Abstract
Bilateral symmetry is visually salient to diverse animals including birds, but whereas experimental studies typically use bilaterally symmetrical two-dimensional patterns that are viewed approximately fronto-parallel; in nature, animals observe three-dimensional objects from all angles. Many animals and plant structures have a plane of bilateral symmetry. Here, we first (experiment I) give evidence that young poultry chicks readily generalize bilateral symmetry as a feature of two-dimensional patterns in fronto-parallel view. We then test the ability of chicks to recognize symmetry in images that would be produced by the transformed view produced by a 40° horizontal combined with a 20° vertical rotation of a pattern on a spherical surface. Experiment II gives evidence that chicks trained to distinguish symmetrical from asymmetrical patterns treat rotated views of symmetrical ‘objects’ as symmetrical. Experiment III gives evidence that chicks trained to discriminate rotated views of symmetrical ‘objects’ from asymmetrical patterns generalize to novel symmetrical objects either in fronto-parallel or rotated view. These findings emphasize the importance of bilateral symmetry for three-dimensional object recognition and raise questions about the underlying mechanisms of symmetry perception.
Keywords: symmetry, vision, object recognition, domestic chick, categorization
1. Introduction
Visual symmetry is important in nature: most animals are bilaterally symmetrical and symmetry is treated as a desirable characteristic of potential mates [1–3]. Also, the assumption that an object has a plane of symmetry can be used to compute its three-dimensional shape from the two-dimensional optical image [4,5]. Animals are sensitive to bilateral symmetry, and often prefer symmetrical patterns [6–10]. For instance, pigeons (Columba livia) and bees can learn to classify bilaterally symmetrical to be distinct from asymmetrical and rotationally symmetrical patterns [11–13].
The ethological literature has two main accounts of symmetry preference in animals. One account argues that symmetry gives information about the signaller's quality, and that symmetry preferences evolved to identify high-quality food or mates [1–3,14]. The alternative ‘aesthetic’ account argues that visual mechanisms of image segregation and object recognition are inherently sensitive to symmetry [15–17]. Support for this latter view comes from evidence that symmetry is an organizational factor in perceptual grouping that facilitates figure-ground segmentation [18,19], and has a role in object recognition [5,20,21]. Similarly, symmetry preference could arise from generalization from multiple views of a given object, and it allows detection and recognition of objects from novel viewpoints [15,16,22,23]. One might expect symmetry detection to involve long-range comparisons of potentially conjugate points in the image, but this process is computationally costly and it has been proposed that symmetry is a local feature comparable with edges and lines [17,24–26].
A limitation of nearly all theoretical and experimental studies of symmetry perception is that they consider two-dimensional bilateral symmetry in fronto-parallel view, normally with a vertical axis of symmetry [26]. Self-evidently, bilaterally symmetrical patterns and objects do not normally produce a symmetrical retinal image. Two main types of transformations are possible: skewed projections caused by viewing a symmetrical two-dimensional pattern out of the fronto-parallel plane [27], and the more complex transformations produced by rotating a three-dimensional symmetrical object [5]. It is relevant here that the three-dimensional structure can often be inferred from a two-dimensional view, given a prior assumption that an object is symmetrical [4,5]. To our knowledge, study of perception of transformations of bilaterally symmetrical two-dimensional patterns and three-dimensional objects is limited to human subjects; these confirm that humans identify bilateral symmetry in skewed projections of two-dimensional patterns [28–32].
Clearly, consideration of how non-human animals classify asymmetrical two-dimensional stimuli that are (potentially) generated by symmetrical three-dimensional objects is relevant to understanding symmetry in natural vision and visual signalling, while behavioural capabilities can have implications for the underlying neural mechanisms. The present study investigates how poultry chicks categorize symmetry as a visual feature [13], and then how they generalize from two-dimensional retinal projections of three-dimensional symmetrical objects. Experiment I shows that young chicks generalize bilateral symmetry with two-dimensional fronto-parallel patterns. Experiments II and III go on to look at simulation of rotations of three-dimensional objects, by testing generalization between symmetrical training patterns and stimuli that simulate the transformation produced by combining a 40° horizontal with a 20° vertical rotation of a pattern on a spherical surface.
2. Methods
(a). Subjects
For experiment I, subjects were 88 male domestic chicks (Gallus gallus; Strain ‘Bovans Goldline’) from a commercial hatchery (Joice & Hill, Peterborough, UK). For experiments II and III, subjects were, respectively, 88 and 90 male chicks (White Leghorn, Hybro strain) from a commercial hatchery (Agricola Berica, Montegalda, Vicenza, Italy).
(b). Exposure stimuli and apparatus
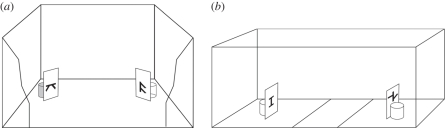
The exposure apparatus was a standard metal home cage (40 × 28 × 30 cm) with two screens (6 × 9 cm) located in two adjacent corners (figure 1a). One of the screens had a symmetrical pattern (2.5 × 4 cm) printed on both sides of it, the other an asymmetrical pattern. A food dish was hidden behind each screen; one dish was empty and the other contained food (chicken starter feed). For each pair of chicks, the symmetrical stimulus was always associated either with the full or with the empty dish.
Figure 1.
(a) The exposure apparatus: a standard metal home cage with the exposure stimuli on two screens hiding the food dishes, one of which (coloured) contained food. (b) The test apparatus with the testing stimuli presented at its opposite ends.
A set of three pairs of shapes was available, so that each pair of chicks could be exposed alternately to two of them and subsequently tested with the third, and previously unseen, one.
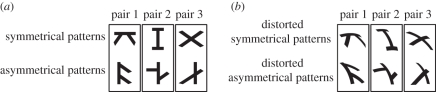
For experiments I and II, the exposure stimuli were two pairs of symmetrical/asymmetrical shapes consistent with a fronto-parallel view (figure 2a); for experiment III, the exposure stimuli were a transformed version of the same symmetrical and asymmetrical shapes, consistent with rotation of three-dimensional objects (figure 2b; appendix A gives the algorithm used for generating such stimuli).
Figure 2.
(a) The three pairs of shapes used in experiment I; all were balanced for dimension and area. (b) The set of ‘distorted’ shapes used in experiments II and III. These are produced by subjecting the symmetrical training stimuli to a transformation that would be produced by a 40° horizontal combined with a 20° vertical rotation of the pattern on a spherical surface viewed from a large distance relative to the diameter of the sphere (according to the algorithm described in appendix A).
(c). Test stimuli and apparatus
The test stimuli consisted of the symmetrical shape and the asymmetrical shape that the pair of chicks had not previously seen. The test apparatus (figure 1b) was a white-plywood runway (75 × 20 × 30 cm), subdivided virtually into a central area (15 cm long) and two side areas (each one 30 cm long), with the test stimuli placed at the two ends, 22.5 cm from the centre.
(d). Procedure
On their arrival at the laboratory (on day 1 of life), chicks were placed in pairs in the exposure apparatus (see above). Two screens, each hiding one of two food dishes, were placed in two corners of the cage. Depending on the pair of chicks, the symmetrical stimulus was associated with either the full or the empty dish. In experiment I, the exposure period ran from the afternoon of day 1 until the morning of day 4. For each pair of chicks, two pairs of stimuli (each pair consisting of one symmetrical and one asymmetrical stimulus) were used during the exposure period, the pair of screens being alternated every 2 h (i.e. two times for the afternoon of day 1 post-hatching and the morning of day 4, four times a day for days 2 and 3, with food being freely available in a standard dish overnight). Which two pairs of stimuli (out of the three pairs) and their initial location in the cage were balanced across subjects. The positions of the two stimuli within the cage were swapped regularly for each pair of chicks. Experiments II and III followed the same training protocol, saving that exposure to the training stimuli continued overnight on days 2 and 3 (exposure stimuli being different for the two nights).
On day 4, in the early afternoon, each pair of chicks was taken from its cage and placed in the central area of the test apparatus (figure 1b). Chicks were tested in pairs because the presence of a companion minimizes the typical freezing response to a novel environment and facilitates exploratory and feeding behaviours [33]. The symmetrical and asymmetrical shapes not seen before by the chicks were placed at opposite ends of the runway, each printed on a screen hiding an empty food dish.
The chicks were observed for six consecutive minutes. The behaviour of individual chicks in a pair is not independent because of social facilitation of exploratory responses. Therefore, for the data analysis, each pair of chicks was treated as a single unit.
If both chicks remained in the mid-compartment or expressed opposite choices (i.e. one of the chicks chose one of the end-side compartments meanwhile the other chick chose the other end-side compartment), this was deemed to indicate no preference. If at least one of the chicks went into an end-side compartment, this was regarded as a preference for the object placed at that end of the runway.
A computer-driven event recorder allowed the experimenter to score the time (seconds) spent by the pair of chicks in each of the three compartments. The proportion of time spent near the stimulus belonging to the same category which had been associated with food during the exposure phase was computed as:
The index values ranged from 0 (full choice for the ‘not-reinforced’ category) to 100 (full choice for the ‘reinforced’ category).
Data were analysed with repeated-measures ANOVA; departures from random choice were estimated by one-sample (two-tailed) t-tests (SPSS statistical package).
3. Results
(a). Experiment I
This experiment examined how chicks trained to discriminate bilaterally symmetrical from asymmetrical patterns generalize the presence of symmetry to novel patterns. For each pair of chicks, symmetry was associated with either the full or an empty food dish. Twenty-four pairs of chicks had food associated with the symmetrical shapes (i.e. the reinforced category was ‘symmetry’) and 20 pairs had food associated with asymmetrical shapes (i.e. reinforced category: ‘asymmetry’). The test stimuli-pair consisted of the fronto-parallel symmetrical shape and the fronto-parallel asymmetrical shape that the pair of chicks had not previously seen (figure 2a).
A repeated-measures ANOVA with reinforced category (symmetry versus asymmetry) as between-subject factors, and time (from 1 to 6 min) as within-subject factor revealed a significant effect of time (F5,210 = 3.362, p = 0.006). No other effect or interaction was significant (reinforced category: F1,42 = 0.857, p = 0.360; time × reinforced category: F5,210 = 0.570, p = 0.723).
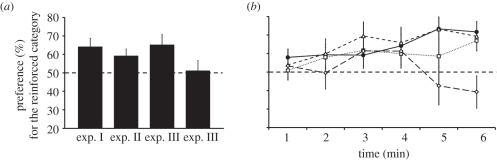
Overall, in the 6 min test, chicks significantly chose to approach and stay closer to the category reinforced at training (mean ± s.e.m. = 64.288 ± 4.371; one-sample t-test: t43 = 3.269, p = 0.002; figure 3a). As shown in figure 3b, such a preference increased in the last 3 min of the test (fourth minute: mean ± s.e.m. = 64.196 ± 6.112; one-sample t-test: t43 = 2.323, p = 0.025; fifth minute: mean ± s.e.m. = 73.392 ± 5.458; one-sample t-test: t43 = 4.286, p = 0; sixth minute: mean ± s.e.m. = 71.738 ± 5.588; one-sample t-test: t43 = 3.890, p = 0), while it was not above chance in the first 3 min of the test.
Figure 3.
(a) Average (mean ± s.e.m.) percentage of time for the 6 min test spent by the chicks in experiments I, II and III (third bar: chicks tested with non-distorted stimuli; fourth bar: chicks tested with distorted stimuli) near the test stimulus of the category reinforced in training. The dotted line represents chance level (50%). (b) Time courses of preference scores show a tendency for selectivity to increase during the test. Filled circles, experiment I; open squares, experiment II; open triangles, experiment III–not distorted; open diamonds, experiment III–distorted.
(b). Experiment II
The purpose of this experiment was to test whether chicks trained to discriminate bilaterally symmetrical from asymmetrical patterns generalize to novel asymmetrical patterns according to whether or not they are consistent with being rotated views of bilaterally symmetrical three-dimensional objects. Twenty-six pairs of chicks had food associated with the symmetrical shapes (i.e. reinforced category: symmetry), and 18 pairs had food associated with the asymmetrical shapes (i.e. reinforced category: asymmetry). The test stimuli consisted of a rotated view of the symmetrical and asymmetrical shapes never experienced before (figure 2b).
A repeated-measures ANOVA with reinforced category (symmetry versus asymmetry) as the between-subject factor, and time (from 1 to 6 min) as the within-subject factor did not reveal any significant effect or interaction (time: F5,210 = 1.644, p = 0.150; reinforced category: F1,42 = 0.100, p = 0.753; time × reinforced category: F5,210 = 1.353, p = 0.243).
Overall, chicks approached and stayed closer to the reinforced stimulus-type significantly more than the unreinforced (mean ± s.e.m. = 59.307 ± 3.635; one-sample t-test: t43 = 2.560, p = 0.014; figure 3). Such a preference was above chance in the third and in the sixth minute of the test (third minute: mean ± s.e.m. = 61.673 ± 5.205; one-sample t-test: t43 = 2.243, p = 0.030; sixth minute: mean ± s.e.m. = 66.936 ± 5.522; one-sample t-test: t43 = 3.067, p = 0.004); it was not marginally significant on minute four (mean ± s.e.m. = 59.909 ± 5.668; one-sample t-test: t43 = 1.748, p = 0.088), and at chance on all other minutes of the test.
(c). Experiment III
This key experiment examined whether chicks, trained to discriminate asymmetrical patterns according to whether or not they are consistent with being rotated views of bilaterally symmetrical three-dimensional objects, generalize to novel patterns according to the same criterion.
Twenty pairs of chicks had food associated with rotated views of symmetrical objects (figure 2b; i.e. reinforced category: symmetry), 25 pairs had food associated with rotated views of asymmetrical shapes (figure 2b; i.e. reinforced category: asymmetry). Chicks were divided into two groups: 25 pairs were tested with the fronto-parallel version of the symmetrical and asymmetrical shapes to which they had not been exposed to previously (i.e. stimuli in figure 2a); 20 pairs of chicks were tested with the distorted version of the symmetrical and asymmetrical shapes never experienced before (stimuli in figure 2b).
A repeated-measures ANOVA with reinforced category (symmetry versus asymmetry) as between-subject factor, type of test stimuli (fronto-parallel versus distorted) and time (from 1 to 6 min) as within-subject factors did not reveal any significant effect or interaction (time: F5,205 = 1.045, p = 0.392; reinforced category: F1,41 = 2.800, p = 0.102; type of test stimuli: F1,41 = 3.016, p = 0.090; reinforced category × type of test stimuli: F1,41 = 0.070, p = 0.792; time × reinforced category: F5,205 = 0.091, p = 0.994; time × type of test stimuli: F5,205 = 1.785, p = 0.117; time × reinforced category × type of test stimuli: F5,205 = 0.539, p = 0.747).
Overall, subjects significantly chose to approach and stayed closer to the stimulus-category which was reinforced at training significantly more than to the non-reinforced (mean ± s.e.m. = 59.024 ± 4.086; one-sample t-test: t44 = 2.208, p = 0.032; figure 3). Although the effect of the type of test stimuli was not significant at the ANOVA, it is worth noting that the subgroup of chicks tested with the distorted stimuli, in spite of these being more similar to the stimuli employed during training, performed at chance in every one of the 6 min of the test (figure 3). While the subgroup of chicks tested with the non-distorted version of the stimuli performed above chance or nearly so starting from the third minute of the test (third minute: mean ± s.e.m. = 69.483 ± 7.583; one-sample t-test: t24 = 2.569, p = 0.017; fourth minute: mean ± s.e.m. = 65.788 ± 8.467; one-sample t-test: t24 = 1.865, p = 0.074; fifth minute: mean ± s.e.m. = 73.178 ± 8.152; one-sample t-test: t24 = 2.843, p=0.009; sixth minute: mean ± s.e.m. = 69.294 ± 7.846; one-sample t-test: t24 = 2.459, p = 0.022).
4. Discussion
Experiment I demonstrates that 4 day old domestic chicks can learn and generalize symmetry as a visual category. Experiment II gives evidence that chicks trained to discriminate symmetrical from asymmetrical patterns successfully classify rotated views of novel objects according to whether or not they could have been generated by a symmetrical three-dimensional object. Finally, experiment III gives evidence that chicks trained to discriminate asymmetrical patterns generalize to novel patterns according to whether or not the training stimuli could have been generated by a symmetrical three-dimensional object. To our knowledge, this is the first evidence for such generalization in a non-human species.
These findings are of interest from at least two points of view.
First, these data are the first clear evidence that the domestic chick, like the bee (Apis mellifera) [13], can categorize symmetry as a visual feature and generalize this learning to novel shapes (experiment I). Previous work [8] showed that newly hatched, and hence visually naïve chicks can detect symmetry and develop a preference to peck at symmetrical displays. This finding is consistent with previous evidence in pigeons (C. livia) [11], which used much more extensive training, and so emphasizes the facility with which birds learn to recognize symmetry.
Second, this is the first time, to our knowledge, a two-dimensional asymmetrical stimulus consistent with the rotation of a three-dimensional object (experiments II and III) has been used to assess symmetry perception in a non-human species. Chicks trained to discriminate bilaterally symmetrical two-dimensional patterns from asymmetrical patterns, generalized to stimuli that were consistent with rotated views of symmetrical or asymmetrical patterns on a three-dimensional spherical surface (experiment II). Perhaps most strikingly, experiment III gives evidence that chicks can detect the underlying three-dimensional symmetrical structure of a two-dimensional asymmetrical pattern and generalizes this to a novel pattern, either in a fronto-parallel or in a rotated view. These results are consistent with the hypothesis that chicks recognized the inherent structure of the objects regardless of their viewing angle, by correctly identifying and responding to the category a shape belongs to, even when its symmetry could not be perceived in a fronto-parallel perspective.
Our findings favour the view that symmetry is important for detection and recognition of shapes from several viewpoints [4]. This is consistent with the idea that symmetry preferences in animal display arise because symmetry is a property of biologically significant objects (i.e. other living organisms) [15,17], rather than as an honest signal of quality—of course, these accounts are not mutually exclusive. However, if birds can indeed recognize that a three-dimensional object has a plane of symmetry, regardless of viewing angle, this leaves very much open the question of what mechanisms might be involved. It seems likely that local mechanisms, comparable with edge and line detectors, that have been proposed for symmetrical two-dimensional patterns would face problems [17,26].
For experiments II and III, the clear discrimination by the chicks between the rotated views of symmetrical objects from other asymmetrical patterns implies that their symmetry preference is robust against rotational transformations of three-dimensional objects. Moreover, training the subjects with distorted shapes and then testing them with either distorted or non-distorted shapes (experiment III) increases the cue-level differences between training and testing stimuli and gives additional evidence for generalization of symmetrical objects to their rotated view.
Thus, we give evidence that 4 day old chicks can classify objects according to whether or not they have a plane of symmetry. While this observation is relevant to natural behaviour, it is in a sense purely qualitative. In the absence of a specific hypothesis about the mechanism of symmetry detection, it is difficult to compare the degree of asymmetry in the test stimuli that represent rotated symmetrical objects to the asymmetrical alternatives. More evidence is needed to understand the mechanism that would allow animals to exploit symmetry as a visual cue to identify three-dimensional objects (i.e. mates or food) seen from multiple viewing angles. The fact that chicks generalize the presence of symmetry to novel objects rules out the possibility—suggested for primate face recognition [33–35]—that they recognize objects simply by storing multiple views.
It would be interesting to investigate the ecological value of such symmetry perception. For example, which symmetrical objects (insects, seeds, predators, etc.) are ecologically relevant for each species. This would require quantitative analysis of symmetry in natural objects and experimental stimuli. It would also be interesting to learn how the preference for bilateral symmetry is affected by distortions caused by non-fronto-parallel views. Moreover, in further research, a wide range of experimental stimuli should be used to confirm the results of the present paper.
Finally, the possibility of performing controlled-rearing studies with chicks opens the door to direct investigation of the neural and genetic basis of such symmetry detection mechanism.
Acknowledgements
This work was carried out at the Universities of Sussex and Padua in accordance with the relevant National animal welfare legislation and University Ethics guidelines.
We thank Lucas Wilkins for creating the rotated stimuli of experiments II and III and for writing the appendix. This research was funded by the project ‘EDCBNL: Evolution and Development of Cognitive, Behavioural and Neural Lateralization’ (2006/2009) supported by the Commission of the European Communities within the framework of the specific research and technological development programme ‘Integrating and strengthening the European Research Area’ (initiative ‘What it Means to be Human’).
Appendix A
Initially, the image (I (x,y) ∈ {0,1,}) is orthogonally projected into the surface of unit sphere parametrized by the Cartesian coordinates (a,b,c). r is the radius of the unit sphere.
The rotation used was chosen so as to preserve the effective orientation of the image. For this reason, the rotation was performed by two consecutive rotations, first around the vertical axis of the image plane and second around the horizontal.
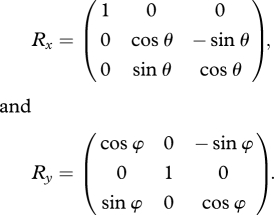 |
References
- 1.Møller A. P. 1992. Female swallow preference for symmetric male sexual ornaments. Nature 357, 238–240 10.1038/357238a0 (doi:10.1038/357238a0) [DOI] [PubMed] [Google Scholar]
- 2.Swaddle J. P., Cuthill I. C. 1994. Preference for symmetrical males by female zebra finches. Nature 367, 165–166 10.1038/367165a0 (doi:10.1038/367165a0) [DOI] [Google Scholar]
- 3.Møller A. P., Thornhill R. 1998. Bilateral symmetry and sexual selection: a meta-analysis. Am. Nat. 151, 174–192 10.1086/286110 (doi:10.1086/286110) [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Pizlo Z., Steinman R. M. 2009. A computational model that recovers the 3D shape of an object from a single 2D retinal representation. Vis. Res. 49, 979–991 10.1016/j.visres.2008.05.013 (doi:10.1016/j.visres.2008.05.013) [DOI] [PubMed] [Google Scholar]
- 5.Pizlo Z., Sawada T., Li Y., Kropatsch W. G., Steinman R. M. 2010. New approach to the perception of 3D shape based on veridicality, complexity, symmetry and volume. Vis. Res. 50, 1–11 10.1016/j.visres.2009.09.024 (doi:10.1016/j.visres.2009.09.024) [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez I., Gumbert A., Hempel de Ibarra N., Kunze J., Giurfa M. 2004. Symmetry is in the eye of the ‘beeholder’: innate preference for bilateral symmetry in flower-naïve bumblebees. Naturwissenchaften 91, 374–377 [DOI] [PubMed] [Google Scholar]
- 7.Wignall A. E., Heiling A. M., Cheng K., Herberstein M. E. 2006. Flower symmetry preferences in honeybees and their crab spider predators. Ethology 112, 510–518 10.1111/j.1439-0310.2006.01199.x (doi:10.1111/j.1439-0310.2006.01199.x) [DOI] [Google Scholar]
- 8.Clara E., Regolin L., Vallortigara G. 2007. Preference for symmetry is experience dependent in newborn chicks (Gallus gallus). J. Exp. Psychol. Anim. Behav. Process. 33, 12–20 10.1037/0097-7403.33.1.12 (doi:10.1037/0097-7403.33.1.12) [DOI] [PubMed] [Google Scholar]
- 9.Swaddle J. P., Ruff D. A., Page L. C., Frame A. M., Long V. A. 2008. A test of receiver perceptual performance: European starlings' ability to detect asymmetry in a naturalistic trait. Anim. Behav. 76, 487–495 10.1016/j.anbehav.2008.05.005 (doi:10.1016/j.anbehav.2008.05.005) [DOI] [Google Scholar]
- 10.Tudor M. S., Morris M. R. 2009. Experience plays a role in female preference for symmetry in the swordtail fish Xiphophorus malinche. Ethology 115, 812–822 10.1111/j.1439-0310.2009.01676.x (doi:10.1111/j.1439-0310.2009.01676.x) [DOI] [Google Scholar]
- 11.Delius J. D., Habers G. 1978. Symmetry: can pigeons conceptualize it? Behav. Biol. 22, 336–342 10.1016/S0091-6773(78)92411-2 (doi:10.1016/S0091-6773(78)92411-2) [DOI] [PubMed] [Google Scholar]
- 12.Horridge G. A. 1996. The honeybee (Apis mellifera) detects bilateral symmetry and discriminates axis. J. Insect Physiol. 42, 755–764 10.1016/0022-1910(96)00026-1 (doi:10.1016/0022-1910(96)00026-1) [DOI] [Google Scholar]
- 13.Giurfa M., Eichmann B., Menzel R. 1996. Symmetry perception in an insect. Nature 382, 458–461 10.1038/382458a0 (doi:10.1038/382458a0) [DOI] [PubMed] [Google Scholar]
- 14.Møller A. P. 1990. Fluctuating asymmetry in male sexual ornaments may reliably reveal male quality. Anim. Behav. 40, 1185–1187 10.1016/S0003-3472(05)80187-3 (doi:10.1016/S0003-3472(05)80187-3) [DOI] [Google Scholar]
- 15.Enquist M., Arak A. 1994. Symmetry, beauty and evolution. Nature 372, 169–172 10.1038/372169a0 (doi:10.1038/372169a0) [DOI] [PubMed] [Google Scholar]
- 16.Johnstone R. A. 1994. Female preference for symmetrical males as a by-product of selection for mate recognition. Nature 372, 172–175 10.1038/372172a0 (doi:10.1038/372172a0) [DOI] [PubMed] [Google Scholar]
- 17.Osorio D. 1996. Symmetry detection by categorization of spatial phase: a model. Proc. R. Soc. Lond. B 263, 105–110 10.1098/rspb.1996.0017 (doi:10.1098/rspb.1996.0017) [DOI] [Google Scholar]
- 18.Köhler W. 1929. Gestalt psychology. New York, NY: Liveright [Google Scholar]
- 19.Wertheimer M. 1938. Laws of organization in perceptual forms. In A source book of Gestalt psychology (ed. Ellis W.), pp. 71–88 New York, NY: Harcourt [Google Scholar]
- 20.Marr D. 1982. Vision. San Francisco, CA: W. H. Freeman and Company [Google Scholar]
- 21.Troscianko T., Benton C. P., Lovell G., Tolhurst D. J., Pizlo Z. 2009. Camouflage and visual perception. Phil. Trans. R. Soc. B 364, 449–461 10.1098/rstb.2008.0218 (doi:10.1098/rstb.2008.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enquist M., Johnstone R. 1997. Generalization and the evolution of symmetry preferences. Proc. R. Soc. Lond. B 264, 1345–1348 10.1098/rspb.1997.0186 (doi:10.1098/rspb.1997.0186) [DOI] [Google Scholar]
- 23.Jansson L., Forkman B., Enquist M. 2002. Experimental evidence of receiver bias for symmetry. Anim. Behav. 63, 617–621 10.1006/anbe.2001.1936 (doi:10.1006/anbe.2001.1936) [DOI] [Google Scholar]
- 24.Rainville S. J. M., Kingdom F. A. A. 2000. The functional role of oriented spatial filters in the perception of mirror symmetry: psychophysics and modeling. Vis. Res. 40, 2621–2644 10.1016/S0042-6989(00)00110-3 (doi:10.1016/S0042-6989(00)00110-3) [DOI] [PubMed] [Google Scholar]
- 25.Scognamillo R., Rhodes G., Morrone C., Burr D. 2003. A feature-based model of symmetry detection. Proc. R. Soc. Lond. B 270, 1727–1733 10.1098/rspb.2003.2434 (doi:10.1098/rspb.2003.2434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier F. J. A. M., Wilson H. R. 2009. A biologically plausible model of human shape symmetry perception. J. Vis. 10, 9. 10.1167/10.1.9 (doi:10.1167/10.1.9) [DOI] [PubMed] [Google Scholar]
- 27.Wagemans J., Van Gool L., Swinnen V., Van Horebeek J. 1993. Higher-order structure in regularity detection. Vis. Res. 33, 1067–1088 10.1016/0042-6989(93)90241-N (doi:10.1016/0042-6989(93)90241-N) [DOI] [PubMed] [Google Scholar]
- 28.Wagemans J. 1993. Skewed symmetry: a nonaccidental property used to perceive visual forms. J. Exp. Psychol. Hum. Percept. Perform. 19, 364–380 10.1037/0096-1523.19.2.364 (doi:10.1037/0096-1523.19.2.364) [DOI] [PubMed] [Google Scholar]
- 29.Szlyk J. P., Rock I., Fisher C. B. 1995. Level of processing in the perception of symmetrical forms viewed from different angles. Spat. Vis. 9, 139–150 10.1163/156856895X00151 (doi:10.1163/156856895X00151) [DOI] [PubMed] [Google Scholar]
- 30.McBeath M. K., Schiano D. J., Tversky B. 1997. Three-dimensional bilateral symmetry bias in judgments of figural identity and orientation. Psychol. Sci. 8, 217–223 10.1111/j.1467-9280.1997.tb00415.x (doi:10.1111/j.1467-9280.1997.tb00415.x) [DOI] [Google Scholar]
- 31.Saunders J. A., Knill D. C. 2001. Perception of 3D surface orientation from skew symmetry. Vis. Res. 41, 3163–3183 10.1016/S0042-6989(01)00187-0 (doi:10.1016/S0042-6989(01)00187-0) [DOI] [PubMed] [Google Scholar]
- 32.Van der Vloed G., Csathó A., Van der Helm P. A. 2005. Symmetry and repetition in perspective. Acta Psychol. 120, 74–92 10.1016/j.actpsy.2005.03.006 (doi:10.1016/j.actpsy.2005.03.006) [DOI] [PubMed] [Google Scholar]
- 33.Vallortigara G., Zanforlin M. 1988. Open-field behavior of young chicks (Gallus gallus): antipredatory responses, social reinstatement motivation, and gender affect. Anim. Learn. Behav. 16, 359–362 10.3758/BF03209088 (doi:10.3758/BF03209088) [DOI] [Google Scholar]
- 34.Hill H., Schyns P. G., Akamatsu S. 1997. Information and viewpoint dependence in face recognition. Cognition 62, 201–222 10.1016/S0010-0277(96)00785-8 (doi:10.1016/S0010-0277(96)00785-8) [DOI] [PubMed] [Google Scholar]
- 35.Perrett D. I., Oram M. W., Ashbridge E. 1998. Evidence accumulation in cell populations responsive to faces: an account of generalisation of recognition without mental transformations. Cognition 67, 111–145 10.1016/S0010-0277(98)00015-8 (doi:10.1016/S0010-0277(98)00015-8) [DOI] [PubMed] [Google Scholar]