Abstract
In many species, each female pairs with a single male for the purpose of rearing offspring, but may also engage in extra-pair copulations. Despite the prevalence of such promiscuity, whether and how multiple mating benefits females remains an open question. Multiple mating is typically thought to be favoured primarily through indirect benefits (i.e. heritable effects on the fitness of offspring). This prediction has been repeatedly tested in a variety of species, but the evidence has been equivocal, perhaps because such studies have focused on pre-reproductive survival rather than lifetime fitness of offspring. Here, we show that in a songbird, the dark-eyed junco (Junco hyemalis), both male and female offspring produced by extra-pair fertilizations have higher lifetime reproductive success than do offspring sired within the social pair. Furthermore, adult male offspring sired via extra-pair matings are more likely to sire extra-pair offspring (EPO) themselves, suggesting that fitness benefits to males accrue primarily through enhanced mating success. By contrast, female EPO benefited primarily through enhanced fecundity. Our results provide strong support for the hypothesis that the evolution of extra-pair mating by females is favoured by indirect benefits and shows that such benefits accrue much later in the offspring's life than previously documented.
Keywords: extra-pair mating, multiple mating, lifetime reproductive success, indirect fitness benefits, sexual selection
1. Introduction
Promiscuity, or the tendency to mate with multiple partners, has been observed in most species in which it has been looked for, suggesting that multiple mating is often favoured by selection [1]. Although the benefits of multiple mating are obvious for males, female reproductive success tends to be limited by resources rather than sperm [2], and it is therefore less clear why females should seek copulations with more than one male. An evolutionary advantage to female promiscuity may arise from a direct fitness benefit if engaging in multiple mating increases the number of offspring a female can produce (e.g. if males provide nuptial gifts) [3]. However, in many species, males contribute only genetic material, suggesting that direct benefits cannot suffice as a general explanation for female promiscuity [4]. Females that mate with multiple males may also receive indirect benefits through an increase in the genetic quality of their offspring. Such a scenario may occur if the males chosen as sires possess alleles that are either universally favoured in the population (‘good genes’) or lead to favourable combinations with the female's alleles (‘compatible genes’) [5–8].
The indirect benefits hypothesis has been used in particular to explain the evolution of extra-pair mating in species that form social pair bonds [9]. Extra-pair mating behaviour in such socially monogamous species is common and is often female-initiated [10–12], but evidence suggests that females rarely, if ever, benefit directly from extra-pair mating [13,14]. Therefore, extra-pair mating is thought to be advantageous to females primarily via indirect benefits (i.e. the effects of the genetic contribution of extra-pair males on offspring fitness) [4,9,13].
The main prediction of the indirect benefits hypothesis is that extra-pair offspring (EPO) should have higher lifetime fitness than their within-pair counterparts (WPO). This prediction arises because with an even sex ratio, nearly all females will be able to form social pairs, but genetically superior or compatible males will represent a small subset of available social males. Therefore, if females use extra-pair copulations to genetically ‘trade up’ when possible, on average EPO should be sired by superior or more compatible males. Although tests of this prediction are common, their results are mixed, and a recent meta-analysis of over 120 studies concluded that they offer only equivocal support [15]. However, all but a few of these studies [16–18] have estimated offspring fitness using some proxy for total fitness rather than by directly quantifying adult reproductive success. In particular, most have focused on pre-reproductive fitness components such as size at independence or survival to first breeding, which may only be weakly correlated to lifetime fitness [15]. Direct measures of the reproductive success of offspring (i.e. the number of offspring they themselves produce) are needed to fully evaluate the contribution of indirect benefits to the evolution of multiple mating by females [19,20].
In this study, we use a long-term dataset on the demography, reproduction and paternity of a free-living population of dark-eyed juncos (Junco hyemalis), a songbird with an appreciable rate of extra-pair paternity (EPP; 28% EPO [21]), to directly compare the reproductive performance of adult EPO and WPO (hereafter F1 EPO and F1 WPO; individuals in the preceding and following generations will be denoted F0 and F2, respectively; electronic supplementary material, figure S1). Only one previous study (of another songbird species) has analysed the effect of paternity on the reproduction of F1 offspring [16–18]. However, this study was unable to assess the extra-pair siring success of F1 males, which can comprise a major component of lifetime fitness, and thus of the potential indirect benefit of promiscuity [22–24]. In this study, we present for the first time a comparison of the lifetime genetic reproductive success of both male and female F1 EPO and F1 WPO, including fitness variance arising through extra-pair mating among F1 individuals, providing a unique and robust test of the indirect benefits hypothesis.
2. Material and methods
(a). Field methods
This study is based on a population of dark-eyed juncos (J. h. carolinensis) that breeds at and around the Mountain Lake Biological Station in Giles County, western Virginia, USA [25]. Data on capture rate, demography and reproduction in this population have been collected since 1983, while blood sampling for DNA analysis began in 1990 (see Reed et al. [26] for details of field methodology). In brief, during the years of 1990–2007, adults were captured in baited mist nets during the pre-breeding season (early April to 15 May), given a unique colour band combination, and a small blood sample was drawn from the wing vein.
Throughout the breeding season, researchers searched for and located nests. At each nest, social parents were identified based on observations of parental care and nest-defence behaviours. Blood samples from nestlings were collected 6 days post-hatching, and nest fate continued to be tracked until the nestlings left the nest (‘fledged’) at 10–12 days post-hatching. Only nestlings that were seen/captured on or after fledging day were considered in these analyses as having fledged successfully. Longevity of all adult birds was determined by the minimum number of years that they were known to be alive on our study site, based on capture and nesting records.
(b). Genotyping and paternity analyses
Blood samples from 1990 to 1996 were genotyped using a combination of minisatellites and microsatellites; paternity analyses for these years were conducted using band-sharing analyses [27]. Samples from 1997 to 2007 were genotyped at nine microsatellite loci (see electronic supplementary material, table S1 for microsatellite information); only alleles that amplified consistently in multiple genotyping runs were used in subsequent paternity analyses. On average, 95 per cent of individuals were typed at each locus, and the combined non-exclusion probability for all nine loci was 5.98 × 10−10 for each parental pair (see electronic supplementary material, table S2 for genotyping information).
Paternity was determined using the program CERVUS [28,29]. Only individuals that were genotyped at four or more loci were included; we estimated the proportion of sampled males to be 0.9. Each year was analysed separately, with all living males in that year included as potential sires for all nestlings. Males were considered to be an offspring's genetic sire when the trio of offspring–mother–putative sire was assigned by CERVUS with a positive likelihood of detection (LOD) and a ΔLOD with a confidence value of 95 per cent. If no sire was assigned by CERVUS at that confidence level, but the LOD of the social father was low (less than −8.0; average LOD for assigned fathers: 6.33 ± 0.15), then the nestling was assumed to be an EPO but was not assigned a genetic father.
(c). Measures of adult reproduction
Only those F1 offspring that returned to the population as adults were included in these analyses. In order to keep values of genetic reproductive success comparable between the sexes, we calculated all measures of F1 reproductive success as the number of genotyped F2 offspring produced. Analyses of male extra-pair and within-pair reproductive success included all returning males, as some individuals that did not have a social mate still produced offspring via extra-pair fertilizations. However, loss of paternity was calculated only for males whose social mate produced at least one genotyped offspring. Measures of F2 EPO and F2 WPO production were calculated both for all returning females (so as to measure the contribution of each type of F2 offspring to total reproductive success) and for females that produced at least one genotyped offspring (so as to compare the effect of F1 paternity on F2 paternity distribution).
(d). Testosterone treatment
During portions of the 18 years of our study, individuals were given exogenous testosterone (T) implants (males: 1993–2000; females: 2001–2002 and 2005–2007) [26,30,31]. Because testosterone significantly increases reproductive success in males [26] and decreases reproductive success in females [30], we included the number of years an individual received a testosterone implant (as the percentage of total breeding tenure; ‘%T years’) as a covariate in all analyses of reproductive performance based on paternity.
(e). Statistical analyses
All statistical analyses were conducted using ASReml3 [32]. Measures of F1 reproductive success, longevity and extra-pair behaviour were compared using a generalized linear model (GLM) with a Poisson error structure, with birth year, mother identity and nest identity included as random effects to control for maternal and environmental effects, paternity as a categorical fixed effect, and Julian hatch date, maternal age at hatching and %T years included as continuous fixed effects. For analyses in which the sexes were considered together, sex, sex × paternity and sex × %T years were also included as fixed effects, although this last interaction term was non-significant and was removed from the final model [33]. In the text, we report on the primary variable of interest (paternity); we provide the full results of the GLM for each variable in the electronic supplementary material, tables S3–S6.
3. Results
Out of 2182 offspring of known paternity produced between 1990 and 2007, 35 EPO (17 females and 18 males) and 108 WPO (48 females and 60 males) returned to our population as adults, an overall return rate of 6.6 per cent; the relative proportion of successful fledglings that returned as adults was independent of whether they were EPO or WPO (females: 17.4% of EPO, 19.0% of WPO, χ2 = 0.12, p = 0.725; males: 16.1% of EPO, 19.5% of WPO, χ2 = 0.65, p = 0.419). It was rare that more than one individual from a nest returned to our population; the 143 returning F1 offspring were from 129 nests produced by 118 different F0 mothers. However, to account for effects of early environment and maternal genetics, we statistically controlled for nest and maternal identity, as well as other potential confounding factors, in all comparisons below. While the significance of several of these factors varied between analyses, the percentage of breeding years during which a bird was implanted with testosterone did not significantly predict any of our measures of reproductive success or extra-pair behaviour (we report the effects of paternity below; see the electronic supplementary material for full model results).
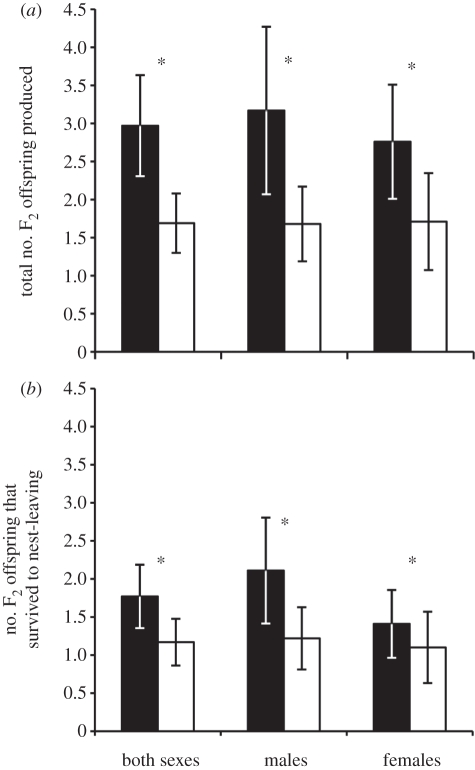
As determined by paternity analysis of 6-day-old offspring, F1 EPO that returned to our population as adults produced more genetic F2 offspring than did F1 WPO, regardless of their sex (figure 1a; this and all subsequent tests are a GLM: sexes combined: paternity F1,136 = 16.71, p < 0.001; sex F1,136 = 0.40, p = 0.527; males: F1,45.2 = 5.60, p = 0.022; females: F1,60 = 16.21, p < 0.001). The paternity of F1 individuals also had a significant effect on the number of genetic F2 offspring that survived to nest-leaving when the sexes were considered together (figure 1b; paternity F1,136 = 6.57, p = 0.012; sex F1,136 = 1.52, p = 0.222). When the sexes were considered separately, this effect held for both sexes but was stronger in females (figure 1b; males: F1,50.7 = 4.02, p = 0.050; females: F1,60 = 6.27, p = 0.015). This greater lifetime reproductive success of adult male and female F1 EPO was not the result of differences in longevity, as paternity did not predict the number of years that an individual was present in our population (mean ± s.e.m.: EPO = 2.00 ± 0.20; WPO = 1.80 ± 0.11; GLM: paternity F1,136 = 1.15, p = 0.287; sex F1,136 = 0.64, p = 0.424).
Figure 1.
Adult reproductive success of extra-pair and within-pair offspring. Bars represent uncorrected mean ± s.e.m.; for full model details see electronic supplementary material, tables S3–S5. Asterisks indicate significance at p < 0.05. (a) F1 EPO (black bars) produced significantly more total F2 offspring than did F1 WPO (white bars), both when the sexes were considered together (n = 143) and separately (males, n = 78; females, n = 65). (b) When considering only those F2 offspring that survived to nest-leaving, F1 EPO had higher reproductive success than did F1 WPO both when the sexes were considered together and separately.
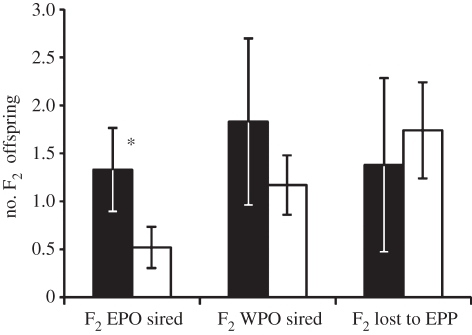
In males, increased reproductive success of EPO was largely driven by differences in extra-pair siring success. Adult male F1 EPO sired more F2 EPO than did adult male F1 WPO (figure 2; F1,24.1 = 8.15, p = 0.009). This increase in extra-pair siring success also meant that F1 EPO males had marginally higher mating success (i.e. number of different females with which they sired offspring) than did F1 WPO males (mean ± s.e.m.: EPO = 1.00 ± 0.26; WPO = 0.60 ± 0.14; GLM: paternity F1,44 = 3.87, p = 0.055).
Figure 2.
Male extra-pair and within-pair siring success. Adult male F1 EPO (black bars) sired significantly more F2 EPO than did male F1 WPO (white bars), but they did not differ in the number of F2 WPO they sired. Among males whose social mates produced at least one genotyped offspring (n = 27), male F1 EPO did not differ from F1 WPO in the number of their social offspring that were lost to extra-pair paternity (EPP). In all cases, bars represent uncorrected means ± s.e.m.; for full model details see electronic supplementary material, tables S4 and S6. Asterisks indicate significance at p < 0.05.
Adult male F1 EPO had greater genetic and extra-pair reproductive success than male F1 WPO but did not differ in their reproductive success in their home nests. That is, male F1 EPO did not differ from F1 WPO in the total number of F2 WPO they sired (figure 2; F1,55.6 = 2.04, p = 0.158). Furthermore, among males whose mates produced at least one genotyped social offspring, male F1 EPO did not differ significantly from F1 WPO in the number of their social offspring that were lost to EPP (figure 2; F1,22 = 0.77, p = 0.390).
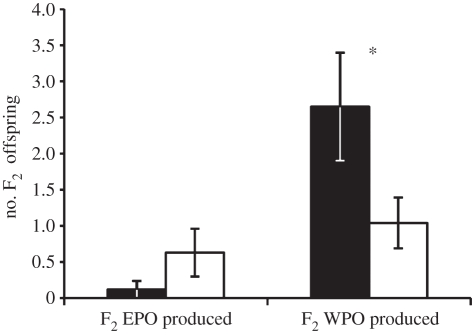
In contrast, the reproductive superiority of female F1 EPO derived primarily from increased production of F2 WPO. Female F1 EPO produced significantly more F2 WPO than did female F1 WPO (figure 3; F1,60 = 17.84, p < 0.001). This effect probably arises because female F1 EPO produced more nests or eggs in their lifetime (i.e. they were more fecund) or because their offspring were more successful at reaching the nestling stage, perhaps owing to enhanced parental care or nest defence. Female F1 EPO and F1 WPO did not differ in the number of F2 EPO they produced (figure 3; F1,60 = 0.80, p = 0.374).
Figure 3.
Female extra-pair and within-pair reproductive success. F1 EPO females (black bars) did not differ from F1 WPO (white bars) in their number of F2 EPO, but female F1 EPO produced significantly more F2 WPO than did female F1 WPO. Bars represent uncorrected means ± s.e.m.; for full model details see electronic supplementary material, tables S5 and S6. Asterisks indicate significance at p < 0.05.
Among females that produced at least one genotyped offspring, F1 EPO and F1 WPO did not differ in the distribution of paternity of their own offspring; that is, F1 EPO females did not significantly differ from F1 WPO females in their number of F2 EPO (mean ± s.e.m.: F1 EPO = 0.22 ± 0.22; F1 WPO = 2.14 ± 1.05; GLM: paternity F1,18 = 2.64, p = 0.121) or F2 WPO (mean ± s.e.m.: F1 EPO = 5.00 ± 0.80; F1 WPO = 3.57 ± 0.92; GLM: paternity F1,16.5 = 0.48, p = 0.497). Furthermore, these F1 EPO females did not differ significantly from F1 WPO females in the number of males that sired their offspring (mean ± s.e.m.: EPO = 1.44 ± 0.24; WPO = 2.00 ± 0.49; GLM: paternity F1,18 = 0.87, p = 0.364).
4. Discussion
In this study, we show for both sexes of the dark-eyed junco that EPO have higher lifetime reproductive success than WPO. To our knowledge, this is the first case in which EPP has been shown to increase lifetime reproductive success of adult offspring in a free-living songbird. In the only similar previous study, the reproductive success of adult female F1 EPO coal tits (Parus ater) did not differ from that of female F1 WPO, and adult male F1 EPO had lower apparent (social) reproductive success than did male F1 WPO. However, extra-pair siring success of F1 males was not measured, and therefore adult genetic reproductive success could not be calculated [16–18].
In many species, there is no obvious benefit to female fitness from mating with an extra-pair male, and some have even hypothesized that extra-pair mating should carry a cost to offspring production or survival [34,35] (although not in juncos [21]; see also [36]). This lack of a cost to females, combined with the observed twofold benefit in F2 offspring production by F1 EPO versus F1 WPO, indicates that extra-pair mating is adaptive for females because it allows them to produce offspring of higher reproductive quality.
The difference between F1 EPO and F1 WPO in adult reproductive success persists even when controlling for both maternal and early environmental effects (electronic supplementary material, tables S3–S6), suggesting that this difference probably depends upon the genetic contribution of extra-pair sires. Such a contribution may arise either from alleles that are universally favoured (‘good genes’) or those that form favourable combinations with a female's alleles (‘compatible genes’) [8,9,37]. Although our data strongly suggest the presence of genetic indirect benefits, they do not allow us to distinguish between these two competing hypotheses. Such a test requires partitioning indirect benefits into additive and non-additive genetic components [38], which is not feasible given the low rate of return of nestlings to our population (10.4% of banded fledglings, which is typical of most songbirds).
Another possibility is that EPO outperform WPO for a reason unrelated to genetic quality, such as a maternal effect that allows females to bias care or provisioning towards EPO. For example, in several species, EPO have been shown to be clustered in the first-laid eggs, and thus have a size and survival advantage over their nest-mates [39–42]. However, species in which this pattern has been found are also species in which clutches hatch asynchronously; in the junco, the majority of clutches hatch synchronously (i.e. less than 24 h between first- and last-hatched), and thus there is no detectable size difference between nestlings based on hatching order [43]. Similarly, while older females tend to be both better parents and more likely to engage in extra-pair mating [44–46], our findings remained significant when controlling for F0 maternal age (electronic supplementary material, tables S3–S6). In fact, males (but not females) produced by older mothers were actually less successful, contrary to the predicted relationship (electronic supplementary material, table S4). Although we have no direct data on provisioning of individual offspring in the junco, evidence from other avian species does not support the idea that parents can differentially allocate care within a brood based on offspring paternity [47–51]. However, while we have accounted for some potential sources of maternal or environmental variation, we cannot completely rule out the possibility that females that produce EPO also vary in some way that affects offspring quality. To do so would require a larger sample of returning maternal half-sibs than is feasible in a free-living population with a low rate of recruitment.
In male juncos, the increased reproductive success of F1 EPO was primarily owing to increased extra-pair siring success. This relationship between paternity and adult male extra-pair behaviour may have several explanations that are not mutually exclusive. If extra-pair copulations are primarily initiated by males, then the higher rate of extra-pair fertilizations gained by adult male F1 EPO may indicate a heritable tendency to engage in multiple mating, a phenomenon that has been reported in male red squirrels [52] and female song sparrows [53]. This relationship between a male having been sired by an extra-pair male and later exhibiting success as an extra-pair sire himself may thus arise owing to heritable variation in allocation to mating effort.
Alternatively, if extra-pair copulations are primarily initiated by females, our results may indicate that extra-pair sires tend to produce more attractive sons [24,54]. While we did not find differences between 1-year-old F1 EPO and F1 WPO in any of the phenotypic traits we measured (electronic supplementary material, table S7), they may differ in some other attractive trait, such as song. However, regardless of its cause, this multi-generational tendency of males to sire EPO supports the idea that the evolution of female promiscuity may be at least partially driven by the mating success of her extra-pair sons [54,55].
Females that engage in extra-pair mating acquire indirect fitness benefits not only through their sons, but also through their daughters. Female F1 EPO benefited through increased nestling and fledgling production, suggesting that they may be more fecund, better parents or both. Although our sample size was small, our data do not suggest that paternity affects the allocation of an F1 female to F2 WPO versus F2 EPO. This may indicate that there is no additive genetic variation for extra-pair mating by females, either as a result of strong past selection, or because extra-pair matings are initiated opportunistically by males. Similarly, the fact that extra-pair fathers are not more likely to have daughters that engage in extra-pair mating suggests that female and male participation in extra-pair behaviour may not share a common genetic basis as it does in zebra finches [56]. Alternatively, if female F1 EPO are of higher quality, as indicated by their increased reproductive success, it may be the case that they are more likely to pair with preferred high-quality or compatible social males, and therefore would not gain an additional genetic benefit from mating with extra-pair males.
Regardless of the mechanism by which F1 EPO outperform F1 WPO, our results in the junco provide support for the idea that F0 females acquire indirect fitness benefits through extra-pair mating by producing F1 offspring with higher reproductive output, a process that should favour the evolution of female extra-pair behaviour. Most studies to date that have tested the indirect benefits hypothesis of extra-pair mating have compared the relative fitness of WPO and EPO using proxies such as nestling size and juvenile survival to measure offspring quality. There have been many such studies, but collectively their results have been inconclusive, perhaps because traits measured during an individual's first year of life are not always accurate predictors of adult reproductive success [15]. Indeed, in the junco, we found no differences between EPO and WPO in any measure of their quality in their first year of life, including mass, immune function or survival [57], suggesting that fitness differences based on genetic quality may arise later in life than typically assumed. Additional studies in which F1 offspring fitness is measured directly by quantifying lifetime reproductive success, rather than indirectly by using proxy measures of quality, will be crucial for understanding the evolution of extra-pair behaviour and mate choice, and assessing the mechanisms and consequences of sexual selection [15,20].
Acknowledgements
All procedures used in this study were approved by the Bloomington Institutional Animal Care and Use Committee.
Thanks to E. Brodie III and members of the Ketterson research group for comments on the manuscript; Mountain Lake Biological Station and the Mountain Lake Hotel for facilitating fieldwork on their properties; B. Harr for providing microsatellite primers; the Center for the Integrative Study of Animal Behavior for assistance in the laboratory; E. Snajdr for help with data compilation; and the many people who contributed to collecting the long-term field data. This work was supported by NSF BSR 91-114980, NSF IBN 94-08061, NSF IBN 97-28384 and NSF IBN 02-16091, NSF BSC-05-19211 and NSF IOS-0820055 to E.D.K., NSF DDIG 0808051 to N.M.G., and NSF DDIG 0508692 to J.W.M.
References
- 1.Birkhead T. R., Møller A. P. 1998. Sperm competition and sexual selection. London, UK: Academic Press [Google Scholar]
- 2.Bateman A. J. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 10.1038/hdy.1948.21 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 3.Arnqvist G., Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 10.1006/anbe.2000.1446 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 4.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Iwasa Y., Pomiankowski A. 1991. The evolution of costly mate preferences. II. The handicap principle. Evolution 45, 1431–1442 10.2307/2409890 (doi:10.2307/2409890) [DOI] [PubMed] [Google Scholar]
- 6.Colegrave N., Kotiaho J. S., Tomkins J. L. 2002. Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection. Evol. Ecol. Res. 4, 911–917 [Google Scholar]
- 7.Mays H. L., Jr, Hill G. E. 2004. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 19, 554–559 10.1016/j.tree.2004.07.018 (doi:10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- 8.Pryke S. R., Rollins L. A., Griffith S. C. 2010. Females use multiple mating and genetically loaded sperm competition to target compatible genes. Science 329, 964–967 10.1126/science.1192407 (doi:10.1126/science.1192407) [DOI] [PubMed] [Google Scholar]
- 9.Jennions M. D., Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 75, 21–64 10.1017/S0006323199005423 (doi:10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 10.Griffith S. C., Owens I. P. F., Thuman K. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212 10.1046/j.1365-294X.2002.01613.x (doi:10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 11.Drevon T., Slagsvold T. 2005. When and from whom do female pied flycatchers (Ficedula hypoleuca) solicit copulations. Behaviour 142, 1059–1076 10.1163/156853905774405335 (doi:10.1163/156853905774405335) [DOI] [Google Scholar]
- 12.Double M. C., Cockburn A. 2000. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. Lond. B 267, 465–470 10.1098/rspb.2000.1023 (doi:10.1098/rspb.2000.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westneat D. F., Sherman P. W., Morton M. L. 1990. The ecology and evolution of extra-pair copulations in birds. Curr. Ornithol. 7, 331–369 [Google Scholar]
- 14.Westneat D. F., Stewart I. 2003. Extra-pair paternity in birds: causes, correlates and conflict. Annu. Rev. Ecol. Evol. Syst. 34, 365–396 10.1146/annurev.ecolsys.34.011802.132439 (doi:10.1146/annurev.ecolsys.34.011802.132439) [DOI] [Google Scholar]
- 15.Akçay E., Roughgarden J. 2007. Extra-pair paternity in birds: review of the genetic benefits. Evol. Ecol. Res. 9, 855–868 [Google Scholar]
- 16.Schmoll T., Dietrich-Bischoff V., Winkel W., Epplen J. T., Lubjuhn T. 2003. Long-term fitness consequences of female extra-pair matings in a socially monogamous passerine. Proc. R. Soc. Lond. B 270, 259–264 10.1098/rspb.2002.2216 (doi:10.1098/rspb.2002.2216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmoll T., Dietrich-Bischoff V., Winkel W., Epplen J. T., Schurr F., Lubjuhn T. 2005. Paternal genetic effects on offspring fitness are context dependent within the extrapair mating system of a socially monogamous passerine. Evolution 59, 645–657 [PubMed] [Google Scholar]
- 18.Schmoll T., Schurr F. M., Winkel W., Epplen J. T., Lubjuhn T. 2009. Lifespan, lifetime reproductive performance and paternity loss of within-pair and extra-pair offspring in the coal tit Periparus ater. Proc. R. Soc. B 276, 337–345 10.1098/rspb.2008.1116 (doi:10.1098/rspb.2008.1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt J., Bussiere L. F., Jennions M. D., Brooks R. 2004. What is genetic quality? Trends Ecol. Evol. 19, 329–333 10.1016/j.tree.2004.03.035 (doi:10.1016/j.tree.2004.03.035) [DOI] [PubMed] [Google Scholar]
- 20.Arnqvist G., Kirkpatrick M. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 165, S26–S37 10.1086/429350 (doi:10.1086/429350) [DOI] [PubMed] [Google Scholar]
- 21.Ketterson E. D., Parker P. G., Raouf S. A., Nolan V., Jr, Ziegenfus C., Chandler C. R. 1997. The relative impact of extra-pair fertilizations on variation in male and female reproductive success in dark-eyed juncos (Junco hyemalis). Ornithol. Monogr. 49, 81–101 [Google Scholar]
- 22.Fisher R. A. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- 23.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 10.1073/pnas.78.6.3721 (doi:10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokko H., Brooks R., McNamara J., Houston A. 2002. The sexual selection continuum. Proc. R. Soc. Lond. B 269, 1331–1340 10.1098/rspb.2002.2020 (doi:10.1098/rspb.2002.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler C. R., Ketterson E. D., Nolan V., Jr, Ziegenfus C. 1994. Effects of testosterone on spatial activity in free-ranging male dark-eyed juncos, Junco hyemalis. Anim. Behav. 47, 1445–1455 10.1006/anbe.1994.1191 (doi:10.1006/anbe.1994.1191) [DOI] [Google Scholar]
- 26.Reed W., Clark M., Parker P. G., Raouf S. A., Arguedas N., Monk D., Snajdr E. A., Nolan V., Jr, Ketterson E. D. 2006. Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness. Am. Nat. 167, 667–683 10.1086/503054 (doi:10.1086/503054) [DOI] [PubMed] [Google Scholar]
- 27.Raouf S. A., Parker P. G., Ketterson E. D., Nolan V., Jr, Ziegenfus C. 1997. Testosterone affects reproductive success by influencing extra-pair fertilizations in male dark-eyed juncos (Aves: Junco hyemalis). Proc. R. Soc. Lond. B 264, 1599–1603 10.1098/rspb.1997.0223 (doi:10.1098/rspb.1997.0223) [DOI] [Google Scholar]
- 28.Marshall T., Slate J., Kruuk L. E. B., Pemberton J. M. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655 10.1046/j.1365-294x.1998.00374.x (doi:10.1046/j.1365-294x.1998.00374.x) [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski S., Taper M. L., Marshall T. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 10.1111/j.1365-294X.2007.03089.x (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 30.O'Neal D. M., Reichard D. G., Pavilis K., Ketterson E. D. 2008. Experimentally-elevated testosterone, female parental care, and reproductive success in a songbird, the dark-eyed junco (Junco hyemalis). Horm. Behav. 54, 571–578 10.1016/j.yhbeh.2008.05.017 (doi:10.1016/j.yhbeh.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 31.Clotfelter E. D., O'Neal D. M., Gaudioso J. M., Casto J. M., Parker-Renga I. M., Snajdr E. A., Duffy D. L., Nolan V., Jr, Ketterson E. D. 2004. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 46, 171–178 10.1016/j.yhbeh.2004.03.003 (doi:10.1016/j.yhbeh.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 32.Gilmour A. R., Gogel B. J., Cullis B. R., Thompson R. 2009. ASReml user guide release 3.0. Hemel Hempstead, UK: VSN International Ltd [Google Scholar]
- 33.Engqvist L. 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971 10.1016/j.anbehav.2005.01.016 (doi:10.1016/j.anbehav.2005.01.016) [DOI] [Google Scholar]
- 34.Whittingham L. A., Taylor P. D., Robertson R. J. 1992. Confidence of paternity and male parental care. Am. Nat. 139, 1115–1125 10.1086/285376 (doi:10.1086/285376) [DOI] [Google Scholar]
- 35.Owens I. 1993. When kids just aren't worth it: cuckoldry and parental care. Trends Ecol. Evol. 8, 269–271 10.1016/0169-5347(93)90251-J (doi:10.1016/0169-5347(93)90251-J) [DOI] [PubMed] [Google Scholar]
- 36.Gerlach N. M., McGlothlin J. W., Parker P. G., Ketterson E. D. Interpreting positive Bateman gradients in female and male dark-eyed juncos. Submitted. [Google Scholar]
- 37.Hettyey A., Hegyi G., Puurtinen M., Hoi H., Torok J., Penn D. J. 2010. Mate choice for genetic benefits: time to put the pieces together. Ethology 116, 1–9 10.1111/j.1439-0310.2009.01704.x (doi:10.1111/j.1439-0310.2009.01704.x)21132114 [DOI] [Google Scholar]
- 38.Neff B. D., Pitcher T. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38 10.1111/j.1365-294X.2004.02395.x (doi:10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 39.Magrath M. J. L., Vedder O., van der Velde M., Komdeur J. 2009. Maternal effects contribute to the superior performance of extra-pair offspring. Curr. Biol. 19, 792–797 10.1016/j.cub.2009.03.068 (doi:10.1016/j.cub.2009.03.068) [DOI] [PubMed] [Google Scholar]
- 40.Ferree E. D., Dickinson J., Rendell W., Stern C., Porter S. 2010. Hatching order explains an extrapair chick advantage in western bluebirds. Behav. Ecol. 21, 802–807 10.1093/beheco/arq056 (doi:10.1093/beheco/arq056) [DOI] [Google Scholar]
- 41.Cordero P. J., Wetton J. H., Parkin D. T. 1999. Within-clutch patterns of egg viability and paternity in the house sparrow. J. Avian Biol. 30, 103–107 10.2307/3677249 (doi:10.2307/3677249) [DOI] [Google Scholar]
- 42.Krist M., Nadvornik P., Uvirova L., Bures S. 2005. Paternity covaries with laying and hatching order in the collared flycatcher Ficedula albicollis. Behav. Ecol. Sociobiol. 59, 6–11 10.1007/s00265-005-0002-2 (doi:10.1007/s00265-005-0002-2) [DOI] [Google Scholar]
- 43.Nolan V., Jr, Ketterson E. D., Cristol D. A., Rogers C. M., Clotfelter E. D., Titus R. C., Schoech S. J., Snajdr E. 2002. Dark-eyed junco (Junco hyemalis). In The birds of North America, no. 716 (eds Poole A., Gill F.). Philadelphia, PA: The Birds of North America, Inc [Google Scholar]
- 44.Gowaty P. 1996. Battles of the sexes and origins of monogamy. In Partnerships in birds: the study of monogamy (ed. Black J. M.), pp. 21–52 Oxford, UK: Oxford University Press [Google Scholar]
- 45.Bouwman K. M., Komdeur J. 2005. Old female reed buntings (Emberiza schoeniclus) increase extra-pair paternity in their broods when mated to young males. Behaviour 142, 1449–1463 10.1163/156853905774831819 (doi:10.1163/156853905774831819) [DOI] [Google Scholar]
- 46.Grant P. R., Grant B. R. 2011. Causes of lifetime fitness of Darwin's finches in a fluctuating environment. Proc. Natl Acad. Sci. USA 108, 674–679 10.1073/pnas.1018080108 (doi:10.1073/pnas.1018080108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke T., Davies N. B., Bruford M. W., Hatchwell B. J. 1989. Parental care and mating behaviour of polyandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting. Nature 338, 249–251 10.1038/338249a0 (doi:10.1038/338249a0) [DOI] [Google Scholar]
- 48.Westneat D. F., Clark A. B., Rambo K. C. 1995. Within brood patterns of paternity and paternal behavior in red-winged blackbirds. Behav. Ecol. Sociobiol. 37, 349–356 10.1007/BF00174140 (doi:10.1007/BF00174140) [DOI] [Google Scholar]
- 49.Briskie J. V., Montgomerie R., Poldmaa T., Boag P. T. 1998. Paternity and paternal care in the polygynandrous Smith's longspur. Behav. Ecol. Sociobiol. 43, 181–190 10.1007/s002650050479 (doi:10.1007/s002650050479) [DOI] [Google Scholar]
- 50.Peterson K., Thusius K., Whittingham L. A., Dunn P. O. 2001. Allocation of male parental care in relation to paternity within and among broods of the common yellowthroat (Geothlypis trichas). Ethology 107, 573–586 10.1046/j.1439-0310.2001.00676.x (doi:10.1046/j.1439-0310.2001.00676.x) [DOI] [Google Scholar]
- 51.Bouwman K. M., Lessells C. K. M., Komdeur J. 2005. Male reed buntings do not adjust parental effort in relation to extrapair paternity. Behav. Ecol. 16, 499–506 10.1093/beheco/ari021 (doi:10.1093/beheco/ari021) [DOI] [Google Scholar]
- 52.McFarlane S. E., Lane J. E., Taylor R. W., Gorrell J. C., Coltman D. W., Humphries M. M., Boutin S., McAdam A. G. 2010. The heritability of multiple male mating in a promiscuous mammal. Biol. Lett. 7, 368–371 10.1098/rsbl.2010.1003 (doi:10.1098/rsbl.2010.1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid J. M., Arcese P., Sardell R. J., Keller L. F. 2011. Heritability of female extra-pair paternity rate in song sparrows (Melospiza melodia). Proc. R. Soc. B 278, 1114–1120 10.1098/rspb.2010.1704 (doi:10.1098/rspb.2010.1704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weatherhead P. J., Robertson R. J. 1979. Offspring quality and the polygyny threshold: ‘the sexy son hypothesis’. Am. Nat. 113, 201–208 10.1086/283379 (doi:10.1086/283379) [DOI] [Google Scholar]
- 55.Kokko H., Jennions M. D., Brooks R. 2006. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66 10.1146/annurev.ecolsys.37.091305.110259 (doi:10.1146/annurev.ecolsys.37.091305.110259) [DOI] [Google Scholar]
- 56.Forstmeier W., Martin K., Bolund E., Schielzeth H., Kempenaers B. 2011. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl Acad. Sci. USA 108, 10 608–10 613 10.1073/pnas.1103195108 (doi:10.1073/pnas.1103195108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerlach N. M., McGlothlin J. W., Parker P. G., Ketterson E. D. Dark-eyed junco extra-pair offspring are not of higher quality than within-pair offspring in their first year of life. In preparation. [Google Scholar]