Abstract
Variation in the physical characteristics of the environment should impact the movement energetics of animals. Although cognizance of this may help interpret movement ecology, determination of the landscape-dependent energy expenditure of wild animals is problematic. We used accelerometers in animal-attached tags to derive energy expenditure in 54 free-living imperial cormorants Phalacrocorax atriceps and construct an energy landscape of the area around a breeding colony. Examination of the space use of a further 74 birds over 4 years showed that foraging areas selected varied considerably in distance from the colony and water depth, but were characterized by minimal power requirements compared with other areas in the available landscape. This accords with classic optimal foraging concepts, which state that animals should maximize net energy gain by minimizing costs where possible and show how deriving energy landscapes can help understand how and why animals distribute themselves in space.
Keywords: energy landscape, movement ecology, metabolic power, area use, foraging efficiency
1. Introduction
The concept that animals should forage optimally [1] has been pivotal in giving biologists a framework with which to examine the mechanisms behind energy acquisition [2]. A central tenet is that animals should minimize energy expenditure with respect to energy acquisition, maximizing their net rate of energy gain [2]. Foraging costs may be couched in terms of time or energy [3] but those calculated [4] generally ignore the variation in the physical manifestation of the landscape that may profoundly affect movement costs. For example, although it is widely accepted that many birds enhance their flight capacities by making use of predictable sources of rising air [5] and that terrestrial animals expend more energy moving over soft substrate than hard [6], general consideration of the energetic costs of animals moving through their variable landscapes is minimal (but see [7]). Landscapes vary in character in both space and time with, for example, heterogeneous vegetation landscapes changing during succession [8,9] and over the growing season [10], becoming correspondingly more problematic for animals to move through [11]. Indeed, the degree of variation in the landscape (e.g. incline, substrate- and vegetation-type) [12] will be responsible for varying movement costs and this variation translates into an effective energy landscape for animals foraging through, or in, it [7]. Ultimately, the costs of moving in particular landscapes should prove important for informing movement ecology [13] and help us understand why and how animals distribute themselves in space [14]. We expect variability in the energy landscape to exert selection pressure on animals to modulate their foraging strategies accordingly although to our knowledge this has not been examined explicitly in an optimal foraging context. Specifically, where food is not distributed in a manner that links to the energy landscape, we would expect animals to preferentially use areas of their energy landscape which result in minimized power costs in accordance with maximizing their net energetic gain during foraging.
This study examines animals foraging in a variable energy landscape using animal-attached devices to derive the energetic costs of a foraging, benthic-feeding diver, the imperial cormorant Phalacrocorax atriceps feeding near Punta Leon, Chubut, Argentina. These birds can be captured readily and equipped with tags to record position and depth [15] as well as new devices used to record tri-axial acceleration [16]. Tri-axial acceleration data can be used to calculate a powerful linear proxy for metabolic power, overall dynamic body acceleration (ODBA) [17], which can be further converted directly into energy expenditure [17]. Although imperial cormorants may occasionally feed in groups on pelagic school fish in the upper water layers [18], they generally hunt solitarily, executing benthic dives to the seabed [19]. Such dives to the seabed are executed virtually exclusively by birds at Punta Leon [20] in a foraging area consisting exclusively of an extensive sandy substrate [21]. Here, they exploit benthic prey such as Raneya fluminensis, Triathalassothia argentina and Octopus tehuelchus) [22], all species which are widely distributed in coastal waters over the Patagonian Shelf (www.fishbase.org) [23]. The birds forage at variable distances from their colony, exploit water of different depths and thus operate in a simple, well-defined energy landscape because both distance from the colony and water depth relate to energy expenditure exploiting prey. We hypothesize that birds should preferentially use areas where foraging costs are minimal, moving to the more demanding regions as prey become depleted.
2. Material and methods
(a). Device deployment
During the austral summers of 2004, 2005, 2007 and 2008, 132 imperial cormorants, P. atriceps brooding small chicks at Punta Leon, Argentina were fitted with logging devices. A total of 74 birds carried global positioning system (GPS) devices (Ocean Earth Technologies, Inc., Kiel, Germany) recording position at 1 Hz with an accuracy of better than 7 m, whereas 54 birds carried units measuring, among other things, pressure and tri-axial acceleration (‘daily diaries’) [16] at 6 Hz with depth resolution of better than 1 cm. Birds were released and devices recovered after a single forging trip before data were downloaded.
(b). Calculation of position and energy
GPS positions were sorted to determine the position of foraging birds, which were defined by low travelling speeds (<5 km h−1) at sea with fixes punctuated by loss of GPS fixes for periods which exceeded 20 s, indicating foraging behaviour [15]. These positions were mapped onto the area using ArcMap and examined in relation to the bathymetry (derived from local charts) and derivation of the energy used for foraging (see below).
Foraging energy and behaviour were quantified using custom-written software that identified descent, bottom and ascent phases of cormorant dives as well as their inter-dive pause durations. The durations of these phases were determined with respect to maximum depths reached during the dive as were their ODBA totals and means (see [24] for details). ODBA (in g) was calculated using the sum of the absolute values of dynamic acceleration from each of the three spatial axes (corresponding to surge, heave and sway) after subtracting the static acceleration from the raw acceleration values, itself derived using a running mean over 2 s [20] so that
| 2.1 |
where Ax, Ay and Az are the derived dynamic accelerations at any point in time corresponding to the three orthogonal axes of the accelerometer.
Extensive recent work has shown a linear relationship between ODBA and metabolic rate in all species examined to date, which includes fish [25], amphibia [26], mammals and birds [27–32], and this has been explicitly defined in cormorants for resting, diving and walking by Gomez Laich et al. [33] as
| 2.2 |
where MP is the mass-specific power (W kg−1). We used this relationship to define a measure of the energy-based foraging costs (φ) as the energy used per unit time spent on the seabed according to
| 2.3 |
with units of J kg−1 s−1.
Both mean mass-specific power (W kg−1) during foraging and the energy used per second bottom duration (J kg−1 s−1) were used to construct energy and foraging cost landscape maps based on the bathymetry of the marine area surrounding the colony at Punta Leon (figure 1).
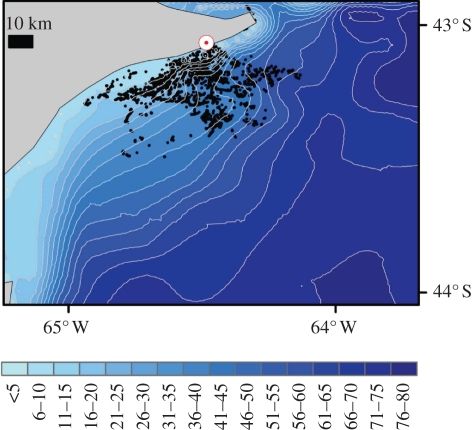
Figure 1.
Distribution of 74 imperial cormorants foraging from their colony at Punta Leon (white circle with red dot) over 4 years in relation to bottom bathymetry (depths in metres).
In a second step, and to incorporate the costs associated with travel from the central place (the breeding site) to the foraging site, the energetic costs for flight were built into a general model. Although flight costs could theoretically be taken from the ODBA values, as yet no validation has been undertaken to show that measured costs for flight accord with the otherwise linear relationship between ODBA and rate of oxygen consumption for diving and walking birds [33]. Thus, flight costs were simply taken to be 102 W kg−1 [33] and incorporated into a time budget of imperial cormorants provisioning small chicks [15] by modifying equation (2.3) so that
| 2.4 |
where Φ represents the mass-specific foraging costs per second bottom time (J kg−1 s−1), incorporating all costs incurred between leaving the colony and returning to it at the end of the foraging period.
This model assumed that birds were limited to a total of 6 h foraging (studies at this site show means of 5.7 (s.d. 2.2) and 6.1 h (s.d. 1.3) for females and males, respectively [15]) that flight speeds were 60 km h−1 [15], and that at every foraging site within the area considered (figure 1), birds would only dive there and otherwise fly directly to it from the colony and back again at the above speeds and calculated energy costs [15]. Time spent diving was derived by subtracting flight durations (directly proportional to the distance between the colony and foraging site) from 6 h, and the number of dive cycles executed was determined by dividing this residual time by the dive cycle duration for the prescribed depth. The mean, mass-specific power use and energy-based foraging costs for imperial cormorants incorporating the transit costs from and to the colony were then calculated by summing the total energy expended for the foraging period and dividing by 6 h, and by calculating the total energy expended for the foraging period and dividing by the total bottom duration, respectively.
3. Results
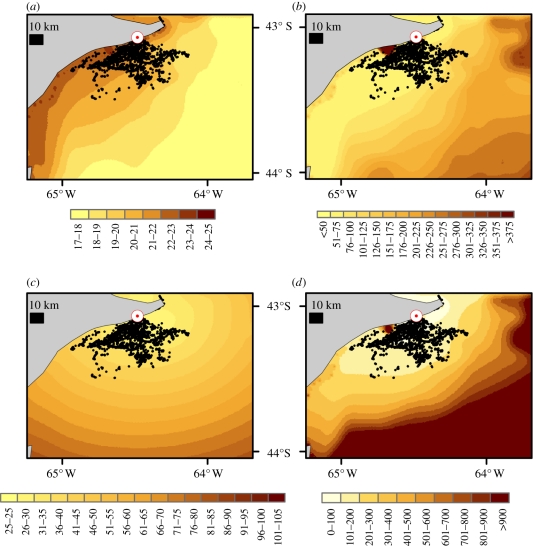
Seventy-four GPS-equipped imperial cormorants showed considerable variation in two primary foraging parameters—depth and distance from the colony. They dived in water depths varying between 3.8 and 62.1 m and at distances of between 1.1 and 52.6 km from the colony (figure 1). Detailed data on diving behaviour from a further 58 birds showed that the durations of the descent, bottom, ascent and inter-dive pauses were all highly correlated with maximum depth reached during the dive (table 1) as was the proxy for metabolic power, ODBA (table 1). Conversion of ODBA (g) to energy expenditure (J kg−1 s−1) revealed that, where flight costs from the colony were not considered, mean (mass-specific) power use (during all periods ascribed to foraging, including time resting at the surface between dives) was highest in shallowest waters (figure 2a) but that the energy-based foraging costs (expressed as the costs in joules, expended over the full dive cycle, for each second spent at the sea bed—equation (2.3)) showed a reverse trend (figure 2b). There was no obvious relationship between the foraging areas used by birds and depth, distance (figure 1), mean power use (figure 2a) or simple energy-based foraging costs (considering the mean mass-specific energy invested per second of bottom time after incorporating all other costs involved in the dive cycle—figure 2b). Inclusion of flight costs to determine the effect of distance of the foraging locality from a central place (the colony) showed that flight was critical in modulating overall power costs (figure 2c, cf. figure 2a), while calculation of energy-based foraging costs incorporating both depth and distance from the colony (equation (2.4)) indicated that birds used a virtually homogeneous energy landscape (figure 2d, cf. figure 2b).
Table 1.
Relationship between dive parameters and maximum dive depth (D) for 58 imperial cormorants foraging during chick-rearing at Punta Leon, Argentina between 2004 and 2008. All durations are expressed in seconds, all overall dynamic body acceleration (ODBA) values in g and all depths in metre. p-Values for all functions are <0.001.
| parameter | function | r2 |
|---|---|---|
| descent duration | y = 0.78D + 1.6 | 0.97 |
| bottom duration | y = −0.0185D2 + 3.12D − 5.0 | 0.79 |
| ascent duration | y = 0.70D + 1.9 | 0.91 |
| pause duration | y = 12.31e0.0603D | 0.57 |
| descent ODBA | y = 0.368D + 1.59 | 0.91 |
| bottom ODBA | y = 0.826D0.956 | 0.66 |
| ascent ODBA | y = 1.24e0.0309D | 0.58 |
Figure 2.
Distribution of foraging imperial cormorants (cf. figure 1) (a) with respect to the calculated mean mass-specific power (W kg−1) uniquely for diving at the relevant site and (b) with respect to the overall mass-specific energy invested per second of bottom duration (J kg−1 s−1). Insets (c) and (d) show the same as (a) and (b), respectively, but additionally incorporate the energetic costs of commuting to and from the breeding colony (shown by the white circle with red dot).
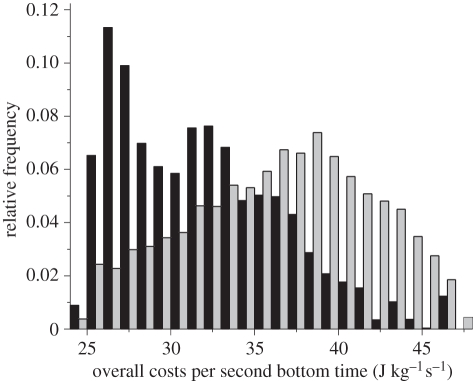
Within this landscape, however, birds preferentially used the areas and depths that resulted in lower energy-based foraging costs: consideration of the foraging costs of real birds compared with a theoretical population of evenly spaced individuals exploiting the available foraging area (based on a semicircle with a radius corresponding to the maximum shown by the tagged birds) showed that the real imperial cormorants had markedly lower energy-based foraging costs than the evenly spaced individuals (figure 3). Beyond this, where cormorants occurred, bird density decreased linearly with increasing energy-based foraging cost (φ, in joules per second bottom duration) according to
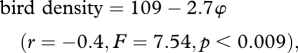 |
2.4 |
where bird density is given by the number of birds per 100 km2 and φ values are means for the respective grid squares.
Figure 3.
Frequency usage of particular energy costs of foraging (mass-specific energy invested per second of bottom duration) resulting from the sea areas and depths frequented by imperial cormorants breeding at Punta Leon (black bars) compared with theoretical birds foraging, regularly spaced within the area available to imperial cormorants from the colony (grey bars).
4. Discussion
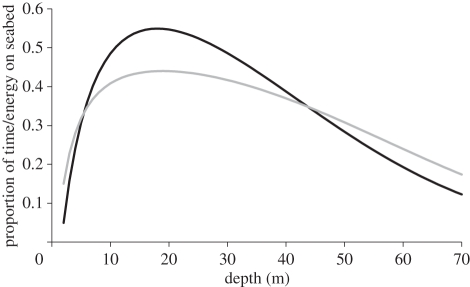
Our energy landscape for birds diving shows reduced power costs for deeper water (figure 2a), something that is not intuitively obvious. However, buoyancy is a major factor affecting energy expenditure in diving birds [34] and the higher pressures experienced by deeper diving birds compress respiratory and feather-associated air more so that the effort to counteract this buoyancy is reduced [32]. In fact, in a demonstration of this, Quintana et al. [35] calculated that an imperial cormorant descending the water column at a constant speed (1.5 ms−1) uses about three times as much power when it is at a depth of 2 m as it does at 30 m. Decreasing energy expenditure costs with depth are, however, more than compensated by decreasing time-based efficiency. As exploitation of greater depths requires longer dive durations owing to increased transit between the surface and the seabed where birds forage [24], birds must also compensate by increasing the bottom duration and surface recovery period, the latter of which increases as an exponential function of dive duration [24,36]. All this makes imperial cormorants, and many other divers [37], rapidly less time-efficient with increasing depth. The energy-based foraging costs, which must equate the total energy used to maintain and transport the bird to and from the seabed with the time available to forage while on the seabed, reverses the simple power used to dive (figure 2a) as a function of depth so that depths of ca 10–30 m become the most efficient (cf. figure 1 and figure 2b). In fact, cognizance of the difference between time- and energy-based efficiency may fundamentally change our understanding of optimum strategies [20]. For example, authors examining diving capacity in air-breathers conventionally use the proportion of time that animals remain in the bottom phase as a fraction of the whole dive cycle duration to measure efficiency [38] (figure 4). The energetic equivalent of this (the fraction of energy used in the bottom phase compared with that for the whole dive cycle) necessarily shows an approximately similar pattern (figure 4), because animals expend energy all the time, and therefore do so as a function of time. However, the precise form varies according to the variation in metabolic costs, which, in the case of the cormorant, changes with depth, producing an efficiency versus depth pattern that decreases much less rapidly with depth than the time-based efficiency scenario (figure 4). The extent of differences between time-based and energy-based efficiency is primarily modulated by the amount of air held within breath-hold diving vertebrates, which is hugely variable depending on taxon [39,40] although thermoregulation may also play a significant role [41].
Figure 4.
Time- (black line) and energy-based (grey line) efficiency of imperial cormorant foraging as a function of depth based on the regressions shown in table 1. The graphs show the proportion of time or energy allocated to foraging along the seabed in relation to the total time, or energy, used in the full dive cycle.
This energy landscape scenario would be applicable only to imperial cormorants if they remained continuously in the foraging zone, as many overwintering seabirds may do [42]. However, the central place aspect of their ecology, necessitating commuting between the colony and the foraging site, means that the energetic costs of flight should be incorporated into the energy landscape, which changes it dramatically (figure 2c), and particularly when the complete energetic costs of foraging along the seabed are considered (figure 2d).
The energy-based foraging cost translates into an effective index of necessary prey density because higher foraging costs require higher prey densities for them to be energetically tenable. Thus, movement of birds out to areas with higher costs implies that the closer areas have been depleted of prey [43–45]. Nonetheless, we would expect the distribution of cormorants around the colony to show generally decreasing densities of birds exploiting prey from energetically more costly environments, as we observed. More specifically, bird density may be expected to follow an ideal free distribution [46] with individuals attempting to maximize net energy gain by exploiting areas with minimal-associated costs first [47,48]. Severe prey depletion in areas with low-cost energy landscapes could, in fact, result in those areas being avoided by birds, something that is not readily apparent in our observations. In such cases, we would expect birds to populate other low-cost energy landscape areas where prey density was not diminished first before moving to high-cost energy landscapes as resources became scarcer, consistently, however, maximizing net energy gain.
This work points to the critical nature of the interaction of colony location and water depth in modulating the coastal distribution of diving seabirds. Clearly, not all sites are appropriate for nesting [49] and birds must balance the advantages of nesting on a particular land mass with the costs of foraging around it [50]. Beyond that, however, the approach provides a framework to examine how the foraging costs of adjacent, potentially competing colonies might interact with density to limit bird distribution at sea [51].
Our examination of the imperial cormorant energy landscape is simplistic but demonstrates mechanisms for deriving costs associated with animals operating in their environment (cf. [7]). Although many energy landscapes may be more complex to derive, with power values varying with parameters such as topography, terrain, substrate and vegetation (cf. [52]), such landscapes can elucidate spatially linked strategies adopted by animals as well as the energetic consequences of having to change them. This should help inform optimality models but also, perhaps, find particular resonance in conservation science where the animal allocation of energies in a changing world may be pivotal for species survival.
Acknowledgements
This study was supported by the Wildlife Conservation Society of New York, Rolex Awards for Enterprise, National Geographic and Agencia Nacional de Promoción Científica y Tecnológica de Argentina. We are grateful to Agustina Gómez Laich, Emily Shepard, Marcela Uhart and Tito Svagelj for help and expertise in the field.
References
- 1.Pyke G. H. 1984. Optimal foraging theory: a critical review. Ann. Rev. Ecol. Syst. 15, 523–575 10.1146/annurev.es.15.110184.002515 (doi:10.1146/annurev.es.15.110184.002515) [DOI] [Google Scholar]
- 2.Stephens D. W., Brown J. S., Ydenberg R. C. 2007. Foraging behavior and ecology. Chicago and London: University of Chicago Press [Google Scholar]
- 3.Lemon W. C. 1991. Fitness consequences of foraging behavior in the zebra finch. Nature 352, 153–155 10.1038/352153a0 (doi:10.1038/352153a0) [DOI] [Google Scholar]
- 4.Niaccarone A. D., Brzorad J. N., Stone H. M. 2008. Characteristics and energetics of great egret and snowy egret foraging flights. Waterbirds 31, 541–549 [Google Scholar]
- 5.Leshem Y., YomTov Y. 1996. The use of thermals by soaring migrants. Ibis 138, 667–674 [Google Scholar]
- 6.Wilson R. P., Culik B., Adelung D., Coria N. R., Spairani H. J. 1991. To slide or stride: when should Adelie penguins (Pygoscelis adeliae) toboggan. Can. J. Zool. 69, 221–225 10.1139/z91-033 (doi:10.1139/z91-033) [DOI] [Google Scholar]
- 7.Wall J., Douglas-Hamilton I., Vollrath F. 2006. Elephants avoid costly mountaineering. Curr. Biol. 16, R527–R529 10.1016/j.cub.2006.06.049 (doi:10.1016/j.cub.2006.06.049) [DOI] [PubMed] [Google Scholar]
- 8.Marteinsdottir B., Svavarsdottir K., Thorhallsdottir T. E. 2010. Development of vegetation patterns in early primary succession. J. Veg. Sci. 21, 531–540 10.1111/j.1654-1103.2009.01161.x (doi:10.1111/j.1654-1103.2009.01161.x) [DOI] [Google Scholar]
- 9.Searle K. R., Hobbs N. T., Jaronski S. R. 2010. Asynchrony, fragmentation and scale determine benefits of landscape heterogeneity to mobile herbivores. Oecologia 163, 815–824 10.1007/s00442-010-1610-8 (doi:10.1007/s00442-010-1610-8) [DOI] [PubMed] [Google Scholar]
- 10.Walter W. D., et al. 2009. Regional assessment on influence of landscape configuration and connectivity on range size of white-tailed deer. Landscape Ecol. 24, 1405–1420 10.1007/s10980-009-9374-4 (doi:10.1007/s10980-009-9374-4) [DOI] [Google Scholar]
- 11.Obermaier E., Heisswolf A., Poethke H. J., Randlkofer B., Meiners T. 2008. Plant architecture and vegetation structure: two ways for insect herbivores to escape parasitism. Eur. J. Entomol. 105, 233–240 [Google Scholar]
- 12.Rubenson J., Henry H. T., Dimoulas P. M., Marsh R. L. 2006. The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris). J. Exp. Biol. 209, 2395–2408 10.1242/jeb.02310 (doi:10.1242/jeb.02310) [DOI] [PubMed] [Google Scholar]
- 13.Nathan R., Getz W. M., Revilla E., Holyoak M., Kadmon R., Saltz D., Smouse P. E. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059 10.1073/pnas.0800375105 (doi:10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holyoak M., Casagrandi R., Nathan R., Revilla E., Spiegel O. 2008. Trends and missing parts in the study of movement ecology. Proc. Natl Acad. Sci. USA 105, 19 060–19 065 10.1073/pnas.0800483105 (doi:10.1073/pnas.0800483105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintana F., Wilson R. P., Dell'Arciprete P., Shepard E. L. C., Gomez Laich A. 2010. Women from Venus, men from Mars: inter-sex foraging differences in the Imperial cormorant, a colonial seabird. Oecologia 120, 350–358 [Google Scholar]
- 16.Wilson R., Shepard E. L. C., Liebsch N. 2008. Prying into the intimate details of animal lives: use of a daily diary on animals. Endang. Spec. Res. 4, 123–137 10.3354/esr00064 (doi:10.3354/esr00064) [DOI] [Google Scholar]
- 17.Gleiss A. C., Wilson R. P., Shepard E. L. C. 2011. Making dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2, 23–33 10.1111/j.2041-210X.2010.00057.x (doi:10.1111/j.2041-210X.2010.00057.x) [DOI] [Google Scholar]
- 18.Punta G. E., Saravia J. R. C., Yorio P. M. 1993. The diet and foraging behaviour of two Patagonian cormorants. Mar. Ornithol. 21, 27–36 [Google Scholar]
- 19.Quintana F., Yorio P., Lisnizer N., Gatto A., Soria G. 2004. Diving behavior and foraging areas of the Neotropic Cormorant at a marine colony in Patagonia, Argentina. Wilson Bull. 116, 83–88 10.1676/0043-5643(2004)116[0083:DBAFAO]2.0.CO;2 (doi:10.1676/0043-5643(2004)116[0083:DBAFAO]2.0.CO;2) [DOI] [Google Scholar]
- 20.Shepard E. L. C., Wilson R. P., Halsey L. G., Quintana F., Laich A. G., Gleiss A. C., Liebsch N., Myers A. E., Norman B. 2009. Derivation of body motion via appropriate smoothing of acceleration data. Aquat. Biol. 4, 235–241 10.3354/ab00104 (doi:10.3354/ab00104) [DOI] [Google Scholar]
- 21.Parker G., Paterlini M. C., Volante R. A. 1996. El Fondo Marino. In El Mar Argentino y sus Recursos Pesqueros, vol. 1 (ed. Boschi E.), pp. 65–87 Pesca y Alimentación, Mar del Plata, Argentina: Instituto Nacional de Investigacion y Desarrollo Pesquera, Secretaría de Agricultura [Google Scholar]
- 22.Malacalza V. E., Poretti T. I., Bertollotti N. M. 1994. La dieta de Phalacrocorax albiventer en Punta Leon (Chubut, Argentina) durante la temporada reproductiva. Ornitologia Neotropical 5, 91–97 [Google Scholar]
- 23.Narvarte M., Gonzalez R., Fernandez M. 2006. Comparison of Tehuelche octopus (Octopus tehuelchus) abundance between an open-access fishing ground and a marine protected area: evidence from a direct development species. Fish. Res. 79, 112–119 10.1016/j.fishres.2006.02.013 (doi:10.1016/j.fishres.2006.02.013) [DOI] [Google Scholar]
- 24.Shepard E. L. C., Wilson R. P., Quintana F., Laich A. G., Forman D. W. 2009. Pushed for time or saving on fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proc. R. Soc. B 276, 3149–3155 10.1098/rspb.2009.0683 (doi:10.1098/rspb.2009.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleiss A. C., Dale J. J., Holland K. N., Wilson R. P. 2010. Accelerating estimates of activity-specific metabolic rate in fishes: testing the applicability of acceleration data-loggers. J. Exp. Mar. Biol. Ecol. 385, 85–91 10.1016/j.jembe.2010.01.012 (doi:10.1016/j.jembe.2010.01.012) [DOI] [Google Scholar]
- 26.Halsey L. G., White C. R. 2010. Measuring energetics and behaviour using accelerometry in cane toads Bufo marinus. PLoS ONE 5, e10170. 10.1371/journal.pone.0010170 (doi:10.1371/journal.pone.0010170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahlman A., Wilson R., Svard C., Rosen D. A. S., Trites A. W. 2008. Activity and diving metabolism correlate in Steller sea lion Eumetopias jubatus. Aquat. Biol. 2, 75–84 10.3354/ab00039 (doi:10.3354/ab00039) [DOI] [Google Scholar]
- 28.Green J. A., Halsey L. G., Wilson R. P., Frappell P. B. 2009. Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. J. Exp. Biol. 212, 745–746 10.1242/jeb.030049 (doi:10.1242/jeb.030049) [DOI] [PubMed] [Google Scholar]
- 29.Halsey L. G., Green J. A., Wilson R. P., Frappell P. B. 2009. Accelerometry to estimate energy expenditure during activity: best practice with data loggers. Physiol. Biochem. Zool. 82, 396–404 10.1086/589815 (doi:10.1086/589815) [DOI] [PubMed] [Google Scholar]
- 30.Halsey L. G., Shepard E. L. C., Hulston C. J., Venables M. C., White C. R., Jeukendrup A. E., Wilson R. P. 2008. Acceleration versus heart rate for estimating energy expenditure and speed during locomotion in animals: tests with an easy model species, Homo sapiens. Zoology 111, 231–241 10.1016/j.zool.2007.07.011 (doi:10.1016/j.zool.2007.07.011) [DOI] [PubMed] [Google Scholar]
- 31.Halsey L. G., Shepard E. L. C., Quintana F., Laich A. G., Green J. A., Wilson R. P. 2009. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Phys. A 152, 197–202 10.1016/j.cbpa.2008.09.021 (doi:10.1016/j.cbpa.2008.09.021) [DOI] [PubMed] [Google Scholar]
- 32.Wilson R. P., White C. R., Quintana F., Halsey L. G., Liebsch N., Martin G. R., Butler P. J. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081–1090 10.1111/j.1365-2656.2006.01127.x (doi:10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 33.Gomez Laich A., Wilson R. P., Gleiss A. C., Shepard E. L. C., Quintana F. 2011. Use of overall dynamic body acceleration for estimating energy expenditure in cormorants. Does locomotion in different media affect relationships? J. Exp. Mar. Biol. Ecol. 399, 151–155 [Google Scholar]
- 34.Lovvorn J. R., Watanuki Y., Kato A., Naito Y., Liggins G. A. 2004. Stroke patterns and regulation of swim speed and energy cost in free-ranging Brunnich's guillemots. J. Exp. Biol. 207, 4679–4695 10.1242/jeb.01331 (doi:10.1242/jeb.01331) [DOI] [PubMed] [Google Scholar]
- 35.Quintana F., Wilson R. P., Yorio P. 2007. Dive depth and plumage air in wettable birds: the extraordinary case of the imperial cormorant. Mar. Ecol. Progr. Ser. 334, 299–310 10.3354/meps334299 (doi:10.3354/meps334299) [DOI] [Google Scholar]
- 36.Butler P. J., Jones D. R. 1997. Physiology of diving birds and mammals. Physiol. Rev. 77, 837–899 [DOI] [PubMed] [Google Scholar]
- 37.Zimmer I., Wilson R. P., Beaulieu M., Ropert-Coudert Y., Kato A., Ancel A., Plotz J. 2010. Dive efficiency versus depth in foraging emperor penguins. Aquat. Biol. 8, 269–277 10.3354/ab00213 (doi:10.3354/ab00213) [DOI] [Google Scholar]
- 38.Carbone C., Houston A. 1996. The optimal allocation of time over the dive cycle: an approach based on aerobic and anaerobic respiration. Anim. Behav. 51, 1247–1255 10.1006/anbe.1996.0129 (doi:10.1006/anbe.1996.0129) [DOI] [Google Scholar]
- 39.Wilson R. P., Hustler K., Ryan P. G., Burger A. E., Noldeke E. C. 1992. Diving birds in cold water: do Archimedes and Boyle determine energetic costs? Am. Nat. 140, 179–200 10.1086/285409 (doi:10.1086/285409) [DOI] [Google Scholar]
- 40.Williams T. M., Davis R. W., Fuiman L. A., Francis J., le Boeuf B. L., Horning M., Calambokis J., Croll D. A. 2000. Sink or swim: strategies for cost efficient diving by marine mammals. Science 288, 133–136 10.1126/science.288.5463.133 (doi:10.1126/science.288.5463.133) [DOI] [PubMed] [Google Scholar]
- 41.Wilson R. P., Vargas F. H., Steinfurth A., Riordan P., Ropert-Coudert Y., MacDonald D. W. 2008. What grounds some birds for life? Movement and diving in the sexually dimorphic Galapagos Cormorant. Ecol. Monogr. 78, 633–652 10.1890/07-0677.1 (doi:10.1890/07-0677.1) [DOI] [Google Scholar]
- 42.Kubetzki U., Garthe S., Fifield D., Mendel B., Furness R. W. 2009. Individual migratory schedules and wintering areas of northern gannets. Mar. Ecol. Prog. Ser. 391, 257–265 10.3354/meps08254 (doi:10.3354/meps08254) [DOI] [Google Scholar]
- 43.Ballance L. T., Ainley D. G., Ballard G., Barton K. 2009. An energetic correlate between colony size and foraging effort in seabirds, an example of the Adelie penguin Pygoscelis adeliae. J. Avian Biol. 40, 279–288 10.1111/j.1600-048X.2008.04538.x (doi:10.1111/j.1600-048X.2008.04538.x) [DOI] [Google Scholar]
- 44.Birt V. L., Birt T. P., Goulet D., Cairns D. K., Montevecchi W. A. 1987. Ashmole Halo: direct evidence for prey depletion by a seabird. Mar. Ecol. Prog. Ser. 40, 205–208 10.3354/meps040205 (doi:10.3354/meps040205) [DOI] [Google Scholar]
- 45.Elliott K. H., Woo K. J., Gaston A. J., Benvenuti S., Dall'Antonia L., Davoren G. K. 2009. Central-place foraging in an Arctic seabird evidence for Storer–Ashmole's halo. Auk 126, 613–625 10.1525/auk.2009.08245 (doi:10.1525/auk.2009.08245) [DOI] [Google Scholar]
- 46.Fretwell S. D. 1972. Populations in a seasonal environment. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 47.Olsson O., Brown J. S., Helf K. L. 2008. A guide to central place effects in foraging. Theor. Popul. Biol. 74, 22–33 10.1016/j.tpb.2008.04.005 (doi:10.1016/j.tpb.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 48.van Gils J. A., Tijsen W. 2007. Short-term foraging costs and long-term fueling rates in central-place foraging swans revealed by giving-up exploitation times. Am. Nat. 169, 609–620 10.1086/513114 (doi:10.1086/513114) [DOI] [PubMed] [Google Scholar]
- 49.Brown C. R., Stutchbury B. J., Walsh P. D. 1990. Choice of colony size in birds. Trends Ecol. Evol. 5, 398–403 10.1016/0169-5347(90)90023-7 (doi:10.1016/0169-5347(90)90023-7) [DOI] [PubMed] [Google Scholar]
- 50.Forbes L. S., Jajam M., Kaiser G. W. 2000. Habitat constraints and spatial bias in seabird colony distributions. Ecography 23, 575–578 10.1034/j.1600-0587.2000.230508.x (doi:10.1034/j.1600-0587.2000.230508.x) [DOI] [Google Scholar]
- 51.Gremillet D., Dell'Omo G., Ryan P. G., Peters G., Ropert-Coudert Y., Weeks S. J. 2004. Offshore diplomacy, or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of Cape gannets from neighbouring colonies. Mar. Ecol. Prog. Ser. 268, 265–279 10.3354/meps268265 (doi:10.3354/meps268265) [DOI] [Google Scholar]
- 52.Thorpe S. K. S., Crompton R. H., Alexander R. M. 2007. Orangutans use compliant branches to lower the energetic cost of locomotion. Biol. Lett. 3, 253–256 10.1098/rsbl.2007.0049 (doi:10.1098/rsbl.2007.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]