Abstract
Africa hosts a single breeding species of penguin today, yet the fossil record indicates that a diverse array of now-extinct taxa once inhabited southern African coastlines. Here, we show that the African penguin fauna had a complex history involving multiple dispersals and extinctions. Phylogenetic analyses and biogeographic reconstructions incorporating new fossil material indicate that, contrary to previous hypotheses, the four Early Pliocene African penguin species do not represent an endemic radiation or direct ancestors of the living Spheniscus demersus (blackfooted penguin). A minimum of three dispersals to Africa, probably assisted by the eastward-flowing Antarctic Circumpolar and South Atlantic currents, occurred during the Late Cenozoic. As regional sea-level fall eliminated islands and reduced offshore breeding areas during the Pliocene, all but one penguin lineage ended in extinction, resulting in today's depleted fauna.
Keywords: biogeography, South Atlantic Gyre, fossil, Pliocene, phylogeny
1. Introduction
An outstanding question in seabird evolution is what drivers influenced biogeography, diversification and extinction in modern (crown clade) penguins. As flightless marine birds capable of travelling for thousands of kilometres [1,2], yet tied to coastal margins for breeding, penguins responded to changes in the southern oceans in ways unique from other seabirds [3–6]. Major shifts in ocean systems appear to have been intimately involved in shaping penguin distribution across the Cenozoic. On the one hand, key currents have aided the biogeographic expansion of penguins by serving as vectors and providing nutrient-rich areas of upwelling that create foraging zones [4,7,8]. On the other hand, thermal clines and south-drifting currents are thought to present a barrier that has prevented penguin dispersal across the equator [9,10]. These factors account for the absence of penguins in the Northern Hemisphere despite their remarkably widespread range in the Southern Hemisphere.
Africa is a notable exception to a general pattern of Early Cenozoic biogeographic expansion by penguins. The fossil record reveals that penguins rapidly dispersed from New Zealand to reach Antarctica, South America and Australia by the Late Eocene [3,11–13]. Several early colonizations ultimately ended in extinctions, but penguins have repeatedly dispersed between continents throughout the Cenozoic, and so maintained their wide austral distribution to the present day [3,6]. Africa appears to have been the last southern continent to be colonized, as the oldest penguin fossils from Africa are approximately 30 Ma younger than the oldest fossils from other major landmasses. Pliocene marine deposits from Africa are nonetheless rich in penguin fossils and record a high level of diversity. Four contemporaneous species are known from Early Pliocene localities [8,14–17] of the celebrated Varswater Formation [18]. The presence of bones from unfledged chicks (figure 1g–j) further indicates that at least one species was breeding in South Africa during the Pliocene.
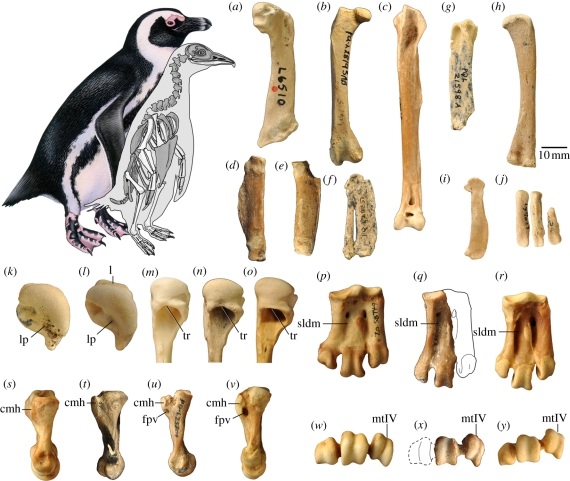
Figure 1.
Skeletal reconstruction of Inguza predemersus (known elements in white) with extant Spheniscus demersus for scale. Fossil material of I. predemersus includes the (a) humerus (L6510, holotype), (b) femur (PQL28195), (c) tibiotarsus (PQL23012), (d) ulna (PQL23003), (e) radius (PQL28254) and (f) carpometacarpus (PQL28251). Bones of unfledged chicks tentatively assigned to this species include the (g) humerus (PQL21598), (h) femur (PQL28432A), (i) carpometacarpus (PQL23015) and (j) tarsometatarsus (PQL28467). Phylogenetically informative characters are illustrated in the bottom two rows. Humeri of (k,n) I. predemersus, (l,o) S. demersus and (m) Eudyptula minor are shown in proximal (k,l) and medial (m–o) views. A straight (versus concave) dorsal margin of the tricipital fossa (tr) is a synapomorphy uniting Spheniscus muizoni and the four extant Spheniscus species [19], and the projection of the lip bounding the fossa (l) is a synapomorphy uniting the four extant Spheniscus species [6,19]. Inguza retains the primitive state for both characters. Presence of a ligament pit (lp) characterizes crown clade penguins. Tarsometatarsi of (p,s,w) Pygoscelis antarctica, (q,u,x) I. predemersus, (t) Nucleornis insolitus and (r,v,y) S. demersus are shown in dorsal (p–r), medial (s–v) and distal (w–y) views. A deep sulcus longitudinalis dorsalis medialis (sldm) is a synapomorphy uniting Nucleornis + clade A. Perforation of the crista medialis hypotarsi (cmh) by the medial foramen proximale vasculare (fpv) is a synapomorphy uniting clade A. Nucleornis retains the primitive condition in which the medial fpv exits distal to the cmh. Dorsal displacement of trochlea metatarsi IV (mtIV) is a synapomorphy of the extant species of Spheniscus. Inguza retains the primitive condition in which the trochleae are subparallel in distal view. Image (t) is reversed to aid comparison. Spheniscus artwork by Barbara Harmon and Inguza reconstruction by Kristin Lamm. Scale bar under (h) applies to (a–j); other images not to scale.
Evolutionary relationships of the African fossil taxa have remained shrouded in uncertainty and have not previously benefited from phylogenetic analysis. Alternate hypotheses have interpreted one or more of these species as direct ancestors of the extant Spheniscus demersus, members of the extinct South American genus Palaeospheniscus, or low-latitude relatives of extant Antarctic/sub-Antarctic penguins [14,15,20]. Alternatively, all four species have been considered to represent an endemic radiation [21].
Key to interpreting the history of the African penguin fauna is deciphering whether these species arrived in Africa through multiple waves of dispersal or radiated following a single colonization event. Resolving the relationships of these species could also provide clues to the timing of each radiation or dispersal. Understanding the origins and history of South African penguins across a period of pronounced climate shifts and sea-level fluctuation may be one of the keys to predict the response of the currently endangered S. demersus [22] to global change.
2. Material and methods
(a). Fossil material
We examined over 200 penguin specimens in the Iziko South African Museum collections (figure 1). Specimens considered in the phylogenetic analysis were all collected from the Muishond Fontein Phosphatic Sand Member (MFPSM) of the Varswater Formation (i.e. ‘Peletal Phosphate Member’) exposed at East Quarry, Langebaanweg (32.98° S, 18.15° E), and Koeberg (33.67° S, 18.44° E). The Varswater Formation is the product of latest Miocene to Pliocene transgression, and the MFPSM was deposited in an estuarine or littoral setting [23]. The synthesis of biostratigraphy, palaeomagnetic data and global sea-level reconstructions indicates an age of 5.15 ± 0.1 Ma (Early Pliocene, Zanclean Stage) for the MFPSM [24].
(b). Phylogenetic and biogeographic analyses
We conducted a phylogenetic analysis using a combined (total evidence) matrix including 228 morphological characters and molecular data from the genes RAG-1, 12S, 16S, COI and cytochrome b (see the electronic supplementary material for character descriptions and citations). Taxon sampling included 56 penguin taxa and 15 outgroup species from Procellariiformes and Gaviiformes with trees rooted to Gavia. Two African fossil penguin taxa (‘Palaeospheniscus’ huxleyorum and Dege hendeyi) erected on single-element holotypes were included in an initial analysis. This resulted in a completely unresolved strict consensus tree for Spheniscidae, so both species were excluded from the final analysis. The final dataset was evaluated using PAUP* v. 4.0b10 [25] with a heuristic search strategy (10 000 replicates of random taxon addition with TBR branch swapping; TBR, tree bisection and reconnection). All characters were equally weighted, multi-state codings were used only to represent polymorphism and branches of minimum length 0 were collapsed.
Ancestral area reconstructions were conducted in a parsimony framework using Fitch optimization as implemented in MacClade v. 4.08 [26], and in a Bayesian framework using RASP [27] with 100 000 cycles specified and root distribution set to ‘outgroup’. Taxa were assigned ranges based on breeding area for living taxa and fossil distribution for extinct taxa, with 10 areas delineated following Bertelli & Giannini [7]. Fitch parsimony, which has previously been applied to extant penguin biogeography [7], allows only single area ancestral reconstructions and may be appropriate when there is little concern with underestimating the number of range changes [28]. This method is thus conservative with regards to testing for multiple dispersals to a single area. Bayesian analysis allows wider ancestral areas and incorporates uncertainty into results. See the electronic supplementary material for further details.
3. Results
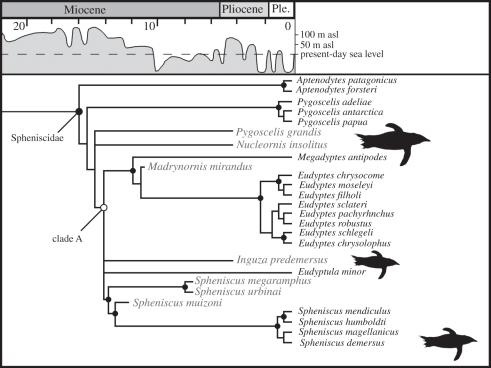
Results of the phylogenetic analyses (figure 2) reject a close relationship between extant S. demersus and the fossil taxa Inguza and Nucleornis, and imply a minimum of three separate dispersals to Africa. Morphological evidence for placing Inguza predemersus outside of the clade formed by the four extant species of Spheniscus is strong, precluding this fossil taxon as a candidate for sister taxon or direct ancestor of the extant blackfooted penguin. Three unambiguous synapomorphies shared by all extant Spheniscus species are absent in I. predemersus (figure 1). Available evidence supports a more basal position for Nucleornis insolitus, outside the clade uniting Spheniscus, Eudyptula, Eudyptes and Megadyptes (clade A in figure 1), though only a single unambiguous synapomorphy supports this relationship. Both fossil lineages appear to have arrived from either South America or Austro-New Zealand. Parsimony ancestral area reconstructions using the strict consensus tree were ambiguous owing to polytomies, though all trees combined in the consensus support an Austro-New Zealand ancestral area for at least the I. predemersus lineage (electronic supplementary material, figure S4). In contrast, Bayesian reconstructions favoured a South American ancestral area for both fossil lineages (electronic supplementary material, figure S3). Both methods reconstruct the ancestral area of the extant blackfooted penguin lineage as South American.
Figure 2.
Strict consensus tree for Spheniscidae (crown group penguins) resulting from combined analysis of the molecular + morphological dataset with branch lengths calibrated to the fossil record, minimizing ghost lineages. Nodes that were also recovered by an analysis using only the morphological dataset are indicated by black circles. Fossil taxa are indicated in grey font, and scaled silhouettes indicate the relative size of the African species. Outgroups and stem penguin taxa are not shown; see the electronic supplementary material for additional data.
Based on constraints imposed by the recovered topology and the global fossil record, two separate dispersals by the ancestors of Inguza and Nucleornis occurred by (at latest) the Late Miocene. These two fossil lineages must have branched off prior to the divergence of the more highly nested species Spheniscus muizoni, the oldest fossils of which are estimated at 11–13 Ma in age [19]. This is a minimum constraint, as the divergence of a lineage may predate its oldest fossil owing to the incomplete nature of the fossil record. Assuming speciation occurred subsequent to the Inguza and Nucleornis lineages arriving in Africa, multiple penguin lineages existed in the region for a 6–8 Myr interval while leaving only a single reported bone [21]. Such poor representation is geologically plausible given that African's southern continental margin is tectonically passive and preserves almost no Early Neogene sediments [29,30]. Alternatively, it is possible that the Inguza and/or Nucleornis lineages speciated after dispersing from South America or Austro-New Zealand to intermediate oceanic islands, and only arrived in Africa during the Pliocene. However, no fossil evidence exists to support this more complex scenario. A more recent dispersal resulted in the colonization of Africa by S. demersus. A review of all Plio-Pleistocene material in collections found no evidence for the presence of this species in Pliocene deposits. The oldest verifiable fossils of this species come from Duinefontein localities dated to 250–400 ka [31,32]. Molecular divergence dating estimates for the split between S. demersus and its sister species Spheniscus magellanicus range from 1.9 to 4.9 Ma [9]. Thus, both the fossil record and molecular dates support the arrival of S. demersus in Africa after the deposition of the MFPSM. Blackfooted penguins must have arrived more recently than Inguza and Nucleornis, but whether they overlapped with late surviving members of these fossil lineages remains uncertain.
4. Discussion
Reorganization of southern ocean systems during the Palaeogene–Neogene transition appears to have opened the first viable dispersal route to Africa for penguins. Two major ocean currents may be implicated: the Antarctic Circumpolar Current (ACC) and the South Atlantic Current (SAC). The ACC flows eastward through the Drake Passage and passes well south of Africa. The SAC originates off the Brazilian coast and flows parallel to the ACC, where part of the SAC continues northward along the western coast of Africa as the Benguela Current (BC). The ACC developed during the Oligocene with the opening of the Drake Passage [33], and the Benguela and Brazilian currents were established by the Late Miocene, at which time they were probably connected via the SAC [34,35]. Timing of the origins of the ACC and the SAC is consistent with these currents serving as vectors for Late Neogene penguins to disperse eastward across the Atlantic Ocean. Critically, both currents offer food-rich waters that have probably drawn foraging penguins for millions of years [36]. Bertelli & Giannini [7] inferred that the ancestor of S. demersus crossed the Atlantic from South America, consistent with our results. At present, it remains uncertain whether the ancestors of Inguza and Nucleornis arrived from South America or Austro-New Zealand. Nevertheless, for either ancestral area, the most plausible dispersal direction is west to east, using the ACC/SAC vector.
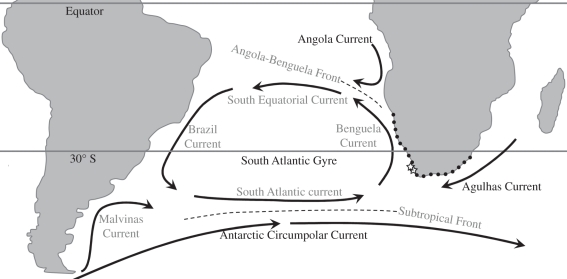
Penguin distribution in Africa appears to have been tightly controlled by major ocean currents from the Miocene through the present. African penguins forage in the Agulhas Current (AGC) and BC to the south and west, but are restricted from the north by the Angola-Benguela Front (ABF; approximately 15°S; figure 3). The warm, nutrient-poor waters of the Angola Current have flowed southward along the African margin to converge with the BC since at least the Late Miocene [39]. Hence, the ABF appears to represent an important physical and nutrient barrier that prevents S. demersus from expanding northwards. Likewise, penguins appear never to have reached Madagascar, which is bounded on both sides by strong southward flowing currents (AGC, Mozambique Current, East Madagascar Current).
Figure 3.
Surface currents in the South Atlantic Ocean. The Antarctic Circumpolar Current and Subtropical Gyre have been important dispersal mechanisms, furnishing Africa with penguins from other austral landmasses (e.g. South America). Pliocene fossil penguin localities are indicated with open stars, and closed circles show the breeding range of S. demersus (after the study by Crawford et al. [37]). Dashed lines indicate ocean fronts. Oceanographic features are from Stramma & England [38].
At issue is the age of the extinction(s) that decimated the Pliocene penguin fauna. Extinction of the four fossil species and arrival of S. demersus occurred sometime in the interval between the Early Pliocene and Middle Pleistocene. Direct causes of extinction remain unclear, but sea-level fluctuations probably impacted penguin diversity [8]. During the Pliocene, higher sea levels submerged large areas of coastal land in the Cape region [24]. An Early Pliocene highstand at 90 m above present-day sea level [23,40] corresponds with the peak in penguin diversity. This highstand created islands at high coastal elevations that probably served as secure nesting sites [24]. At the end of the Early Pliocene, sea levels plunged globally [41], and in a more pronounced manner in southern Africa [24]. A series of marked drops and rises in sea level are recorded from the end of the Early Pliocene to the present in the Cape region (figure 2; [24]). These rapid shifts altered the coastal environment for penguins, with an overall trend towards deteriorating conditions for viable breeding colonies. As receding sea levels assimilated islands into the mainland, we calculate that the total area of offshore island breeding sites in the Cape region would have been cut to a fraction of the area available during the Early Pliocene (electronic supplementary material).
Investigations into the African fossil record demonstrate the complex relationship between penguins and ocean systems. Major currents provided a dispersal vector for Neogene penguins to reach Africa and coastal upwelling permitted the establishment of colonies by these vagrants. At the same time, ocean currents have also restricted the range of penguins to the southern part of the continent. Sea-level flux appears to have led to a lower carrying capacity for penguin species over the past few million years, resulting in the ultimate failure of several colonizing lineages. Carrying capacity has been further reduced in recent years owing to commercial fishing and expansion of fur seals [37]. The currently endangered blackfooted penguin is thus recognized as the last survivor in an environment that has deteriorated for penguins both on a geological and on a historical time scale.
Acknowledgements
We thank Graham Avery and Kerwin van Willingh of the Iziko South African Museum, and Pippa Haarhoff of the West Coast Fossil Park, for access to collections, Barbara Harmon and Kristin Lamm for creating the artwork in figure 1, and two anonymous reviewers for feedback. This research was supported by NSF DEB grant 0949899 (D.T.K.) and the University Research Council of the University of Cape Town Postdoctoral Fellowship (D.B.T.).
References
- 1.Bost C. A., et al. 1997. Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar. Ecol. Prog. Ser. 150, 21–33 10.3354/meps150021 (doi:10.3354/meps150021) [DOI] [Google Scholar]
- 2.Kooyman G. L. 2002. Evolutionary and ecological aspects of some Antarctic and sub-Antarctic penguin distributions. Oecologia 130, 485–495 10.1007/s00442-001-0836-x (doi:10.1007/s00442-001-0836-x) [DOI] [PubMed] [Google Scholar]
- 3.Clarke J. A., Ksepka D. T., Stucchi M., Urbina M., Giannini N., Bertelli S., Narváez Y., Boyd C. A. 2007. Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proc. Natl Acad. Sci. USA 104, 11 545–11 550 10.1073/pnas.0611099104 (doi:10.1073/pnas.0611099104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fordyce R. E., Jones C. M. 1990. Penguin history and new fossil material from New Zealand. In Penguin biology (eds Davis L. S., Darby J. T.), pp. 419–446 San Diego, CA: Academic Press [Google Scholar]
- 5.Jadwiszczak J. 2010. Penguin response to the Eocene climate and ecosystem change in the northern Antarctic Peninsula region. Polar Sci. 4, 229–235 10.1016/j.polar.2010.03.001 (doi:10.1016/j.polar.2010.03.001) [DOI] [Google Scholar]
- 6.Ksepka D. T., Bertelli S., Giannini N. P. 2006. The phylogeny of the living and fossil Sphenisciformes (penguins). Cladistics 22, 412–441 10.1111/j.1096-0031.2006.00116.x (doi:10.1111/j.1096-0031.2006.00116.x) [DOI] [Google Scholar]
- 7.Bertelli S., Giannini N. P. 2005. A phylogeny of extant penguins (Aves: Sphenisciformes) combining morphology and mitochondrial sequences. Cladistics 21, 209–239 10.1111/j.1096-0031.2005.00065.x (doi:10.1111/j.1096-0031.2005.00065.x) [DOI] [Google Scholar]
- 8.Olson S. L. 1985. An Early Pliocene marine avifauna from Duinefontein, Cape Province, South Africa. Ann. S. Afr. Mus. 95, 147–164 [Google Scholar]
- 9.Baker A. J., Pereira S. L., Haddrath O. P., Edge A. 2006. Multiple gene evidence for expansion of extant penguins out of Antarctica due to global cooling. Proc. R. Soc. B 217, 11–17 10.1098/rspb.2005.3260 (doi:10.1098/rspb.2005.3260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson G. G. 1946. Fossil penguins. Bull. Am. Mus. Nat. Hist. 87, 7–99 [Google Scholar]
- 11.Jadwiszczak J. 2009. Penguins past: the current state of knowledge. Pol. Polar Res. 30, 3–28 [Google Scholar]
- 12.Ksepka D. T., Clarke J. A. 2010. The basal penguin (Aves: Sphenisciformes) Perudyptes devriesi and a phylogenetic evaluation of the penguin fossil record. Bull. Am. Mus. Nat. Hist. 337, 1–77 10.1206/653.1 (doi:10.1206/653.1) [DOI] [Google Scholar]
- 13.Slack K. E., Jones C. M., Ando T., Harrison G. L., Fordyce R. E., Arnason U., Penny D. 2006. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol. Biol. Evol. 23, 1144–1155 10.1093/molbev/msj124 (doi:10.1093/molbev/msj124) [DOI] [PubMed] [Google Scholar]
- 14.Simpson G. G. 1971. Fossil penguin from the Late Cenozoic of South Africa. Science 171, 1144–1145 10.1126/science.171.3976.1144 (doi:10.1126/science.171.3976.1144) [DOI] [PubMed] [Google Scholar]
- 15.Simpson G. G. 1973. Tertiary penguins (Sphenisciformes, Spheniscidae) from Ysterplaats, Cape Town, South Africa. S. Afr. J. Sci. 69, 342–344 [Google Scholar]
- 16.Simpson G. G. 1979. Tertiary penguins from the Duinefontein site, Cape Province, South Africa. Ann. S. Afr. Mus. 79, 1–17 [Google Scholar]
- 17.Simpson G. G. 1979. A new genus of Late Tertiary penguin from Langebaanweg, South Africa. Ann. S. Afr. Mus. 78, 1–9 [Google Scholar]
- 18.Hendey Q. B. 1973. Fossil occurrences at Langebaanweg, Cape Province. Nature 244, 13–14 10.1038/244013a0 (doi:10.1038/244013a0) [DOI] [Google Scholar]
- 19.Göhlich U. B. 2007. The oldest fossil record of the extant penguin genus Spheniscus–a new species from the Miocene of Peru. Acta Palaeontol. Pol. 52, 285–298 [Google Scholar]
- 20.Simpson G. G. 1975. Notes on variation in penguins and on fossil penguins from the Pliocene of Langebaanweg, Cape Province, South Africa. Ann. S. Afr. Mus. 69, 59–72 [Google Scholar]
- 21.Olson S. L. 1983. Fossil seabirds and changing marine environments in the Late Tertiary of South Africa. S. Afr. J. Sci. 79, 399–402 [Google Scholar]
- 22.IUCN 2011. IUCN Red List of threatened species. See www.iucnredlist.org. (Version 2011.1. Downloaded on 13 July 2011.)
- 23.Roberts D. L. 2006. Varswater Formation including the Langeeheid Clayey Sand, Konings Vlei Gravel, Langeberg Quartz Sand and Muishond Fontein Phosphatic Sand Members. In Catalogue of South African lithostratigraphic units (ed. Johnson M. R.), pp. 97–931 Pretoria, South Africa: Council for Geoscience [Google Scholar]
- 24.Roberts D. L., et al. 2011. Regional and global context of the Late Cenozoic Langebaanweg (LBW) palaeontological site: west coast of South Africa. Earth Sci. Rev. 106, 191–214 10.1016/j.earscirev.2011.02.002 (doi:10.1016/j.earscirev.2011.02.002) [DOI] [Google Scholar]
- 25.Swofford D. L. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sunderland, MA: Sinauer Associates [Google Scholar]
- 26.Maddison W. P., Maddison D. R. 2005. MacClade. Version 4.08. Sunderland, MA: Sinauer Associates [Google Scholar]
- 27.Yu Y., Harris A. J., He X. J. 2011. RASP (reconstruct ancestral state in phylogenies). See http://mnh.scu.edu.cn/soft/blog/RASP [DOI] [PMC free article] [PubMed]
- 28.Lamm K. S., Redelings B. D. 2009. Reconstructing ancestral ranges in historical biogeography: properties and prospects. J. System. Evol. 47, 369–382 10.1111/j.1759-6831.2009.00042.x (doi:10.1111/j.1759-6831.2009.00042.x) [DOI] [Google Scholar]
- 29.Gallagher K., Brown R., Osmaston M., Ebinger C., Bishop P. 1999. Denudation and uplift at passive margins: the record on the Atlantic margin of southern Africa. Phil Trans. R. Soc. Lond. A 357, 835–859 10.1098/rsta.1999.0354 (doi:10.1098/rsta.1999.0354) [DOI] [Google Scholar]
- 30.Roberts D. L., Botha G. A., Maud R. R., Pether J. 2007. Coastal Cenozoic deposits. In The geology of South Africa (eds Johnson M. R., Anhaeusser C. R., Thomas R. J.), pp. 605–628 Pretoria, South Africa: Council for Geoscience [Google Scholar]
- 31.Cruz-Uribe K., Klein R. G., Avery G., Avery M., Halkett D., Hart T., Milo R. G., Sampson C. G., Volman T. P. 2003. Excavation of buried Late Acheulean (Mid-Quaternary) land surfaces at Duinefontein 2, Western Cape Province, South Africa. J. Archaeol. Sci. 30, 229–575 10.1016/S0305-4403(02)00202-9 (doi:10.1016/S0305-4403(02)00202-9) [DOI] [Google Scholar]
- 32.Klein R. G., Avery G., Cruz-Uribe K., Halkett D., Hart T., Milo R. G., Volman T. P. 1999. Duinefontein 2: an Acheulean site in the Western Cape Province of South Africa. J. Hum. Evol. 37, 153–190 10.1006/jhev.1999.0307 (doi:10.1006/jhev.1999.0307) [DOI] [PubMed] [Google Scholar]
- 33.Lawver L. A., Gahagan L. M. 2003. Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr. Palaeoclimatol. 198, 11–37 10.1016/S0031-0182(03)00392-4 (doi:10.1016/S0031-0182(03)00392-4) [DOI] [Google Scholar]
- 34.Diester-Haass L., Meyers P. A., Vidal L. 2002. The Late Miocene onset of high productivity in the Benguela Current upwelling system as part of a global pattern. Mar. Geol. 180, 87–103 10.1016/S0025-3227(01)00207-9 (doi:10.1016/S0025-3227(01)00207-9) [DOI] [Google Scholar]
- 35.Martínez S., del Río C. J. 2002. Late Miocene molluscs from the southwestern Atlantic Ocean (Argentina and Uruguay): a palaeobiogeographic analysis. Palaeogeogr. Palaeoclimatol. 188, 167–187 10.1016/S0031-0182(02)00551-5 (doi:10.1016/S0031-0182(02)00551-5) [DOI] [Google Scholar]
- 36.Williams T. D. 1995. The penguins. Oxford, UK: Oxford University Press [Google Scholar]
- 37.Crawford R. J. M., Underhill L. G., Upfold L., Dyer B. M. 2007. An altered carrying capacity of the Benguela upwelling ecosystem for African penguins (Spheniscus demersus). ICES J. Mar. Sci. 64, 570–576 10.1093/icesjms/fsm009 (doi:10.1093/icesjms/fsm009) [DOI] [Google Scholar]
- 38.Stramma L., England M. 1999. On the water masses and mean circulation of the South Atlantic Ocean. J. Geophys. Res. 104, 20 863–20 883 10.1029/1999JC900139 (doi:10.1029/1999JC900139) [DOI] [Google Scholar]
- 39.Robert C., Diester-Haass L., Paturel J. 2005. Clay mineral assemblages, siliciclastic input and paleoproductivity at ODP Site 1085 off Southwest Africa: a late Miocene–early Pliocene history of Orange river discharges and Benguela current activity, and their relation to global sea level change. Mar. Geol. 216, 221–238 10.1016/j.margeo.2005.02.024 (doi:10.1016/j.margeo.2005.02.024) [DOI] [Google Scholar]
- 40.Miller K. G., et al. 2005. The Phanerozoic record of global sea-level change. Science 310, 1293–1298 10.1126/science.1116412 (doi:10.1126/science.1116412) [DOI] [PubMed] [Google Scholar]
- 41.Zachos J., Pagani M., Sloan L., Thomas E., Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686. 10.1126/science.1059412 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]