Abstract
Giant suspension feeders such as mysticete whales, basking and whale sharks, and the extinct (indicated by ‘†’) †pachycormiform teleosts are conspicuous members of modern and fossil marine vertebrate faunas. Whether convergent anatomical features common to these clades arose along similar evolutionary pathways has remained unclear because of a lack of information surrounding the origins of all groups of large-bodied suspension feeders apart from baleen whales. New investigation reveals that the enigmatic ray-finned fish †Ohmdenia, from the Lower Jurassic (Toarcian, 183.0–175.6 Ma) Posidonia Shale Lagerstätte, represents the immediate sister group of edentulous †pachycormiforms, the longest lived radiation of large vertebrate suspension feeders. †Ohmdenia bisects the long morphological branch leading to suspension-feeding †pachycormiforms, providing information on the sequence of anatomical transformations preceding this major ecological shift that can be compared to changes associated with the origin of modern mysticetes. Similarities include initial modifications to jaw geometry associated with the reduction of dentition, followed by the loss of teeth. The evolution of largest body sizes within both radiations occurs only after the apparent onset of microphagy. Comparing the fit of contrasting evolutionary models to functionally relevant morphological measurements for whales and †pachycormiform fishes reveals strong support for a common adaptive peak shared by suspension-feeding members of both clades.
Keywords: Cetacea, convergent evolution, filter feeding, Mysticeti, planktivory, stem teleost
1. Introduction
Giant suspension-feeding vertebrates are conspicuous members of recent and ancient marine ecosystems. This guild is represented by baleen whales (mysticetes) and four independent radiations of chondrichthyans (manta rays, and whale, basking and megamouth sharks) in the modern oceans [1], with fossil examples known from the teleost (a subset of †pachycormiform fishes) [2–5] and possibly gnathostome (the arthrodire ‘placoderms’ †Homostius and †Titanichthys) [6,7] stem lineages. Many of these taxa represent the largest members of their respective groups [1,2,8], with mysticetes including the most massive vertebrates ever to have lived. The repeated evolutionary convergence on suspension feeding at large body sizes in phylogenetically disparate vertebrate lineages implies that this ecological strategy represents an important trophic role that has persisted over geological timescales.
Structural specializations shared among giant suspension feeders presumably reflect adaptive solutions to a common lifestyle [1]. However, because of a poor understanding of the origin of microphagy in most of these groups, it remains uncertain whether the evolutionary pathways to similar morphological innovations are also shared between different clades. With their well-studied body fossil record, mysticetes represent the only radiation of large suspension-feeding vertebrates for which the sequence of anatomical transformations documenting the emergence of this trophic strategy can be reconstructed in any detail [9–11]. Cranial modifications found in Oligocene (33.9–23.03 Ma) †aetiocetids have been interpreted as reflecting the transition from the macrophagy primitive to whales [11] to the microphagy characteristic of extant mysticetes [9,10]. These changes include reduction of dentition, alterations to jaw geometry resulting in slender mandibular rami and reduced coronoid processes, and the appearance of palatal vascularization sometimes compared to that associated with baleen plates in extant mysticetes (but see [12]). Further transformations, including the complete loss of dentition, occurred along the mysticete stem crownward of †aetiocetids, but the evolution of the very largest body sizes within mysticetes appears restricted to the living radiation [11,12].
A subset of †pachycormiform fishes represents the clearest example of an extinct radiation of large-bodied vertebrate suspension feeders. Primitively predatory, with overall body geometries similar to modern tunas [3], these Mesozoic stem teleosts include a clade of large (up to at least 8 m in length) [4,5], long-gaped and toothless species interpreted as planktivores [2]. Together, these fishes appear to have filled the ecological role of giant suspension feeders for a 100 Myr interval during the Jurassic and Cretaceous, prior to their disappearance near the Cretaceous–Palaeogene boundary (66 Ma) along with many other large-bodied fish taxa [5,13,14]. This successful radiation occupies an isolated position within †pachycormiform phylogeny; its monophyly is strongly supported, but there are few indications of the pattern of anatomical changes preceding the presumptive origin of suspension feeding in this group [5].
Here, I show that the enigmatic actinopterygian †Ohmdenia, from the Lower Jurassic (Toarcian) Posidonia Shale of Germany [15], is the immediate sister group of edentulous †pachycormiforms. †Ohmdenia bisects the long morphological branch leading to this specialized radiation, helping to establish a sequence of character changes associated with a major shift in trophic ecology. More importantly, †Ohmdenia and its relatives provide a complementary example to fossil and living mysticetes, permitting comparative investigation of the evolution of large-bodied, suspension-feeding vertebrates.
2. Material and methods
(a). Phylogenetic analysis
†Ohmdenia was included in an expanded version of the character matrix presented by Friedman et al. [5]. This matrix contains 121 characters coded for 29 taxa. For all analyses, the stem neopterygian †Pteronisculus was specified as the outgroup. Parsimony analyses were conducted in PAUP* 4.0b10 [16] using the branch-and-bound search algorithm. Nodal support was assessed with character bootstrapping and Bremer decay analysis, the latter using scripts produced by TreeRot [17]. Bayesian analyses were conducted in MrBayes 3.1.2 [18]. Two simultaneous analyses were run, starting from different, randomly generated trees. Each run included four independent Markov chains: one cold chain and three incrementally heated chains with the ‘temp’ value set to 0.20. Priors were kept at their default settings for standard (i.e. morphological) analyses. The analysis was run for 1 × 107 generations. Samples were taken every 1 × 102 generations, resulting in a total of 1 × 105 samples for each of the parallel analyses. The first 2.5 × 104 samples for each run, representing the ‘burn-in’ period, were discarded. A complete phylogeny and character optimizations are given in electronic supplementary material, figure S1.
(b). Morphospace construction
I assembled a list of eight biomechanically and ecologically relevant features and measurements, many of which have been functionally linked to suspension feeding [1,7], and assessed these for a range of †pachycormiforms and cetaceans (including members of both the mysticete and odontocete total groups, as well as the cetacean stem) using primary literature, published figures and direct observation of original material. These characters include: relative gape; relative orbit size; jaw-closing mechanical advantage; mandibular aspect ratio (a proxy for the second moment of area); ratio of tooth height to tooth base (a proxy for tooth cusp angle); relative tooth height; condition of the symphysis; and body size (measured as the log of body length). Full details of these measurements are given in the electronic supplementary material.
All measurements were Z-transformed, then used to calculate an intertaxon distance matrix that was subjected to principal coordinates analysis (PCO). The first 20 ordination axes were retained, with higher axes associated with negative eigenvalues. The negative eivenvalue with the greatest magnitude is −14.8, compared with a sum of 396.5 for all positive eigenvalues. Measured intertaxon distances and ordination distances are strongly correlated (Pearson product–moment correlation; r = 0.96, p < 2.2×10−16), indicating that the PCO preserves much of the underlying distance data without major distortion. Variance summarized by retained ordination axes was estimated as the eigenvalue for a given axis divided by the sum of eigenvalues [19]. Anatomical correlates of ordination axes are given in the electronic supplementary material, table S1. A phylogenetic hypothesis was interpolated between terminal taxa in the ordination as an illustrative tool, creating a ‘phylomorphospace’ [20]. Positions of internal nodes were approximated using maximum-likelihood ancestral state reconstruction for continuous characters as implemented in the ape package [21] for R [22] in conjunction with stratigraphically calibrated phylogenies (electronic supplementary material, figure S2).
(c). Model fitting
The first three axes (estimated cumulative variance = 69.3%) were selected for model-fitting exercises based on a break in a scree plot of eigenvalues. In order to test whether baleen whales and edentulous †pachycormiforms might be drawn to the same functional peak associated with suspension feeding, I compared the fits of a series of explicit adaptive models to taxon scores along axes in the functional ordination. Specifically, I explored the following alternatives: (i) no adaptive peaks, with diffusive evolution under a Brownian motion model; (ii) a single adaptive peak for all cetaceans and †pachycormiforms; (iii) a common peak for baleen whales, †aetiocetids, edentulous †pachycormiforms and †Ohmdenia, two separate peaks for all remaining cetaceans and †pachycormiforms, plus an additional unknown regime for the branches linking these two clades. Model fitting was conducted using the package ouch [23] for R [22]. I calculated a sample-size-corrected version of Akaike's information criterion for each model for each axis [24], and converted these to Akaike weights to facilitate comparison between models fitted to the same axis (electronic supplementary material, table S3). Missing observations in the functional trait matrix mean that the resulting multivariate ordination is approximate, as are the scores for taxa along the ordination axes. The results of these model-fitting exercises must therefore be viewed as similarly approximate.
3. Systematic palaeontology
Actinopterygii, Woodward 1891; Neopterygii, Regan 1923; Teleostei, Müller 1846 sensu de Pinna 1996; †Pachycormiformes, Berg 1937; †Pachycormidae, Woodward 1895; †Ohmdenia, Hauff 1953; †Ohmdenia multidentata, Hauff 1953.
(a). Holotype
Institut für Geowissenschaften, Eberhard Karls Universität Tübingen, Tübingen, Germany, GPIT 1017/1 (figure 1). Disrupted skeleton missing portions of the anal and dorsal fins, skull roof and braincase. The left mandible was found approximately 1.5 m from the skeleton and re-mounted in association with other cranial remains. All other bones are preserved in the position in which they were found. The absence of most dorsal components of the skeleton has been attributed to the individual lying partly exposed on the seafloor, leaving the upper part of the body uncovered prior to complete burial [15,25].
Figure 1.
†Ohmdenia multidentata, holotype, GPIT 1017/1, Lower Jurassic (Lower Toarcian), Posidonia Shale, Germany. (a) Specimen photograph. (b) Interpretive drawing. (c) Reconstruction. Belemnites associated with abdominal region are shaded in grey and marked with an asterisk (‘*’) in (b). a.f, anal fin; ang, angular; c.f, caudal fin; cle, cleithrum; den, dentary; ?epb, possible epibranchials; hym, hyomandibular; ipb, infrapharyngobranchial; max, maxilla; op, opercle; p.f, pectoral fin; pop, preopercle; qu, quadrate; rad, pectoral radial; sang, surangular; scl, supracleithra; sclr, sclerotic ring.
(b). Horizon and locality
Lower Jurassic (Lower Toarcian, bifrons zone, commune subzone), Posidonia Shale, Ohmden, Baden-Württemberg, Germany [26]. The specimen derives from a horizon of 150–200 mm above the boundary between the ε (III) and ε (II12) stratigraphic divisions [27].
(c). Amended diagnosis
†Pachycormiform differing from other members of that clade in the following combination of characters: marginal dentition of lower jaw comprising a pavement of stout, conical teeth; length of dentary exceeds distance between tips of dorsal and anteroventral arms of the cleithrum; anterior and posterior margins of hyomandibular parallel, with no excavations; anterior pectoral rays fused along the leading edge of fin; pelvic fins absent; mineralized squamation absent.
(d). Remarks
†Ohmdenia was initially assigned to †Birgeriidae [15,27], a Mesozoic clade that lies outside the neopterygian crown but whose relationships are otherwise uncertain [28–30]. This placement of †Ohmdenia has been regarded as dubious [31], but persists [32–34] despite a lack of compelling anatomical support. By contrast, there is clear evidence that †Ohmdenia is a crown neopterygian, and more specifically a member of the stem-teleost clade †Pachycormiformes. Relevant synapomorphies are reviewed in §5 below. A †pachycormiform identification has also been advocated by Lambers [3], who mistakenly regarded †Ohmdenia as a probable junior synonym of †Saurostomus.
4. Description and comparison
The only specimen of †Ohmdenia is disrupted, but individual skeletal components maintain relative positional information. Bones of the skull and pectoral girdle are clustered at one end of the fossil, the caudal fin at the other, with a series of bones from the axial column lying between (figure 1a,b). An earlier description of †Ohmdenia [15] contains some errors of interpretation, leading to an inaccurate picture of anatomical structure in this genus.
Long, slender mandibles bearing unusual dentition represent the most striking feature of †Ohmdenia [15,27,33]. Most of the external surface of the jaw consists of the dentary, with the surangular and angular limited to the posterior half of the mandible. There is no indication of a well-developed coronoid process. The dentition comprises densely set, conical, posteriorly reclined teeth, placed within a band that extends along the dorsal margin of the jaw. With the exception of small cusps located along the labial edge of this band, most teeth are of comparable size. It is unclear whether the larger teeth are located exclusively on the dentary, or if they are also borne by the coronoids. The relatively large size of the jaws in †Ohmdenia is genuine, rather than an illusion arising from disruption to the skeleton; the length of the dentary greatly exceeds the distance between the tips of the anteroventral and dorsal limbs of the cleithrum, contrary to the condition in other †pachycormiforms for which the condition can be assessed.
A series of elongated bones bearing dentition similar to that found on the lower jaw lie between the two mandibles. These ossifications are badly broken and overlie one another, but it is clear they include an incomplete maxilla and probable members of the dermopalatine series. The sclerotic ring lies in the same region. Recognizable remains of the skull roof or dermal cheek are not preserved. Several plate-like ossifications are found near the anterior of the specimen, but these are generally obscured by the overlying matrix or bone, and cannot be identified with confidence.
A quadrate is preserved in association with the preoperculum, but has been displaced considerably from life position, lying near the caudal fin. The crescentic preopercle bears well-developed dorsal and anterior limbs. Distally, the dorsal limb is reduced to a narrow splint, with no associated posterior lamina. The quadrate is triangular in shape and includes a conspicuous articular condyle at its anteroventral corner.
A rectangular bone draped over the posteroventral margin of the right mandible is clearly a member of the hyoid arch. This was previously identified as a ceratohyal [15], but the length of this bone in comparison with the mandible suggests this interpretation is unlikely, based on conditions in closely related taxa [3–5,35]. One end of this hyoid ossification bears two distinct facets, comparable with the arrangement of articular surfaces on the proximal end of the hyomandibular in other †pachycormiforms [5,35]. The similarity in size between this bone and the preopercle lends additional support to interpretation as a hyomandibular. Unusually, the anterior and posterior margins of this bone are nearly straight, giving the bone a profile distinct from the ‘waisted’ shape typical of early neopterygian hyomandibulars. A similarly ‘slab-sided’ hyomandibular is found in †Bonnerichthys and, to a lesser degree, †Rhinconichthys [5], two †pachycormiforms interpreted as suspension feeders. The size of the hyomandibular relative to the lower jaw in †Ohmdenia closely agrees with conditions in †Asthenocormus [3] and possibly Martillichthys [4], two other edentulous taxa.
Two bar-like bones preserved immediately behind the right mandible are components of the gill skeleton, possibly epibranchials. There is no trace of any elaborated gill rakers associated with these ossifications. Immediately behind these bars lies a tri-radiate infrapharyngobranchial that closely resembles equivalent bones in †Pachycormus [35] and †Bonnerichthys [5]. An additional infrapharyngobranchial lies in the region framed by the two mandibles. A large, asymmetrical, plate-like bone with rounded margins is interpreted as an opercle.
Even though many cranial bones remain unknown, available material provides clues about skull geometry in †Ohmdenia. Together, the hyomandibular and preopercle constrain the depth of the head, and when this information is combined with the dimensions of the mandible, it is clear that the skull in †Ohmdenia was very shallow relative to its length (figure 1c).
Bones of the pectoral girdle and fin are well represented in †Ohmdenia. The best-preserved components of the dermal girdle are the cleithra and supracleithra. The crescent-shaped cleithra closely resemble those of other †pachycormiforms [5]. There are no indications of clavicles, or attachment areas for these bones on the cleithra (contra [15]). The supracleithra are slender and distinctively kinked, giving them an ‘L’-shaped profile. I have been unable to identify scapulocoracoids, but the region around the cleithra and supracleithra is littered with several pectoral radials. Typical of †pachycormiforms, these are flat and strongly pinched at mid-length with greatly expanded distal ends, giving them a paddle-shaped profile. Both pectoral fins are preserved. One is represented only by a series of rays comprising the leading edge, but a more complete example indicates that these fins were long and slender. Rays fuse distally along the anterior margin of the pectoral fin. More posterior rays bifurcate, with no clear indications of joints or segmentation. There is no trace of pelvic fins or girdles.
Numerous slender ossifications, representing the remains of the axial column (haemal/neural arches and spines, supraneurals), lie between the caudal fin and the concentration of cranial and pectoral bones. Ossified centra and mineralized scales are absent. The first feature is common to most †pachycormiforms, but the latter is restricted to edentulous taxa [3–5]. The symmetrical caudal fin is disrupted, with posterior rays of the slender upper and lower lobes separated from more anterior rays. The proximal tips of dorsal and ventral rays nearly contact in the articulated posterior portion of the fin, indicating that the caudal endoskeleton was almost completely enveloped by dermal rays. Caudal rays are segmented, and more posterior rays in both lobes bifurcate distally.
With a probable total length approaching 2.5 m, †Ohmdenia is among the largest ray-finned fishes of the Lower Jurassic, surpassed only by the giant stem chondrosteans †Stronglyosteus [27,33] and †Gyrosteus [36]. Taken together, the shape inferred for the skull of †Ohmdenia, the geometry of its pectoral girdle and the preserved position of the caudal fin relative to cranial remains imply that the overall body form of this genus was slender, similar in profile to edentulous †pachycormiforms for which the postcranium is known (†Martillichthys, †Asthenocormus) [4]. The combination of a shallow, enlogated skull with a narrow body is unique to these taxa among †pachycormiforms. Other members of this clade characterized by slender postcrania bear skulls that are proportionally much shorter (e.g. †Euthynotus incognitus, †‘Hypsocormus’ macrodon).
5. Systematics
†Ohmdenia is clearly a neopterygian based on characters including: the absence of a clavicle capping the ventral end of the cleithrum; a crescentic preoperculum; a slender maxilla free from the cheek; and epaxial caudal fin rays [37]. Large, scythe-like pectoral fins with a distinctive pattern of bifurcation and reduced segmentation, symmetrical upper and lower lobes of the caudal fin, extreme hypurostegy and paddle-shaped pectoral radials represent derived features shared between †Ohmdenia and other †pachycormiforms [3,5,35].
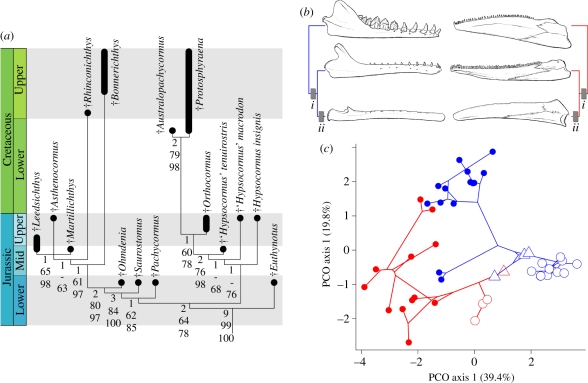
To establish the position of this genus within neopterygian phylogeny, I included it in a revised and expanded version of the character-by-taxon matrix presented by Friedman et al. [5], which was then subjected to maximum parsimony and Bayesian phylogenetic analyses. Both approaches returned consistent results within †pachycormiforms (electronic supplementary material, figure S1). Despite highly incomplete preservation, the placement of †Ohmdenia as a †pachycormiform receives clear support, as does the position of the genus as the immediate sister taxon of all edentulous members of this radiation (Bremer decay index = 2; bootstrap support frequency = 80; Bayesian clade credibility = 97; figure 2a). The sister-group relationship between †Ohmdenia and endentulous †pachycormiforms is unambiguously supported by the geometry of the hyomandibular and the complete absence of squamation. Although not considered as a character in these analyses, the apparently derived body form combining a slender postcranium with an elongated, low-profile skull would seem to represent another feature underpinning (†Ohmdenia + edentulous †pachycormiforms). †Saurostomus is in turn united with this pairing to the exclusion of †Pachycormus by a parasphenoid that is broad anterior to the ascending process, elongated posterior processes of the dermopterotics, a slender ascending ramus of the preopercle lacking a posterior lamina, a hypural plate with a very long vertical axis relative to its horizontal axis, and slender, splint-shaped supracleithra.
Figure 2.
(a) Stratigraphically calibrated cladogram illustrating †pachycormiform interrelationships, based on maximum parsimony and Bayesian analysis of morphological data. Numbers to the left of nodes represent, from top to bottom: Bremer decay index; bootstrap support; Bayesian clade credibility. ‘-’ indicates bootstrap percentage or Bayesian clade credibility <50. Additional statistics for maximum parsimony solution: tree length, 223; consistency index, 0.55; rescaled consistency index, 0.42; retention index, 0.77. Crown teleost, holostean and stem neopterygian outgroups not shown. Complete tree and character optimizations are given in electronic supplementary material, figure S1. (b) Congruent series of transformations in mandibular structure in mysticetes (left) and †pachycormiforms (right). For each cladogram, internode i corresponds to decreased jaw-closing mechanical advantage, change in mandibular aspect ratio, and reduction of tooth crown height, while internode ii corresponds to the loss of dentition. Evolution of the largest body sizes in both groups occurs within the clade subtended by internode ii. Taxa, from top to bottom, are: †Basilosaurus, †Aetiocetus, †Parietobalaena (cetaceans), and †Pachycormus, †Ohmdenia, †Martillichthys (†pachycormiforms). (c) Phylomorphospace for †pachycormiforms (red) and whales (blue), based on principal coordinates analysis (PCO) of anatomical features related to suspension feeding [1,7]. Large hollow triangles represent †Ohmdenia and †aetiocetids; large hollow circles represent suspension-feeding †pachycormiforms and mysticete whales. Parenthetical values in axis labels refer to the estimated percentage of overall variance explained by that coordinate axis.
6. Discussion and conclusions
(a). Feeding in †Ohmdenia
†Ohmdenia is coeval with the earliest known †pachycormiforms [3], indicating that considerable morphological and trophic diversification had already taken place by the first appearance of this radiation in Lower Jurassic deposits. The long gape of †Ohmdenia, combined with its peculiar dentition, allude to a distinctive feeding ecology. Other tooth-bearing †pachycormiforms bear elongated, needle-like teeth (e.g. †Pachycormus and †Euthynotus) or large carinate fangs (e.g. †Protosphyraena, †Australopachycormus and †Hypsocormus) that imply piercing and cutting of prey items, respectively. By contrast, the multiple rows of comparatively stout, low-crowned teeth of †Ohmdenia suggest grasping rather than penetration of prey items. This tooth geometry is commonly found in whales and marine reptiles that specialize on soft-bodied cephalopods [38]. The elongated mandibles of †Ohmdenia reflect a reduction in jaw-closing mechanical advantage relative to other toothed †pachycormiforms, indicating a reduced emphasis on the transmission of force and a relatively less powerful bite [39]. Ammonites are found scattered across the same bedding plane as †Ohmdenia, but only two belemnites are present, both associated with the abdominal region of the specimen (figure 1b,c). It is possible that these guards represent gut contents corroborating inferences of feeding ecology drawn from jaw and tooth structure, but this interpretation must be viewed cautiously owing to the disrupted nature of the skeleton.
(b). The evolution of large vertebrate suspension feeders: towards a comparative perspective
The identification of †Ohmdenia as the sister group of suspension-feeding †pachycormiforms provides important new clues about the evolution of this trophic strategy. As in whales [9–11], the evolution of suspension feeding in †pachycormiforms appears to have first entailed changes in mandibular aspect ratio and jaw-closing mechanical advantage accompanied by alterations to dental structure (figure 2b). The complete loss of teeth followed these initial modifications, with the evolution of the largest body sizes representing a final step, postdating the origin of dedicated suspension feeding in both mysticetes [11] and †pachycormiforms [2,4,5]. Thus, these two groups share the following sequence of parallel steps: (i) changes in dentition and mandibular geometry; (ii) loss of teeth, presumably associated with the onset of microphagy; and (iii) evolution of giant size relative to other members of their individual clades (figure 2b).
Modern mysticetes bear baleen plates, while their †pachycormiform analogues are characterized by greatly elaborated gill rakers [2–5]; these anatomically disparate structures are both involved in the retention of small prey items within the oral cavity. There is circumstantial evidence of baleen plates in toothed †aetiocetids [9,10,12], but the origin of specialized gill rakers relative to the loss of marginal dentition in †pachycormiforms is less well constrained. Expanded gill rakers are present in †Asthenocormus, †Leedsichthys, †Martillichthys and †Rhinconichthys [2–5], but there is no evidence for such structures in either †Bonnerichthys [5] or †Ohmdenia. If the absence of elaborated rakers in †Bonnerichthys is genuine, rather than a consequence of the disarticulated material available for this genus, then the evolution of these structures within †pachycormiforms would appear to postdate the loss of marginal teeth.
In order to quantitatively compare the evolutionary trajectories of †pachycormiforms and whales, I constructed a multivariate morphospace for the two groups based on eight anatomical features hypothesized to be functionally related to suspension feeding [1,7] (figure 2c; electronic supplementary material). Edentulous †pachycormiforms, along with †Ohmdenia, lie near a region of this ordination dominated by fossil and living mysticete whales known or hypothesized to be suspension feeders. Intriguingly, †Ohmdenia plots in close proximity to †aetiocetids, an assemblage of stem mysticetes that might document the functional transition from macrophagy to microphagy in whales [9–11].
Interpolating an estimated phylogeny between points representing terminal branches shows that edentulous †pachycormiform and mysticete whales converge on a common region of morphospace from different ancestral conditions. To investigate whether whales and †pachycormiforms might be drawn to a shared adaptive peak, I have combined the first three axes of the morphometric ordination with statistically explicit models of trait evolution. PCO 1, which summarizes an estimated 39.4 per cent of the variation in the dataset, is best fitted by a four-peak model, with separate peaks for: (i) †Ohmdenia and edentulous †pachycormiforms plus mysticetes including †aetiocetids; (ii) all remaining †pachycormiforms; (iii) all remaining whales; (iv) an ‘unknown’ regime on the branches linking †pachycormiforms and whales. The Akaike weight for this model exceeds 0.999, indicating a substantially better fit by this model to data than its simpler competitors: diffusive phenotypic evolution, and a single peak for all whales and †pachycormiforms. This result constitutes strong support for a common adaptive peak shared by some whales and †pachycormiforms along the first coordinate axis, scores along which are strongly correlated (p < 0.001) with most morphometric variables. In contrast to the case for PCO 1, no single model performs substantially better than all its competitors on PCO 2 (19.8% variance), with all Akaike weights lower than 0.75. PCO 3 (10.1% variance) is best fitted by a model with a single peak for all taxa examined.
At the broadest scale of comparison, both the whale and †pachycormiform examples suggest a tendency for large-bodied marine suspension feeders to emerge from within clades of moderately large- to large-bodied pelagic predators. Additional evidence for this assertion comes from basking and megamouth sharks, whose closest living relatives suggest pelagic, predatory ancestral ecologies for these modern chondrichthyan planktivores [9,40,41]. Putative examples of extinct suspension feeders (e.g. the Devonian ‘placoderm’ †Titanichthys [7,42]) imply a comparable evolutionary trajectory. However, the fact that other paths to suspension feeding at large body sizes are possible is clearly illustrated by whale sharks and manta rays, both of which nest within clades primitively characterized by benthic feeding [40,41,43]. More complete documentation of generalities associated with the evolution of large-bodied vertebrate suspension feeders will require quantitative examination of chondrichthyans in a framework similar to that applied here. Such work will reveal a more detailed picture of the anatomical modifications associated with this striking trophic strategy, which in turn will permit a more explicit means for identifying suspension-feeding vertebrates in the fossil record.
Acknowledgements
I thank the numerous collections managers and curators who permitted me to examine material under their care, most especially P. Havlik, who helped to arrange access to †Ohmdenia. M. Brazeau, J. Liston, N. Pyenson, L. Sallan and an anonymous reviewer provided critical assessments that helped improve earlier versions of this contribution. This research was supported by the John Fell Fund, St Hugh's College, Oxford and NERC NE/I005536/1.
References
- 1.Sanderson S. L., Wassersug R. 1993. Convergent and alternative designs for vertebrate suspension feeding. In The skull, vol. 3 (eds Hanken J., Hall B. K.), pp. 37–112 Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Martill D. M. 1988. Leedsichthys problematicus, a giant filter-feeding teleost from the Jurassic of England and France. Neues Jahrb. Geol. P.-M. 11, 670–680 [Google Scholar]
- 3.Lambers P. H. 1992. On the ichthyofauna of the Solnhofen lithographic limestone (Upper Jurassic, Germany). PhD thesis, Rijksuniversiteit Groningen, Groningen, The Netherlands [Google Scholar]
- 4.Liston J. 2008. A review of the characters of the edentulous pachycormiforms Leedsichthys, Asthenocormus and Martillichthys nov. gen. In Mesozoic fishes 4—homology and phylogeny (eds Arratia G., Schultze H.-P., Wilson M. V. H.), pp. 181–198 Munich, Germany: Verlag Dr Friedrich Pfeil [Google Scholar]
- 5.Friedman M., Shimada K., Martin L. D., Everhart M. J., Liston J., Maltese A., Triebold M. 2010. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 327, 990–993 10.1126/science.1184743 (doi:10.1126/science.1184743) [DOI] [PubMed] [Google Scholar]
- 6.Dension R. 1978. Handbook of paleoichthyology 2. Placodermi. Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 7.Anderson P. S. L., Friedman M., Brazeau M. D., Rayfield E. J. 2011. Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature 476, 206–209 10.1038/nature10207 (doi:10.1038/nature10207) [DOI] [PubMed] [Google Scholar]
- 8.Compagno L., Dando M., Fowler S. 2005. Sharks of the world. Princeton, NJ: Princeton University Press [Google Scholar]
- 9.Deméré T. A., Berta A. 2008. Skull anatomy of the Oligocene toothed mysticete Aetiocetus weltoni (Mammalia; Cetacea): implications for mysticete evolution and functional anatomy. Zool. J. Linn. Soc. 154, 308–352 10.1111/j.1096-3642.2008.00414.x (doi:10.1111/j.1096-3642.2008.00414.x) [DOI] [Google Scholar]
- 10.Deméré T. A., McGowen M. R., Berta A., Gatesy J. 2008. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 57, 15–37 10.1111/j.1096-3642.2008.00414.x (doi:10.1111/j.1096-3642.2008.00414.x) [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald E. M. G. 2010. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool. J. Linn. Soc. 158, 367–476 10.1111/j.1096-3642.2009.00572.x (doi:10.1111/j.1096-3642.2009.00572.x) [DOI] [Google Scholar]
- 12.Marx F. J. 2011. The more the merrier? A large cladistic analysis of mysticetes and comments on the transition from teeth to baleen. J. Mammal. Evol. 18, 77–100 10.1007/s10914-010-9148-4 (doi:10.1007/s10914-010-9148-4) [DOI] [Google Scholar]
- 13.Friedman M. 2009. Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proc. Natl Acad. Sci. USA 106, 5218–5223 10.1073/pnas.0808468106 (doi:10.1073/pnas.0808468106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc. R. Soc. B 277, 1675–1683 10.1098/rspb.2009.2177 (doi:10.1098/rspb.2009.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauff B. 1953. Ohmdenia multidentata nov. gen. et nov. sp. Ein neuer groβer Fischfund aus den Posidonienschiefern des Lias ε von Ohmden/Holzmaden in Württemburg. Neues Jahrb. Geol. P.-A. 97, 39–50 [Google Scholar]
- 16.Swofford D. L. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4. Sunderland, MA: Sinauer Associates [Google Scholar]
- 17.Sorenson M. D., Franzosa E. A. 2007. TreeRot, version 3. Boston, MA: Boston University [Google Scholar]
- 18.Ronquist F., Huelsenbeck J. P. 2003. MrBayes v. 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 19.Foote M. 1995. Morphological diversification of Paleozoic crinoids. Paleobiology 21, 273–299 [Google Scholar]
- 20.Sidlauskas B. L. 2008. Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution 62, 3135–3156 10.1111/j.1558-5646.2008.00519.x (doi:10.1111/j.1558-5646.2008.00519.x) [DOI] [PubMed] [Google Scholar]
- 21.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 22.R Core Development 2008. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 23.Butler M. A., King A. A. 2004. Phylogenetic comparative analysis: a modelling approach for adaptive evolution. Am. Nat. 164, 683–695 10.1086/426002 (doi:10.1086/426002) [DOI] [PubMed] [Google Scholar]
- 24.Sugihura N. 1978. Further analysis of the data by Akaike's information criterion and the finite corrections. Commun. Stat. A 7, 13–26 10.1080/03610927808827599 (doi:10.1080/03610927808827599) [DOI] [Google Scholar]
- 25.Martill D. M. 1986. The stratigraphic distribution and preservation of fossil vertebrates in the Oxford Clay of England. Mercian Geol. 10, 161–186 [Google Scholar]
- 26.Röhl H.-J., Schmid-Röhl A., Oschmann W., Frimmel A., Schwark L. 2001. The Posidonia Shale (Lower Toarcian) of SW-Germany: an oxygen-depleted ecosystem controlled by sea level and palaeoclimate. Palaeogeogr. Palaeoclim. 165, 27–52 10.1016/S0031-0182(00)00152-8 (doi:10.1016/S0031-0182(00)00152-8) [DOI] [Google Scholar]
- 27.Hauff B. 1953. Das Holzmadenbuch. Öhringen, Germany: Buchdruckerei H. Wolf [Google Scholar]
- 28.Gardiner B. G., Schaeffer B. 1989. Interrelationships of lower actinopterygian fishes. Zool. J. Linn. Soc. 97, 135–187 10.1111/j.1096-3642.1989.tb00550.x (doi:10.1111/j.1096-3642.1989.tb00550.x) [DOI] [Google Scholar]
- 29.Coates M. I. 1999. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, UK, and the interrelationships of primitive actinopterygians. Phil. Trans. R. Soc. Lond. B 354, 435–462 10.1098/rstb.1999.0396 (doi:10.1098/rstb.1999.0396) [DOI] [Google Scholar]
- 30.Gardiner B. G., Schaeffer B., Masserie J. A. 2005. A review of the lower actinopterygian phylogeny. Zool. J. Linn. Soc. 144, 511–525 10.1111/j.1096-3642.2005.00181.x (doi:10.1111/j.1096-3642.2005.00181.x) [DOI] [Google Scholar]
- 31.Andrews S. M., Gardiner B. G., Miles R. S., Patterson C. 1967. Pisces. In The fossil record (eds Harland W. B., Holland C. H., House M. R., Hughes N. F., Reynolds A. B., Rudwick M. J. S., Satterthwaite G. E., Tarlo L. B. H., Willey E. C.), pp. 637–683 London, UK: Geological Society of London [Google Scholar]
- 32.Carroll R. L. 1988. Vertebrate paleontology and evolution. New York, NY: Freeman [Google Scholar]
- 33.Hauff B., Hauff R. B. 1981. Das Holzmadenbuch. Fellbach, Germany: REPRO-DRUCK [Google Scholar]
- 34.Romano C., Brinkmann W. 2009. Reappraisal of the lower actinopterygian Birgeria stensioei Aldinger, 1931 (Osteichthyes; Birgeriidae) from the Middle Triassic of Monte San Giorgio (Switzerland) and Besano (Italy). Neues Jahrb. Geol. P.-A. 252, 17–31 10.1127/0077-7749/2009/0252-0017 (doi:10.1127/0077-7749/2009/0252-0017) [DOI] [Google Scholar]
- 35.Mainwaring A. J. 1978. Anatomical and systematic review of the Pachycormidae, a family of Mesozoic fossil fishes. PhD thesis, Westfield College, London, UK [Google Scholar]
- 36.Hilton E. J., Forey P. L. 2009. Redescription of †Chondrosteus acipenseroides Egerton, 1858 (Acipenseriformes, †Chondrosteidae) from the lower Lias of Lyme Regis (Dorset, England), with comments on the early evolution of sturgeons and paddlefishes. J. Syst. Palaeontol. 7, 427–453 10.1017/S1477201909002740 (doi:10.1017/S1477201909002740) [DOI] [Google Scholar]
- 37.Patterson C. 1973. Interrelationships of holosteans. In Interrelationships of fishes (eds Greenwood P. H., Miles R. S., Patterson C.), pp. 233–305 London, UK: Academic Press [Google Scholar]
- 38.Massare J. A. 1987. Morphology and prey preference of Mesozoic marine reptiles. J. Vert. Paleontol. 7, 121–137 10.1080/02724634.1987.10011647 (doi:10.1080/02724634.1987.10011647) [DOI] [Google Scholar]
- 39.Westneat M. W. 2004. Evolution of levers and linkages in the feeding mechanisms of fishes. Integr. Comp. Biol. 44, 378–389 10.1093/icb/44.5.378 (doi:10.1093/icb/44.5.378) [DOI] [PubMed] [Google Scholar]
- 40.Shirai S. 1996. Phylogenetic interrelationships of neoselachians (Chondrichthyes: Euselachii). In Interrelationships of fishes (eds Stiassny M. L. J., Parenti L. R., Johnson G. D.), pp. 9–34 San Diego, CA: Academic Press [Google Scholar]
- 41.Maisey J. G., Naylor G. P., Ward D. J. 2004. Mesozoic elasmobranchs, neoselachian phylogeny and the rise of modern elasmobranch diversity. In Mesozoic fishes 3—systematics, paleoenvironments and biodiversity (eds Arratia G., Tintori A.), pp. 17–57 Munich, Germany: Verlag Dr. Friedrich Pfeil [Google Scholar]
- 42.Carr R. K. 1991. A renanalysis of Heintzichthys gouldii (Newberry), an aspinothoracid arthrodire (Placodermi) from the Famennian of northern Ohio, with a review of brachythoracid systematics. Zool. J. Linn. Soc. 103, 349–390 10.1111/j.1096-3642.1991.ttb00909.x (doi:10.1111/j.1096-3642.1991.ttb00909.x) [DOI] [Google Scholar]
- 43.Summers A. P. 2000. Stiffening the stingray skeleton—an investigation of durophagy in myliobatid stingrays (Chondrichthyes, Batoidea, Myliobatidae). J. Morph. 243, 113–126 (doi:10.1002/(SICI)1097-4687(200002)243:2>113::AID-JMOR1<3.0.CO;2-A) [DOI] [PubMed] [Google Scholar]