical variation in community composition to test whether individuals recognize heterospecific alarm calls by: (i) responding to acoustic features shared among alarm calls; (ii) having innate responses to particular heterospecific calls; or (iii) learning specific alarm calls. We found that superb fairy-wrens (Malurus cyaneus) fled to cover to playback of noisy miner (Manorina melanocephala) aerial predator alarm calls only in locations where miners were present, suggesting that learning rather than acoustic structure determines response. Sites with and without miners were well within the dispersal distance of fairy-wrens, and philopatric males and dispersing females showed the same pattern, so that local genetic adaptation is extremely unlikely. Furthermore, where miners were present, fairy-wrens responded appropriately to different miner calls, implying eavesdropping on their signalling system rather than fleeing from miners themselves. Learned eavesdropping on alarm calls enables individuals to harvest ecologically relevant information from heterospecifics on an astonishingly fine spatial scale. Such phenotypic plasticity is valuable in a changing world, where individuals can be exposed to new species.
Keywords: interspecific communication, eavesdropping, alarm calls, acoustic communication, learning
1. Introduction
Heterospecific interactions—such as competition, mutualism, parasitism or encounters between predators and prey—are important in affecting individual fitness, adaptation and coevolution [1]. Such interactions can be mediated by signals, such as aposematic prey signalling their toxicity to predators and flowers signalling to pollinators, but incidental information flow among species is also pervasive, although often overlooked [2,3]. Individuals can gain fitness-enhancing information about resources or threats by observing the behaviour or location of heterospecifics [4], including eavesdropping on signals evolved to communicate with conspecifics [5,6]. Such eavesdropping is a challenge, however, when there is spatial or temporal variation in community composition, which may select for local genetic adaptation or phenotypic plasticity [7].
A great diversity of vertebrates eavesdrop on and respond appropriately to the alarm calls of other species [8,9], which is a valuable yet astonishing feat given the complexity of any individual's acoustic world. The community of species varies geographically and temporally, and in any one location there can be many species, each giving a variety of vocalizations, including alarm calls. Despite these challenges, playback experiments have shown that mammals, birds and lizards can gain information about danger by eavesdropping on other species' alarm calls [10–20]. Some are even known to respond appropriately to heterospecific alarm variants for the type of predator or degree of danger [11,13,21,22]. Eavesdropping on other species can therefore provide detailed information about predators, and so enhance fitness [2,3,19]. But how do individuals recognize heterospecific alarm calls and cope with variation in community composition?
There are three main possibilities, not necessarily mutually exclusive, for heterospecific alarm call recognition. One possibility is that individuals use general rules of alarm call recognition based on acoustic structure. Animals might respond to calls that are similar in detail to conspecific alarm calls [23–25], or recognize general ‘alarm-like’ acoustic properties, regardless of their own alarm call structure [26,27]. Acoustic structure plausibly accounts for widespread eavesdropping because alarm calls given to immediate threats, such as hawks in flight, tend to be of high frequency and narrow frequency range, while mobbing calls, given to predators not posing an immediate threat, tend to be abrupt calls of broad frequency range [23,28,29]. However, alarm call variation among taxa will reduce generality, and individuals do not universally respond to unfamiliar alarm calls [25,30–32], so that general rules based on the acoustic structure are unlikely to fully explain interspecific eavesdropping. A second possibility is that populations evolve to recognize the alarm calls of particular heterospecifics, comparable to innate recognition of specific predators [33]. Evidence from cross-fostered birds suggests that innate recognition is usually (but not always) confined to conspecific calls [34–36]. A third possibility is that individuals learn to recognize heterospecific alarm calls, which would have the advantage of allowing individuals to cope with rapid temporal or small-scale geographical variation in community composition [6,37,38]. Fish can learn to recognize heterospecific chemical cues of danger [39], but comparable learning of acoustic alarms has not been shown for terrestrial species. Overall, local genetic adaptation or learning appear necessary to account fully for the diversity of eavesdropping, and phenotypic plasticity through learning could be a key mechanism allowing populations to track environmental change through time and space [40].
Studies of geographical variation in response to other species' alarm calls can test hypotheses about the mechanism of heterospecific recognition and spatial scale of behavioural diversity [38]. In the most comprehensive study of mammals, bonnet macaques (Macaca radiata) fled to safety after playback of the alarm calls of only the locally common of two langur species at any one site, showing that their response is not determined by acoustic structure and suggesting that they learn to recognize alarm calls [31]. Similarly, in the only comparable study of birds, superb fairy-wrens fled to cover after playback of alarm calls of white-browed scrubwrens (Sericornis frontalis), where both species occur, but did not flee outside the range of scrubwrens [32]. However, in both these cases, sites were separated by hundreds of kilometres, so that local genetic adaptation rather than learning might explain the geographical patterns. Clearly, we need studies on a small spatial scale, where learning can result in adaptive variation in behaviour on a fine scale [38].
We studied micro-geographical variation in the response of superb fairy-wrens to the aerial alarm calls of noisy miners (Manorina melanocephala). Miners commonly feed in the canopy and could provide early warning of danger to species like fairy-wrens that feed on the ground. Miners form temporally and spatially stable colonies, so we compared the behaviour of fairy-wrens at locations with and without miners on an extremely small spatial scale. We also tested whether fairy-wrens fled in response to miner calls in general, perhaps because they are wary of miners themselves, rather than eavesdropping on miner alarm calls specifically.
2. Methods
(a). Study species
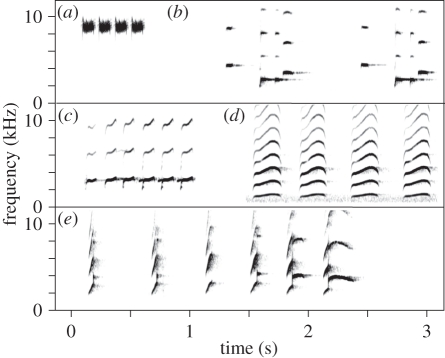
Superb fairy-wrens (Family: Maluridae) are small (9–10 g), sedentary, cooperatively breeding, insectivorous passerines that feed primarily on the ground [41]. Groups consist of a single breeding female and dominant breeding male, and up to five male helpers, with the sexes differing in plumage and bill colour. Groups defend breeding territories, although during the non-breeding season adjacent groups can form temporary flocks. Fairy-wrens produce high-pitched, multi-element aerial alarm calls in response to predatory birds in flight (figure 1) [19,43], and birds almost always immediately flee to cover after these calls or to playbacks consisting of two or more elements [19,22,32,43]. Furthermore, fairy-wrens also flee to cover after playback of aerial alarm calls of the locally common white-browed scrubwrens or New Holland honeyeaters, Phylidonyris novaehollandiae [19,22,32,43].
Figure 1.
Spectrograms of playbacks of (a) superb-fairy wren aerial alarm call, (b) crimson rosella piping call, (c) noisy miner aerial alarm call, (d) noisy miner mobbing alarm call and (e) noisy miner begging call on arrival of an adult with food. Spectrograms were prepared in Raven 1.4 using a Blackman window function, 1.11 ms hop size, 43.1 Hz grid spacing and 3 dB filter bandwidth of 150 Hz [42].
Fairy-wrens have extreme sex-biased dispersal, with philopatric males and dispersing females, which means that the sexes could differ in opportunities for learning. In Canberra, where we studied them, 87 per cent of males remain as helpers on their natal territory and 64 per cent eventually gain a mate there [44]. The remaining males disperse up to three territory widths away (240 m [44]). By contrast, all females leave the natal territory, either late in the breeding season in which they were raised or just before the following breeding season, and those located moved a mean of 11.8 territory widths (940 m), and up to 36 territory widths (2.9 km) [44].
Noisy miners are aggressive, medium-sized (approx. 70 g), sedentary honeyeaters (Family: Meliphagidae) that live in breeding colonies that can persist for many years and occupy up to 40 ha [41]. Miners are aggressive to many species, ranging from hawks to small insectivores, probably reflecting both predator defence and interspecific competition for food [45]. However, there are few records of aggression to ground-feeding species like fairy-wrens [45]. Miner abundance and distribution have increased dramatically in Canberra in the last 10 years [46]. Their preferred habitat is woodland, including both trees (often eucalypts) and open areas [47–49].
Noisy miners are conspicuously vocal, and their repertoire includes two acoustically distinct multi-element alarm calls and loud begging calls [41] (figure 1). Aerial alarm calls are given to raptors in flight [50] and mobbing (‘chur’) alarm calls are given to potential predators that are on the ground or perched [51].
(b). Study sites
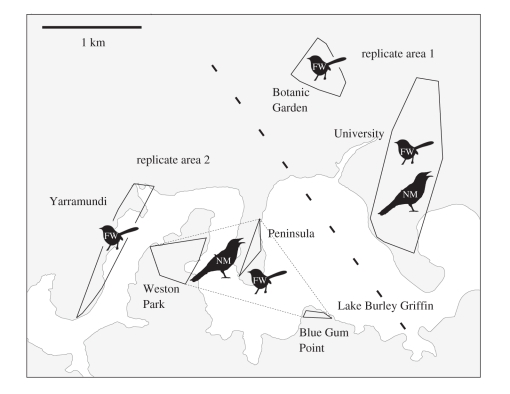
We studied fairy-wren responses to noisy miner calls at six sites in Canberra, Australia, four of which also contained noisy miner colonies (figure 2). All sites included a mix of open areas, shrubs, and eucalypt and other trees. Before carrying out playback experiments below, we surveyed all areas for miners, and during experiments we noted whether we saw or heard miners within 80 m of each group of fairy-wrens. Miners were found during the course of playbacks in every case in ‘miner present’ sites (n = 46 groups) and never at the ‘miner absent’ sites (n = 45 groups). Furthermore, miner presence or absence is stable temporally. From January 2000 to October 2009, Canberra Ornithologists Group surveys detected no miners in either ‘miner absent’ site (0/209 surveys in Botanic Garden; 0/29 in Yarramundi), but detected miners in all ‘miner present’ sites that were surveyed (182/233 University; 20/32 Peninsula; 7/13 Weston Park; Blue Gum Point not surveyed; Canberra Ornithologists Group 2011, unpublished data). Furthermore, miner distribution did not change from November 2009 to April–May 2011, when we again surveyed all sites.
Figure 2.
Locations of playbacks to superb fairy-wrens (FW) at sites where noisy miners (NM) were present or absent. The heavy dashed line separates areas used as replicates in experiment 1. The solid lines are minimum convex polygons around locations of playbacks within a site. The fine dashed line groups smaller sites where miners were present, but where miners did not occur between those sites.
The study sites include both aerial and terrestrial predators. Raptors occur throughout the area [52], including collared sparrowhawks, Accipiter cirrhocephalus, which include both focal species in their diet [53], and which prompt both to give aerial alarm calls (R. D. Magrath, personal observation). Dogs occur at all sites except the Botanic Garden, and foxes, cats and snakes are widespread, and all provoke mobbing calls from fairy-wrens and miners [41,51,54].
(c). General protocols for playback experiments
We carried out three playback experiments in November and December 2009, following methods used in previous experiments on fairy-wrens (electronic supplementary material) [19,22,25,32,43]. Sounds were broadcast to adult fairy-wrens from a distance of about 10 m (range 9–11.5 m). Playbacks were carried out when the focal (closest) bird was feeding on the ground at least 1 m from cover, and we categorized its response as: 0, none; 1, scan for a second or more; or 2, flee to cover. We also noted group identity and the sex of the focal bird. We identified groups by colour bands in the Botanic Garden [55], and elsewhere from spatial location. All sounds broadcast were recorded in Canberra using methods given in the electronic supplementary material.
(d). Experiment 1: do fairy-wrens flee miner alarms only when they are familiar?
Experiment 1 tested the hypothesis that fairy-wrens flee to miner aerial alarm calls only in locations where miners are present, and therefore where fairy-wrens have the opportunity to learn to recognize their calls. Fleeing is the almost invariant response to conspecific aerial alarm calls (above), and is therefore the appropriate measure of aerial alarm call recognition. By contrast, fleeing to these calls regardless of miner presence implies an innate response. The experiment was replicated sequentially in different areas, to ensure that any micro-geographical pattern in one area was not due to unique characteristics of a site rather than the presence or absence of miners.
Replicate experiments matched sites where miners were present with nearby sites where miners were absent (figure 2). The area 1 replicate contrasted the response of fairy-wrens on the University campus (miners present) with the Botanic Garden (miners absent), and the closest playbacks at the two sites were separated by only 600 m, which was also the closest place where miners were seen. There was no barrier to dispersal. The area 2 replicate compared three lakeshore sites where miners were present with a lakeshore site (Yarramundi) where miners were absent. The shortest dispersal distance by land between the ‘miner absent’ and ‘miner present’ playback sites along the lakeshore was 1.2 km, and miners occurred as close as 850 m to ‘miner absent’ playback sites.
In each replicate area, we broadcast five- to six-element noisy miner aerial alarm calls, and two control calls in approximately balanced order to 15 groups at sites where miners were present and 15 groups where miners were absent. A complete set of three playbacks was completed on a group before moving to another, and we alternated between sites with and without miners. Playbacks to a group were separated by a minimum of 5 min (electronic supplementary material). Control calls were four-element aerial alarm calls of fairy-wrens, as a positive control, and the piping contact call of crimson rosellas, Platycercus elegans, which are harmless parrots, as a neutral control (figure 1). In each replicate, all 15 groups with miners received unique exemplars of all playbacks, to avoid pseudoreplication, and the same set of 15 playbacks was used at sites without miners, to avoid any playback differences affecting geographical comparisons. Playbacks were carried out only when miners were not currently nearby, so that fairy-wrens could not take cues from miners themselves.
Playback amplitudes were at natural levels. Miner aerial alarms were broadcast at a mean element amplitude of 66 dB at 10 m. Given the natural mean amplitude of 73 dB at 10 m (electronic supplementary material), from the focal bird's perspective, the amplitude is comparable to the natural context of a miner calling from a nearby tree, about 23 m away. Fairy-wren aerial alarms were broadcast at 54 dB at 10 m and so the amplitude is comparable to a bird calling about 7 m from the focal bird (mean 56.5 dB at 5 m [43]), such as a bird within a foraging group. Rosella contact calls were broadcast at the same amplitude as miner aerial alarms, to control for any effect of amplitude on response.
(e). Experiment 2: do fairy-wrens flee simply because miners are nearby?
In experiment 2, we tested whether fairy-wrens fled to miner aerial alarm calls because they recognized them as alarm calls, or because the calls were a cue that a miner was near. Fairy-wrens might flee simply because miners are aggressive towards other species. Methods were the same as for experiment 1, except that we restricted playback to sites where miners were present and used a set of three different miner calls, all broadcast at an amplitude of 66 dB at 10 m: (i) five- to six-element aerial alarm calls; (ii) four-element mobbing (‘chur’) calls; and (iii) 6–12 element begging calls of young given when an adult arrives with food [56] (figure 1). If fairy-wrens recognize the meaning of these miner signals, they should be more likely to flee to aerial alarm calls signalling immediate danger, than to mobbing calls, signalling potential danger, and should not flee to begging calls, which do not signal anything about predators. By contrast, if fairy-wrens flee simply because miners are near, they should flee to all three playbacks. In this experiment, we also quantified scanning, as increased vigilance might be an appropriate response to mobbing calls.
(f). Experiment 3: do fairy-wrens respond differently to miner calls regardless of experience?
Experiment 3 tested if any differences in fairy-wren response to different miner calls revealed in experiment 2 could be due to their different acoustic properties and not solely because fairy-wrens had learnt to recognize their meaning. We therefore played back the same set of miner calls to fairy-wrens at sites where miners were absent. If the differential response of fairy-wrens to these calls does not depend on experience, then fairy-wrens should respond in a similar way to those in experiment 2.
(g). Statistical analyses
All experiments entailed presenting three different playbacks to each group of fairy-wrens, so where appropriate we used analyses taking into account group identity. In experiment 1, however, birds had almost invariant responses to both control playbacks, and the key prediction was about variation among groups in response to miner aerial alarm calls, so we used generalized linear models (GLM; family binomial with logit link) and Fisher's exact tests for independent comparisons, or McNemar tests with exact binomial probabilities for specific matched comparisons [57,58]. For analysis of experiments 2 and 3, we used generalized linear mixed models (GLMM; family binomial with logit link), with group as the random term [59]. Model selection entailed fitting full models followed by simplification based on dropping individual terms and assessing change in deviance, which follows a χ2-distribution [59]. All analyses were carried out in R 2.7 [60], in which we used the glmmML and lme4 packages for GLMM analyses [61,62].
3. Results
(a). Experiment 1: micro-geographical variation in response to miner aerial alarm calls
Fairy-wrens behaved as expected by fleeing to cover after conspecific aerial alarm calls and never to rosella contact calls, regardless of whether the sites had miners (figure 3). The almost invariant response of fairy-wrens to these control sounds allowed us to restrict analyses to miner aerial alarms.
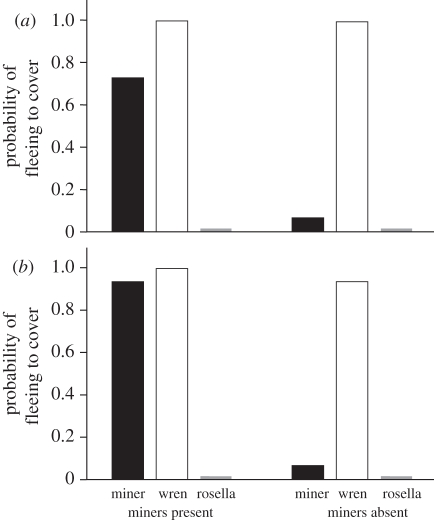
Figure 3.
The probability of superb fairy-wrens fleeing to cover in response to playbacks of noisy miner and superb fairy-wren aerial alarm calls, and crimson rosella contact calls in experiment 1 (a) area 1 and (b) area 2; n = 15 for each bar. Black bars, miner alarm; white bars, wren alarm; grey bars, rosella control.
Fairy-wrens usually fled to cover after playback of noisy miner aerial alarm calls at sites where miners were present, but rarely did so at sites where miners were absent (figure 3; for each replicate area, Fisher's exact test p < 0.001). Analysis including both replicate areas revealed a strong effect of miner presence on response (GLM, miner presence: 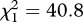 , p < 0.001), but no difference between replicate areas (area:
, p < 0.001), but no difference between replicate areas (area: 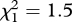 , p = 0.2; interaction of area and miner presence:
, p = 0.2; interaction of area and miner presence: 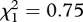 , p = 0.4). Overall, 83 per cent of fairy-wrens fled to miner alarm calls in sites where miners were present, but only 7 per cent fled at sites where they were absent.
, p = 0.4). Overall, 83 per cent of fairy-wrens fled to miner alarm calls in sites where miners were present, but only 7 per cent fled at sites where they were absent.
At sites where miners were present, fairy-wrens responded similarly to miner and conspecific aerial alarms (McNemar tests: area 1, p = 0.13; area 2, p = 1.0; areas combined, p = 0.06). Overall, 25/30 birds fled to both conspecific and miner alarms, and 5/30 fled to conspecific alarms but not miner alarms. By contrast, fairy-wrens were much less likely to flee to miner than conspecific alarms at sites where miners were absent (McNemar tests: p < 0.001 for each area and areas combined). Only 2/30 fled to both species' alarms, whereas 27/30 birds fled to conspecific but not miner alarms, and one fled to neither species's alarm.
Despite the obligate natal dispersal of female fairy-wrens, compared with philopatry of male fairy-wrens, females did not show a weaker micro-geographical pattern of response to miner alarm calls (GLM, replicate areas 1 and 2 combined: 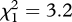 , p = 0.07). The trend, in fact, was the opposite: females fled to none of 11 playbacks at sites where miners were absent and 12 of 13 (92%) at sites where miners were present, while males fled to two of 19 (11%) at sites where miners were absent, and 13 of 17 (76%) at sites where miners were present.
, p = 0.07). The trend, in fact, was the opposite: females fled to none of 11 playbacks at sites where miners were absent and 12 of 13 (92%) at sites where miners were present, while males fled to two of 19 (11%) at sites where miners were absent, and 13 of 17 (76%) at sites where miners were present.
(b). Experiments 2 and 3 response to different noisy miner calls
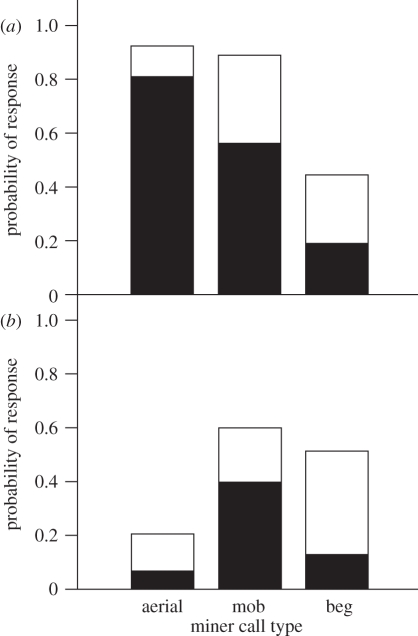
In experiment 2, which was conducted at sites where miners were present, fairy-wrens were most likely to flee after playback of miner aerial alarm calls compared with mobbing alarm or begging calls (figure 4a; GLMM: flee to cover, 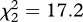 , p < 0.001). Treating playback type as an ordered term, there was a strong linear decline in the probability of fleeing from aerial to mobbing to begging calls (linear term: z = 2.55, p = 0.01), but no nonlinear pattern among playbacks (quadratic term, flee: z = 0.50, p = 0.6). This ordered pattern is that predicted if fairy-wrens recognize the magnitude of threat signalled by the different miner calls, rather than merely fleeing from miners themselves. The pattern was similar for the probability of any response, including both fleeing and scanning (figure 4a; difference among playbacks,
, p < 0.001). Treating playback type as an ordered term, there was a strong linear decline in the probability of fleeing from aerial to mobbing to begging calls (linear term: z = 2.55, p = 0.01), but no nonlinear pattern among playbacks (quadratic term, flee: z = 0.50, p = 0.6). This ordered pattern is that predicted if fairy-wrens recognize the magnitude of threat signalled by the different miner calls, rather than merely fleeing from miners themselves. The pattern was similar for the probability of any response, including both fleeing and scanning (figure 4a; difference among playbacks, 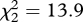 , p < 0.001; linear decline, z = 2.22, p = 0.03; quadratic term, z = 0.94, p = 0.3).
, p < 0.001; linear decline, z = 2.22, p = 0.03; quadratic term, z = 0.94, p = 0.3).
Figure 4.
The response of superb fairy-wrens to playback of noisy miner calls in (a) sites where miners were present, experiment 2 (n = 16), and (b) sites where miners were absent, experiment 3 (n = 15). Black bars, flee; white bars, scan.
Experiment 3, which was conducted at sites where miners were absent, revealed a lower probability of fleeing to cover overall and only a weak effect of miner playback type on fleeing (figure 4b; GLMM: flee, 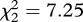 , p = 0.03), with no linear decline from aerial to mobbing to begging (linear term in ordered model: z = 0.68, p = 0.5). Fairy-wrens fled to only one of 15 (7%) miner aerial alarm calls, which was identical to the response in experiment 1 (2/30). Instead, they fled most frequently to miner mobbing calls rather than aerial calls, so there was a weak nonlinear pattern (quadratic term: flee, z = 2.11, p = 0.04). The pattern was similar but weaker still for any response (figure 4b; difference among playbacks,
, p = 0.03), with no linear decline from aerial to mobbing to begging (linear term in ordered model: z = 0.68, p = 0.5). Fairy-wrens fled to only one of 15 (7%) miner aerial alarm calls, which was identical to the response in experiment 1 (2/30). Instead, they fled most frequently to miner mobbing calls rather than aerial calls, so there was a weak nonlinear pattern (quadratic term: flee, z = 2.11, p = 0.04). The pattern was similar but weaker still for any response (figure 4b; difference among playbacks, 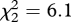 , p = 0.05; linear term, z = 1.82, p = 0.07, with a trend for an increase; quadratic term, z = 1.54, p = 0.12). There was a significantly different pattern of response to the three playbacks between experiments 2 and 3 (GLMM, unordered interaction of playback type and experiment: flee,
, p = 0.05; linear term, z = 1.82, p = 0.07, with a trend for an increase; quadratic term, z = 1.54, p = 0.12). There was a significantly different pattern of response to the three playbacks between experiments 2 and 3 (GLMM, unordered interaction of playback type and experiment: flee, 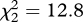 , p = 0.002; any response,
, p = 0.002; any response, 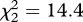 , p < 0.001).
, p < 0.001).
Consistent with the results of experiment 1, the focal bird's sex did not affect its probability of fleeing to playback (GLMM: sex alone and all interactions involving sex, experiment and playback type, p ≥ 0.2). Specifically regarding aerial alarms, when miners were present (experiment 2), six of seven females and seven of nine males fled to cover after playback, whereas when miners were absent (experiment 3) only one of five females and none of 10 males fled to playback (GLM: interaction of sex and experiment: 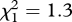 , p = 0.3).
, p = 0.3).
4. Discussion
Fairy-wrens fled to cover after playback of miner aerial alarm calls only at sites where miners were locally present, strongly suggesting that individuals learn to recognize the alarm calls of other species. This result was replicated in two areas, strengthening the conclusion that micro-geographical variation in anti-predator behaviour reflects learning opportunities. Furthermore, at sites where miners were present, fairy-wrens responded as if they recognized the meaning of different types of miner calls, which provides one of few examples of vertebrates discriminating among alarm calls of another species. By contrast, fairy-wrens did not respond appropriately to miner calls of quite different meaning where miners were absent. Our results imply that individuals learn to eavesdrop on the detailed information encoded by other species' calls, and so cope with rapid spatial (and by implication temporal) variability in community composition. Such phenotypic plasticity should affect survival in a changing world [40], especially where range expansion exposes individuals to new predators or prey.
Our results are consistent with fairy-wrens learning to recognize miner aerial alarm calls, rather than with local genetic adaptation, and rules out innate recognition based merely on the acoustic structure of these miner calls. First, gene flow is likely to reduce or eliminate local genetic adaptation [63–65], and yet we found contrasting behaviour of fairy-wrens between nearby sites. Regular gene flow is likely because the distance between sites with and without miners is well within the dispersal distance of female fairy-wrens [44], and most young are the extra-pair offspring of males from other territories, some more than three territories distant [66]. Differential dispersal can reduce the homogenizing effect of gene flow [67] or even exaggerate genetic differences [68], but there would need to be almost complete separation between sites to explain the extreme spatial variation in behaviour. Second, if the behavioural difference between sites is due to genetic difference rather than learning, then females—the dispersing sex—should be more likely to behave maladaptively than males, which are extremely philopatric. Contrary to this prediction, there was no difference in behaviour between the sexes; if anything, females were more likely to behave adaptively than males. The lack of a difference between the sexes implies that females are able to learn to recognize miner aerial alarms after natal dispersal, which is consistent with previous studies showing that adult birds can learn to recognize predators [38,69]. It is also possible that individuals lose their response to miner aerial alarm calls if they disperse from sites with miners to those without, comparable to a decline in response to predator cues that become irrelevant [70]. Finally, the lack of flight to miner aerial alarms at sites where miners were absent shows that fleeing to these alarms in sites where miners were present was not due to similarity to conspecific calls nor to a generic ‘alarming’ acoustic structure. Overall, the strong micro-geographical pattern is consistent with learning and unlikely to be caused by genetic differences among sites.
There have been few studies of geographical variation in response to heterospecific alarm calls and they have been on a much larger spatial scale (see §1) [31,32], which means that genetic variation could affect behaviour. Nonetheless, some studies on a local scale, although not explicitly geographical, show micro-geographical variation in recognition of heterospecific alarm calls consistent with learning. For example, Diana monkeys living within a 50 km2 rainforest were more likely to respond to chimpanzee ‘leopard’ alarm calls by giving their own ‘leopard’ alarm calls if they lived within a chimpanzee group's core home range [71]. This suggests that the monkeys learnt the meaning of chimpanzee calls where the apes were common. The adaptive consequence of learning about other species is beautifully illustrated by territory-by-territory variation in the probability of reed warblers (Acrocephalus scirpaceus) mobbing cuckoos (Cuculus canorus) according to the local risk of brood parasitism [72]. In this case, individuals learn to mob cuckoos at their nest by observing neighbours mobbing them at theirs [38]. Similarly, learning to recognize heterospecific alarm calls should tailor behaviour to local circumstances, and so maximize the use of ecologically relevant information while minimizing false alarms.
Fairy-wrens living in locations with miners responded as if they recognized the meaning of different miner calls, rather than simply fleeing because miners themselves can be aggressive, which would be more akin to predator recognition than to signal eavesdropping. Fairy-wrens were most likely to flee to miner aerial alarm calls (signalling a fast-moving predator) less to mobbing calls (signalling a lower threat) and least to begging calls (indicating an adult arriving with food rather than the presence of any predator). If fairy-wrens had fled merely because they had detected that a miner was nearby, they should have fled to each type of call, because each reveals the presence of a miner. The minimal flight to begging calls implies fairy-wrens do not treat miners themselves as an immediate threat, perhaps because they are rarely aggressive to ground-feeding species ([45]; R. D. Magrath & T. H. Bennett, personal observations).
In contrast to their behaviour when living with miners, fairy-wrens living in sites without miners did not respond appropriately to signal meaning, but there was a small effect of signal structure. Most conspicuously, only one of 15 birds fled to playback of aerial calls, even less than their response to begging calls, and only two others scanned, contrary to the view that alarm calls in general manipulate a listener's attention [27]. Nonetheless, fairy-wrens were slightly more likely to flee after playback of mobbing calls than the other calls, suggesting that these calls had acoustic features, such as being abrupt and broad-band, that capture attention or identify them as alarm calls [23,24,27–29]. However, begging calls also captured attention, judging by increased scanning, as can other calls by juveniles [73]. Overall, an appropriate response to different miner calls appears to rely on learning, but there could be a difference in the relative importance of learning and acoustic structure between call types.
Our results reinforce the growing realization that heterospecific signals are an important source of information [2,3], and that learning can tailor an individual's behaviour to the challenges of a changing world [38,74]. Individuals might learn through direct association of predators and heterospecific alarms or through social learning [37,75], and an understanding of learning mechanisms should help captive release conservation programmes, where we suggest that individuals could be trained to recognize heterospecific alarm calls, and not merely the predators themselves.
Acknowledgements
This study was carried out under an ethics permit from the Australian National University.
We thank Bob Phillips and Jim Forge for help with electronics, Andrew Cockburn and Helen Osmond for information, Isabela Burgher for help with mapping software, Paul Fennell and the Canberra Ornithologists Group for access to census data, and Anastasia Dalziell, Pam Fallow, Tonya Haff, Ben Hatchwell, Brani Igic, Hanna Kokko, Naomi Langmore, Justin Welbergen and two anonymous referees for thorough comments on the manuscript. This work was funded by an Australian Research Council Discovery grant to R.D.M., and carried out under a permit from the Australian National Botanic Gardens.
References
- 1.Ridley M. 2004. Evolution, 3rd edn Oxford, UK: Blackwell [Google Scholar]
- 2.Seppänen J.-T., Forsman J. T., Mönkkönen M., Thomson R. L. 2007. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88, 1622–1633 10.1890/06-1757.1 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- 3.Goodale E., Beauchamp G., Magrath R. D., Nieh J. C., Ruxton G. D. 2010. Interspecific information transfer influences animal community structure. Tree 25, 354–361 [DOI] [PubMed] [Google Scholar]
- 4.Seppänen J.-T., Forsman J. T., Mönkkönen M., Krams I., Salmi T. 2011. New behavioural trait adopted or rejected by observing heterospecific tutor fitness. Proc. R. Soc. B 278, 1736–1741 10.1098/rspb.2010.1610 (doi:10.1098/rspb.2010.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieh J. C., Barreto L. S., Contrera F. A. L., Imperatriz-Fonseca V. L. 2004. Olfactory eavesdropping by a competitively foraging stingless bee, Trigona spinipes. Proc. R. Soc. Lond. B 271, 1633–1640 10.1098/rspb.2004.2717 (doi:10.1098/rspb.2004.2717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page R. A., Ryan M. J. 2005. Flexibility in assessment of prey cues: frog-eating bats and frog calls. Proc. R. Soc. B 272, 841–847 10.1098/rspb.2004.2998 (doi:10.1098/rspb.2004.2998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal A. A. 2001. Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 10.1126/science.1060701 (doi:10.1126/science.1060701) [DOI] [PubMed] [Google Scholar]
- 8.Caro T. M. 2005. Antipredator defences in birds and mammals. Chicago, IL: Chicago University Press [Google Scholar]
- 9.Zuberbühler K. 2009. Survivor signals: the biology and psychology of animal alarm calling. Adv. Stud. Behav. 40, 277–322 10.1016/S0065-3454(09)40008-1 (doi:10.1016/S0065-3454(09)40008-1) [DOI] [Google Scholar]
- 10.Shriner W. M. 1998. Yellow-bellied marmot and golden-mantled ground squirrel responses to heterospecific alarm calls. Anim. Behav. 55, 529–536 10.1006/anbe.1997.0623 (doi:10.1006/anbe.1997.0623) [DOI] [PubMed] [Google Scholar]
- 11.Zuberbühler K. 2000. Interspecies semantic communication in two forest primates. Proc. R. Soc. Lond. B 267, 713–718 10.1098/rspb.2000.1061 (doi:10.1098/rspb.2000.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuberbühler K. 2001. Predator-specific alarm calls in Campbell's monkeys, Cercopithecus campbelli. Behav. Ecol. Sociobiol. 50, 414–422 10.1007/s002650100383 (doi:10.1007/s002650100383) [DOI] [Google Scholar]
- 13.Rainey H. J., Zuberbühler K., Slater P. J. B. 2004. Hornbills can distinguish between primate alarm calls. Proc. R. Soc. Lond. B 271, 755–759 10.1098/rspb.2003.2619 (doi:10.1098/rspb.2003.2619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitousek M. N., Adelman J. S., Gregory N. C., St Clair J. J. H. 2007. Heterospecific alarm call recognition in a non-vocal reptile. Biol. Lett. 3, 632–634 10.1098/rsbl.2007.0443 (doi:10.1098/rsbl.2007.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lea A. J., Barrera J. P., Tom L. M., Blumstein D. T. 2008. Heterospecific eavesdropping in a nonsocial species. Behav. Ecol. 19, 1041–1046 10.1093/beheco/arn064 (doi:10.1093/beheco/arn064) [DOI] [Google Scholar]
- 16.Müller C. A., Manser M. B. 2008. The information banded mongooses extract from heterospecific alarms. Anim. Behav. 75, 897–904 10.1016/j.anbehav.2007.07.012 (doi:10.1016/j.anbehav.2007.07.012) [DOI] [Google Scholar]
- 17.Schmidt K. A., Lee E., Ostfeld R. S., Sieving K. 2008. Eastern chipmunks increase their perception of predation risk in response to titmouse alarm calls. Behav. Ecol. 19, 759–763 10.1093/beheco/arn034 (doi:10.1093/beheco/arn034) [DOI] [Google Scholar]
- 18.Goodale E., Kotagama S. W. 2008. Response to conspecific and heterospecific alarm calls in mixed-species bird flocks of a Sri Lankan rainforest. Behav. Ecol. 19, 887–894 10.1093/beheco/arn045 (doi:10.1093/beheco/arn045) [DOI] [Google Scholar]
- 19.Magrath R. D., Pitcher B. J., Gardner J. L. 2009. An avian eavesdropping network: alarm signal reliability and heterospecific response. Behav. Ecol. 20, 745–752 10.1093/beheco/arp055 (doi:10.1093/beheco/arp055) [DOI] [Google Scholar]
- 20.Ito R., Mori A. 2010. Vigilance against predators induced by eavesdropping on heterospecific alarm calls in a non-vocal lizard Oplurus cuvieri cuvieri (Reptilia: Iguania). Proc. R. Soc. B 277, 1275–1280 10.1098/rspb.2009.2047 (doi:10.1098/rspb.2009.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templeton C. N., Greene E. 2007. Nuthatches eavesdrop on variations in heterospecific chickadee mobbing calls. Proc. Natl. Acad. Sci. USA 104, 5479–5482 10.1073/pnas.0605183104 (doi:10.1073/pnas.0605183104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallow P. M., Magrath R. D. 2010. Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim. Behav. 79, 411–417 10.1016/j.anbehav.2009.11.018 (doi:10.1016/j.anbehav.2009.11.018) [DOI] [Google Scholar]
- 23.Marler P. 1955. Characteristics of some animal calls. Nature 176, 6–8 10.1038/176006a0 (doi:10.1038/176006a0) [DOI] [Google Scholar]
- 24.Marler P. 1957. Specific distinctiveness in the communication calls of birds. Behaviour 11, 13–39 10.1163/156853956X00066 (doi:10.1163/156853956X00066) [DOI] [Google Scholar]
- 25.Fallow P. M., Gardner J. L., Magrath R. D. 2011. Sound familiar? Acoustic similarity provokes responses to unfamiliar heterospecific alarm calls. Behav. Ecol. 22, 401–410 10.1093/beheco/arq221 (doi:10.1093/beheco/arq221) [DOI] [Google Scholar]
- 26.Blumstein D. T., Récapet C. 2009. The sound of arousal: the addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology 115, 1074–1081 10.1111/j.1439-0310.2009.01691.x (doi:10.1111/j.1439-0310.2009.01691.x) [DOI] [Google Scholar]
- 27.Rendall D., Owren M. J., Ryan M. J. 2009. What do animal signals mean? Anim. Behav. 78, 233–240 10.1016/j.anbehav.2009.06.007 (doi:10.1016/j.anbehav.2009.06.007) [DOI] [Google Scholar]
- 28.Johnson F. R., McNaughton E. J., Shelley C. D., Blumstein D. T. 2003. Mechanisms of heterospecific recognition in avian mobbing calls. Aust. J. Zool. 51, 577–585 10.1071/ZO03031 (doi:10.1071/ZO03031) [DOI] [Google Scholar]
- 29.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinauer [Google Scholar]
- 30.Davies N. B., Madden J. R., Butchart S. H. M. 2004. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. Lond. B 271, 2297–2304 10.1098/rspb.2004.2835 (doi:10.1098/rspb.2004.2835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramakrishnan U., Coss R. G. 2000. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata). J. Comp. Psychol. 114, 3–12 10.1037/0735-7036.114.1.3 (doi:10.1037/0735-7036.114.1.3) [DOI] [PubMed] [Google Scholar]
- 32.Magrath R. D., Pitcher B. J., Gardner J. L. 2009. Recognition of other species' aerial alarm calls: speaking the same language or learning another? Proc. R. Soc. B 276, 769–774 10.1098/rspb.2008.1368 (doi:10.1098/rspb.2008.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gall B. G., Mathis A. 2010. Innate predator recognition and the problem of introduced trout. Ethology 116, 47–58 10.1111/j.1439-0310.2009.01718.x (doi:10.1111/j.1439-0310.2009.01718.x) [DOI] [Google Scholar]
- 34.Davies N. B., Madden J. R., Butchart S. H. M., Rutila J. 2006. A host-race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. Proc. R. Soc. B 273, 693–699 10.1098/rspb.2005.3324 (doi:10.1098/rspb.2005.3324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollén L. I., Radford A. N. 2009. The development of alarm call behaviour in mammals and birds. Anim. Behav. 78, 791–800 10.1016/j.anbehav.2009.07.021 (doi:10.1016/j.anbehav.2009.07.021) [DOI] [Google Scholar]
- 36.Magrath R. D., Haff T. M., Horn A., Leonard M. L. 2010. Calling in the face of danger: predation risk and acoustic communication by parent birds and their offspring. Adv. Study Behav. 41, 187–253 10.1016/S0065-3454(10)41006-2 (doi:10.1016/S0065-3454(10)41006-2) [DOI] [Google Scholar]
- 37.Griffin A. S. 2004. Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140 10.3758/BF03196014 (doi:10.3758/BF03196014) [DOI] [PubMed] [Google Scholar]
- 38.Davies N. B., Welbergen J. A. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 10.1126/science.1172227 (doi:10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 39.Mizra R. S., Chivers D. P. 2003. Fathead minnows learn to recognize heterospecific alarm cues they detect in the diet of a known predator. Behaviour 140, 1359–1369 10.1163/156853903771980620 (doi:10.1163/156853903771980620) [DOI] [Google Scholar]
- 40.Charmantier A., McCleery R. H., Cole L. R., Perrins C. M., Kruuk L. E. B., Sheldon B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 41.Higgins P. J., Peter J. M., Steele W. K. 2001. Handbook of Australian, New Zealand and Antarctic Birds. Volume 5: tyrant-flycatchers to Chats. Melbourne, Australia: Oxford University Press [Google Scholar]
- 42.Charif R. A., Waack A. M., Strickman L. M. 2008. Raven Pro 1.3 user's manual. Ithaca, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 43.Magrath R. D., Pitcher B. J., Gardner J. L. 2007. A mutual understanding? Interspecific responses by birds to each other's aerial alarm calls. Behav. Ecol. 18, 944–951 10.1093/beheco/arm063 (doi:10.1093/beheco/arm063) [DOI] [Google Scholar]
- 44.Mulder R. A. 1995. Natal and breeding dispersal in a co-operative, extra-group-mating bird. J. Avian Biol. 26, 234–240 10.2307/3677324 (doi:10.2307/3677324) [DOI] [Google Scholar]
- 45.Dow D. D. 1977. Indiscriminate interspecific aggression leading to almost sole occupancy of space by a single species of bird. Emu 77, 115–121 10.1071/MU9770115 (doi:10.1071/MU9770115) [DOI] [Google Scholar]
- 46.Canberra Ornithologists Group 2011. Annual bird report: 1 July 2009 to 30 June 2010. Canberra Bird Notes 36, 1–80 [Google Scholar]
- 47.Sewell S. R., Catterall C. P. 1998. Bushland modification and styles of urban development: their effects on birds in south-east Queensland. Wildl. Res. 25, 41–63 10.1071/WR96078 (doi:10.1071/WR96078) [DOI] [Google Scholar]
- 48.Ashley L. C., Major R. E., Taylor C. E. 2009. Does the presence of grevilleas and eucalypts in urban gardens influence the distribution and foraging ecology of Noisy Miners? Emu 109, 135–142 10.1071/MU07043 (doi:10.1071/MU07043) [DOI] [Google Scholar]
- 49.Maron M. 2007. Threshold effect of eucalypt density on an aggressive avian competitor. Biol. Conserv. 136, 100–107 10.1016/j.biocon.2006.11.007 (doi:10.1016/j.biocon.2006.11.007) [DOI] [Google Scholar]
- 50.Jurisevic M. A., Sanderson K. J. 1994. The vocal repertoires of six honeyeater (Meliphagidae) species from Adelaide, South Australia. Emu 94, 141–148 10.1071/MU9940141 (doi:10.1071/MU9940141) [DOI] [Google Scholar]
- 51.Kennedy R. A. W., Evans C. S., McDonald P. G. 2009. Individual distinctiveness in the mobbing call of a cooperative bird, the noisy miner Manorina melanocephala. J. Avian Biol. 40, 481–490 10.1111/j.1600-048X.2008.04682.x (doi:10.1111/j.1600-048X.2008.04682.x) [DOI] [Google Scholar]
- 52.Taylor M. 1992. & Canberra Ornithologists Group. In Birds of the Australian Capital Territory: an atlas. Canberra, ACT: Canberra Ornithologists Group Inc [Google Scholar]
- 53.Marchant S., Higgins P. J. 1993. Handbook of Australian, New Zealand and Antarctic birds: raptors to lapwings. Melbourne, Australia: Oxford University Press [Google Scholar]
- 54.Colombelli-Négrel D., Robertson J., Kleindorfer S. 2010. Nestling presence affects the anti-predator response of adult superb fairy-wrens (Malurus cyaneus). Acta Ethol. 13, 69–74 10.1007/s10211-010-0072-7 (doi:10.1007/s10211-010-0072-7) [DOI] [Google Scholar]
- 55.Cockburn A., Sims R. A., Osmond H. L., Green D. J., Double M. C., Mulder R. A. 2008. Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438 10.1111/j.1365-2656.2007.01351.x (doi:10.1111/j.1365-2656.2007.01351.x) [DOI] [PubMed] [Google Scholar]
- 56.O'Brien P. H., Dow D. D. 1979. Vocalizations of nestling noisy miners Manorina melanocephala. Emu 79, 63–70 10.1071/MU9790063 (doi:10.1071/MU9790063) [DOI] [Google Scholar]
- 57.Agresti A. 2007. An Introduction to categorical data analysis. Hoboken, NJ: Wiley [Google Scholar]
- 58.Crawley M. J. 2007. The R book. Chichester, UK: Wiley [Google Scholar]
- 59.Zuur A. F., Ieno E. N., Walker N. J., Saveliev A. A., Smith G. M. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 60.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 61.Bates D., Maechler M., Dai B. 2008. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-26 See http://lme4.r-forge.r-project.org [Google Scholar]
- 62.Broström G. glmmML: generalized linear models with clustering. 2008. R package version 0.81-3. Umeå University, Sweden. [Google Scholar]
- 63.Storfer A., Sih A. 1998. Gene flow and ineffective antipredator behavior in a stream-breeding salamander. Evolution 52, 558–565 10.2307/2411090 (doi:10.2307/2411090) [DOI] [PubMed] [Google Scholar]
- 64.Lenormand T. 2002. Gene flow and the limits to natural selection. Tree 17, 183–189 [Google Scholar]
- 65.Langergraber K. E., et al. 2010. Genetic and ‘cultural’ similarity in wild chimpanzees. Proc. R. Soc. B 277, 408–416 10.1098/rspb.2010.1112 (doi:10.1098/rspb.2010.1112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Double M. C., Peakall R., Beck N. R., Cockburn A. 2005. Dispersal, philopatry and infidelity: dissecting local genetic structure in superb fairy-wrens (Malurus cyaneus). Evolution 59, 625–635 [PubMed] [Google Scholar]
- 67.Postma E., van Noordwijk A. J. 2005. Gene flow maintains large genetic difference in clutch size at a small spatial scale. Nature 433, 65–68 10.1038/nature03083 (doi:10.1038/nature03083) [DOI] [PubMed] [Google Scholar]
- 68.Garant D., Kruuk L. E. B., Wilkin T. A., McCleery R. H., Sheldon B. C. 2005. Evolution driven by differential dispersal within a wild bird population. Nature 433, 60–65 10.1038/nature03051 (doi:10.1038/nature03051) [DOI] [PubMed] [Google Scholar]
- 69.Curio E., Ernst U., Vieth W. 1978. Cultural transmission of enemy recognition: one function of mobbing. Science 202, 899–901 10.1126/science.202.4370.899 (doi:10.1126/science.202.4370.899) [DOI] [PubMed] [Google Scholar]
- 70.Ferrari M. C. O., Brown G. E., Bortoletti G. R., Chivers D. P. 2010. Linking predator risk and uncertainty to adaptive forgetting: a theoretical framework and empirical tests using tadpoles. Proc. R. Soc. B 277, 2205–2210 10.1098/rspb.2009.2117 (doi:10.1098/rspb.2009.2117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuberbühler K. 2000. Causal knowledge of a predator's behaviour in wild Diana monkeys. Anim. Behav. 59, 209–220 10.1006/anbe.1999.1296 (doi:10.1006/anbe.1999.1296) [DOI] [PubMed] [Google Scholar]
- 72.Welbergen J. A., Davies N. B. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240 10.1016/j.cub.2008.12.041 (doi:10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 73.Blumstein D. T., Richardson D. T., Cooley L., Winternitz J., Daniel J. C. 2008. The structure, meaning and function of yellow-bellied marmot pup screams. Anim. Behav. 76, 1055–1064 10.1016/j.anbehav.2008.06.002 (doi:10.1016/j.anbehav.2008.06.002) [DOI] [Google Scholar]
- 74.Seyfarth R. M., Cheney D. L. 2010. Production, usage, and comprehension in animal vocalizations. Brain Lang. 115, 92–100 10.1016/j.bandl.2009.10.003 (doi:10.1016/j.bandl.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 75.Shettleworth S. J. 2010. Cognition, evolution and behavior. Oxford, UK: Oxford University Press [Google Scholar]