Abstract
The study of animal cognition has provided valuable data throughout the years, yet its reliance on laboratory work leaves some open questions. The main question is whether animals employ cognition in daily decision-making. The following discussion uses sperm competition (SC) as a test case for demonstrating the effect of cognition on routine choices, in this case, sexual selection. Cognition is manifested here by males' ability to represent the number of rivals competing with them. I claim that response to SC is driven by quantity estimation and the ability to assess competition magnitude cognitively. Hence, cognition can determine males' response to SC, and consequentially it can be selected within this context. This supports the argument that cognition constitutes an integral part of an individual's toolbox in solving real-life problems, and shows that physical and behavioural phenomena can expose cognition to selection and facilitate its evolution.
Keywords: animal cognition, sperm competition, quantity estimation, evolution, sexual selection, cognition
1. What is quantity estimation?
Quantity estimation (QE) encompasses the range of perceptual and cognitive aptitudes allowing individuals to respond to the quantity aspect of stimuli in various degrees of accuracy [1]. A fundamental distinction is made between quantity and amount: amount describes the physical attributes of stimuli (density, surface area, etc.) and provides information on the magnitude of such continuous indices. In contrast, quantity exclusively refers to the discrete dimension of exact number, and allows increments in integer units only [2]. For example, in foraging, amount reports on the magnitude of physical food features, while quantity conveys information on the number of individual food items. I suggest that the transition from processing amount to processing quantity entails a transition from the automatic and non-cognitive to the progressively more cognitively complex [3]. This is demonstrated by QE's role in sperm competition (SC).
2. What is sperm competition?
SC is the struggle between sperm of different males for the fertilization of a given set of ova [4]. SC affects the evolution of many taxa and induces a variety of adaptations at behavioural, morphological and physiological levels [5–7]. Parker and co-workers [8–11] modelled the resource allocation a male is expected to invest in sperm production when faced with different number of rivals. These models make three predictions: the first states that males should invest minimally in the absence of competition. Secondly, investment should be maximal in the presence of a single competitor. This situation is known as SC risk (SCR), and it is a dichotomy between absence and presence of rivals, thus not requiring QE. Finally, sperm expenditure is expected to progressively decline as the mean number of rivals exceeds one. This is SC intensity (SCI), and here a subtle distinction of quantity is required in order to qualify for the progressive decline condition. These predictions rely on two debatable assumptions: the first is that individuals can assess the magnitude of their competition, be it accurately or vaguely (what I call the ‘estimation conjecture’). The second assumption posits that there exists, in every species, a given knowledge of an average number of competing males (what I call the ‘innate mean conjecture’).
SC models address population-level SC and, consequently, do not consider how individuals perceive, process and act upon the number of rivals. However, it is clear that by alluding to assessment, these models should focus on individuals. Population analyses are limited in explicating the role cognition might play in behaviour, since they average out individual differences in performance. As a result, SC models neglect the questions of the mechanisms of assessment, who performs the assessment, or what is assessed. This is a substantial lacuna, since without the ability to estimate competition size, an organism cannot be said to truly respond to SCI [12]. The following discussion bridges this gap by showing that individuals can gauge the magnitude (intensity) of a competition, by using a range of perceptual and cognitive aptitudes, and that this assessment process determines their response to SC.
3. Linking quantity estimation and sperm competition
Males' ability to estimate competition magnitude determines ejaculate size and composition. Sperm does interesting things post copula, yet those chemical actions are out of cognition's reach. Estimation is not performed by sperm, and once sperm is delivered, cognition takes a back seat. Thus, the time window under investigation here includes only the events leading up to ejaculation. Cognitively speaking, assuming a priori that males can estimate quantity in SC contexts is non-trivial. Schleicherova et al. [13] report that the worm Ophryotrocha diadema modifies its sex allocation through a finely tuned, concentration-based threshold. Here, response to SC is wholly dependent upon amount and requires no cognitive processing. Bonilla et al. [14], report that sperm allocation in the pseudoscorpion Cordylochernes scorpioides decreased almost monotonically as the number of different male olfactory cues increased from 0 to 3. Thomas & Simmons [15] observed that sperm viability decreased in males of the cricket Teleogryllus oceanicus as the number of different male scents on a virgin female partner increased. Evidently, different species rely on different cues to assess SC; those cues are perceived at varying levels of accuracy and sophistication; and assessment determines if and how males modify their behaviour and/or sperm traits (table 1).
Table 1.
Experimental design for species tested for SC intensity. Species: H, hermaphrodite. Competition size: 1v0-2 means a focal male was presented with 0, 1 or 2 other males. 1v1/5 means it was one or five males; m, males; f, females; s, small; l, large. Traits: CD, copula duration; VST, various sperm traits; SE, sperm expenditure. Model: support/refute SCI model predictions. Population-level analyses are not included.
| species | competition | trait inspected | model | ref. |
|---|---|---|---|---|
| Schistocephalus solidus (H) | 1v1/3 | sperm storage volume | support | [16] |
| Macrostomum lignano (H) | 1v1/2/3/7 | sex allocation | support | [17,18] |
| Ophryotrocha diadema (H) | 1v1/11 | sex allocation | support | [19] |
| Helobdella papillomata (H) | 1v0/1/3/7 | testisac volume | support | [20] |
| Ophryotrocha diadema (H) | 1v2/6/12 | sex allocation | refute | [13,21] |
| yellow dung fly | 1v1/3 | testis size | support | [22] |
| fruit fly | 1v0/1/3 | CD | support | [23] |
| 1v1/2/4 | seminal fluid composition | support | [24] | |
| golden egg bug | 1v1/2 | CD, SE | support | [25] |
| rice weevil | 1v1/5/10 | courtship duration, CD | support | [26] |
| monarch butterfly | 1v3, 1s:1l | VST | refute | [27] |
| tropical house cricket | 1v0/1/6 | SE | refute | [28] |
| spring field cricket | 1v0/1/6 | SE | support | [28] |
| Southwestern field cricket | 1v0/1/6 | SE | refute | [28] |
| Australian field cricket | 1v0/1/5 | SE | support | [29] |
| 1v0,1,5,10,15 | VST | refute | [15] | |
| house cricket | 1v0/1/7 | SE | refute | [30] |
| tropical house cricket | 1v0/1/7 | SE | refute | [30] |
| Cordylochernes scorpioides | 1v0-3 | SE | refute | [14] |
| mealworm beetle | 1m:2f/3/4f, 2mv4f | time near scent origin | refute | [31] |
| peppermint shrimp (H) | 1v1/2/5/10 | sex allocation | refute | [32] |
| sailfin mollies | 1m:3f, 3m:1f | SE | refute | [33] |
| guppy | 1v0/1/2/4 | VST | refute | [34] |
| European bitterling | 1v0/1/3/5 | ejaculation rate | support | [35] |
| not specified | SE | support | [36] | |
| grass goby | 1v0/1/2/4 | ejaculate size | support | [37] |
| 1v0/1/4 | territoriality, aggression & SE | refute | [38] | |
| black goby | 1v0/1/2/4 | ejaculate size | support | [37] |
| 1v0/1/4 | territoriality, aggression & SE | support | [38] | |
| rainbow darter | 1v0/1/4 | ejaculate size | refute | [39] |
| freshwater crayfish | 1v0/1/3/ | ejaculate size | refute | [40] |
| red-spotted newt | 1v0/1/3/7 | courting display | support | [41] |
| small-mouthed salamander | 1v0/1/2 | courtship duration, spermatophore number | support | [42] |
| Australian quacking frog | 1v0/1/2/4 | fertilization success | support | [43] |
| 1v0/1/2 | ejaculate & testis size | refute | [12] | |
| meadow vole | 1v5 | SE | support | [44] |
| bank vole | 1v1/4 | VST | support | [45] |
4. The estimation conjecture
SC models provide a framework for examining cognition's role in SC. These models consider two scenarios: in the first, sperm allocation is shaped by the mean level of SC, and males can assess only whether the number of competitors is smaller or greater than such an average [13]. In QE nomenclature, this is called relative quantity judgement. Many species possess basic QE aptitudes, where amount and quantity are significantly confounded (for a review see [46]). In SC, relative quantity judgement is manifested, for example, by males measuring females' reproductive tract content [47,48]. In the second SC scenario, males have precise information concerning the number of competitors (akin to [15]). In QE terminology, this is counting.
To tackle the estimation conjecture, I examine the role quantity plays in males' assessment of SCR/I, and begin by looking at the issue of quantity versus amount. While SCR has substantial experimental support, SCI garners only a fragmented one [49–51] (see also table 1). As an explanation, I suggest that whereas SCR relies on a binary distinction between absence and presence (in which both amount and quantity provide cues of equivalent reliability), SCI demands a representation of the actual number of rivals, entailing the cognitive function of counting. Since counting is more complex, it is expected to be less phylogenetically frequent.
To substantiate the argument for QE, I investigate what is the element assessed by males: if stimuli are continuous (e.g. spermatheca content) and evaluation is concentration-based [27,52], amount is the dominant cue. If stimuli are males themselves, then the cue becomes quantity, and it can be said (given appropriate experimental controls) that males effectively count their rivals.
Next, I use Gelman & Gallistel's counting principles [53], in which ordinality and cardinality are prerequisite to counting. Ordinality is the representation of order within a stimulus array [54]. In QE, ordinality means that tagging of stimuli is continuous and sequential. Cardinality dictates that the last tag assigned represents the sum of all items in an array. To exhibit ordinality in SC, males need to distinguish rivals tallied from rivals to be tallied. Such ability is plausible given several lines of evidence. First, many species can correctly establish mating status [55] or mating order [6] using various mechanisms. Second, males are aware of not simply the presence of observers, but also of their composition ([56] and references therein). Cumulatively, it is safe to presume that males of several species can actively distinguish between individuals, tag them and represent them ordinally. Third, with respect to cardinality, it is observed that, in nature, males are often encountered sequentially [57]. Next, selected reports explain how males can benefit from relegating the execution of sexual behaviour to a later stage: Grant et al. [58] introduced male Japanese medaka to simultaneous and sequential presentations, and concluded that sexual behaviour indices were more prominent following sequential presentation. Reinhardt [59] reports that the summative number of male–male encounters reliably predicts variation in ejaculate size in the grasshopper Chorthippus parallelus. Thus, it is plausible to identify cardinality aptitude employed in SC context. Together, these lines of evidence provide a tentative, albeit indirect, backing to an ability to track and manipulate quantity over time in a manner qualifying to the principles of counting.
Overall, there is experimental support for the existence of a continuum of various fashions of gauging competition magnitude, ranging from crude perceptual sensing of chemical concentration through more elaborate processing of amount cues, and culminating in cognitive processing of quantity and counting. These aptitudes are employed contextually in some individuals and species to respond to SCI.
5. The innate mean conjecture
SC models assume the existence of a given knowledge of an average number of competing males. Clearly, if males use innate means, they need not estimate the number of their rivals, and automatic response to perceptual amount cues (such as concentration thresholds) suffice. Indeed, some species rely upon an a priori value, embedded either prenatally or during a critical period in development [60,61]. This option may apply to males who lack plasticity in accommodating for changes in competition size [23,62]. Nonetheless, innate means cannot be assumed to be global, since the composition and magnitude of competition may vary substantially [12]. Additionally, data show that local responses can rely on the phenotypic plasticity of sperm traits [63–65].
If averages are not used, males need to form their estimation during some point in time, be it prior to or during copula, thus allowing temporal cognition to enter the equation. The role of interval timing in QE has been thoroughly substantiated [2,66,67]. Therefore, identifying interval timing's involvement in SC supports the argument for QE's role in SC. I focus on estimations prior to copula [24,68], since estimations during copula rely predominantly on probing spermatheca content, which is amount estimation. There are two alternatives to the innate mean conjecture concerning timing's role in SC: the first is estimation according to ad hoc, local conditions [69], and the second is decision reached locally via a comparison to a hardcoded value [70,71]. Interval timing is important in both cases, as shown, for example, by the observation that mating duration prolongs as males' exposure to rivals prior to copula progressively extends [72]. Further cementing the role of interval timing in connecting QE and SC is the observation that phenotypic plasticity of various sperm traits is triggered only by a specific stimulus and a threshold exposure time to it [73,74], even at adulthood [75,76].
As an interim conclusion, the QE analysis of SC supports the idea that males can estimate the magnitude of their competition. At the very least, SC assessments require males to perform relative quantity judgements, where males can distinguish between more or less rivals. Occasionally, assessment might entail a more sophisticated QE aptitude, where males need to perform a continuous real-time monitoring of the number of individuals they encounter. Such aptitude involves exact representation, it is sequential (and thus requires handling interval timing), and it meets the requirements of the basic principles of counting [53]. Such an aptitude has yet to be addressed in the QE literature. Thus, current QE models fail to describe fully the impact quantity has on males' assessment of SCI.
As table 1 indicates, this analysis goes beyond Parker et al.'s models: some species' response to SC, while not obeying Parker's predictions, still supports the argument that assessment is crucial for apprehension of competition size. Hence, the assertion that cognitive processes at the individual level tailor behavioural and physiological response to SCR is extended to data that contradicts SC models. Furthermore, the modelling of other phenomena also presupposes males' ability to gauge competition magnitude: mosquito fish males' mate choice [77] and giant danio's and zebra fish's resource defence [78] echo Parker et al.'s assumptions to the dot. Thus, it becomes even more pertinent to explore the role the cognitive functions of QE have in various behaviours and their evolutionary trajectories.
6. Evolutionary analysis of sperm competition and quantity estimation
Based on the suggested link between SC and QE, possible evolutionary corollaries could be considered. To do so, further elaboration of QE theory is needed. QE is a composite behaviour, comprising quasi-independent building blocks (perceptual or cognitive elements; e.g. temporal cognition) brought together by exaptation [2]. The operation of a building block within a cognitive complex does not negate its own independent, parallel and simultaneous effect. Here lies the source of quasi-independence: amount functionality is tapped by various amount networks and by quantity networks of which they are part. If an animal can process both amount and quantity, then stimuli may activate either amount or quantity networks. However, quantity has amount as one of its constituents; thus, the same amount-network is stimulated, but it supports different tasks. This contingent characteristic of the choice between quantity and amount allows various environmental conditions to determine which cue is used per given task. Such an outline suggests that QE aptitudes (such as relative quantity judgement and counting) are punctuated across phylogeny in a way that defies a linear schematization of its evolution: counting most probably have used the QE aptitudes preceding it, and evolved independently in several lineages. Therefore, the evolutionary processes that had shaped the continuum of QE aptitudes are nonlinear and promote convergence [2].
Data show that males' cognitive aptitude directly affects their mating success [79] and that females can infer such aptitudes via behavioural or morphological proxies [80]. Some studies document a direct association between learning and plasticity of sperm traits in SC context [81,82]. A ubiquitous observation in the study of animal behaviour is that individuals differ in cognitive performance. These lines of evidence suggest a viable link between individuals' cognitive capacity and their mating success, thereby exposing their cognitive system to selection, be it directly or indirectly.
Parker et al. argue that males who can accurately assess the number of their competitors use sperm more efficiently than those who cannot. Cognition's role in SC via QE suggests that cognitive traits can be selected [83]. Changes in sperm traits can translate into competitive ability [22,84,85] and sperm competitiveness can become heritable [86,87]. If estimation of quantity is males' assessment mechanism, then cognition is the cause of their response to SC and, consequentially, it could be selected. The interwovenness of quantity and amount dictates that cues generated by rival males activate multiple modalities [88] and processing processes. Such multitude could either improve decision-making [89] and facilitate attainment of perfect knowledge if cues are of the same nature (amount or quantity [14]), or it might stymie the estimation process, and favour averaged responses. Under ecological conditions where amount cannot provide reliable information, and/or when the interaction between quantity and amount jeopardizes swift and trustworthy response, a gradual shift from processing amount towards processing quantity is expected. Pertaining to this, a direct association between learning and plasticity of sperm traits in SC has been reported [81], and even argued to be the only probable cause of an increase in sperm expenditure [82]. Finally, given the observation that profound environmental differences predict vastly different selective forces on sperm traits [90], evolutionary advancements should become possible if some individuals collect a multitude of environmental cues [24,72], and if they can contingently alternate between quantity and amount as salient features. Evolutionarily phrased, QE can complexify if individuals exploit their plastic perceptual and cognitive responses in a way that is canalized towards preferring quantity to amount. Note, however, that currently there is no information on mechanisms translating the cognitive process of QE into actual behavioural or physiological SC modifications.
7. Economics of sperm expenditure
QE does not inform males about rivals' quality. Decision-making in sexual competition scenarios requires additional information, such as female availability and status, time of season, etc. Only the combination of all lines of information forges a reliable appraisal of the investment a male should allocate per competition event. Furthermore, sperm production is costly [91], and an ejaculate's competitive value changes during competition [92]. Hence, sperm expenditure is not trivial. It is probable that an economic regulation principle selects which plastic modification is executed in response to SC, such that it would be the one where minimal energy investment is required [93]. A support for this argument comes from meadow voles, where males' preference for the more receptive female was reversed when males had the choice between the more receptive females accompanied by 0 or 1 males compared with the less receptive females accompanied by five males. Here, the number of males was a cue overriding the chemical signal of female receptiveness [94].
8. Future research
The methodology in the studies reported here has been QE-unaware, hence lacking crucial controls required to consolidate quantity's role in SCI. This leaves the issue of alternative hypotheses to the QE argument open until future studies design methods to incorporate QE data and theories. Numerous ways could assist in achieving this goal.
(a). Design modifications
— It is only the number of males that need be manipulated in future experiments, and done so only in the presence of a single female, in order to eliminate the effect of female availability. Additionally, it has to be the same male who is presented with the choice alternatives, since only the individual level is important, and thus individual differences in performance are crucial.
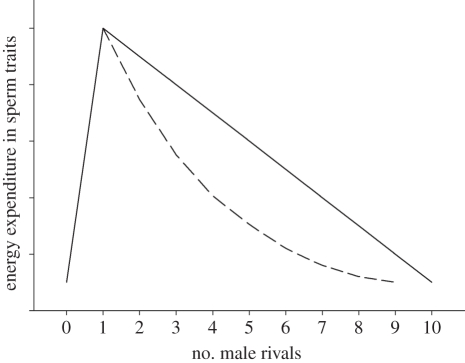
— Future designs should present graded multi-player scenarios, in which both absolute numbers as well as a wide range of ratios are examined, and subjects are forced to perform a comparison (as in [20,75]). Figure 1 graphically depicts the predictions of SC models in terms of QE. From 0 to 1 rivals, the situation is SCR, which is limitedly informative concerning QE. As the number of rivals exceeds 1, SCI can be assessed by either amount or quantity. If males estimate quantity (and conform to SC models' predictions), then we expect a decreasing linear relationship between the number of rivals and energy expenditure towards sperm traits or sexual behaviour. If males process amount, then the relationship is nonlinear, and depends on the nature of the amount cue used (figure 1 uses an arbitrary relationship, illustrating nonlinearity). Future work should be designed to generate data that could be inserted into graphs such as figure 1.
— Current designs boast a substantial variance in choice of observed traits, leading to non-unitary outcomes within and between species [17,26,29,45,93,95]. Future studies should establish a cohesion and standardization (enough to allow comparative studies), and observe a wide range of variables (as in [96]).
Figure 1.
The relation between number of rival males and energy expenditure towards sperm traits as predicted by SC models.
(b). Choice of animal models
-
— Hermaphrodites. Males are a minority in hermaphrodites, thus exposed to relaxed SC [97], yet theory still predicts sex allocation to respond to the actual number of rivals [98]. In simultaneous hermaphrodites, competitors are also potential partners, and therefore competition may occur between related and/or unrelated sperm [99]. In outcrossing simultaneous hermaphrodites, sex allocation depends on mating group size K + 1, where K is the number of different sperm donors [100]. When K = 1 (i.e. a single, self-fertilizing individual), there is local SC and an individual should invest minimally in sex allocation [16]. When K > 1, things get complicated. If K = 2, the effective mating group size depends on mating type: under cross-fertilization, mating group size is one and the optimal investment in sperm depends on the number of eggs of the partner. If both self- and cross-fertilizations occur, mating group size will be two, as sperm from both male functions compete for the fertilization of one's ova. With more individuals, competition scenarios complexify further [16]. While there is considerable amount of data on hermaphrodite sex allocation, investigating more closely the SC aspect, with an emphasis on the QE issue, should provide interesting observations.
An intriguing case is protandric-simultaneous hermaphrodites, where individuals reproduce first as males and later as simultaneous hermaphrodites [18,32]. Here there are two profoundly different QE phases, since in the first QE is performed by a male and in the second by a hermaphrodite. Exposing the same individual under these two phases to different number of rivals could provide precious data.
— Spermatophore depositing species. Spermatophores are interesting for QE because they are sperm encapsulated into discrete entities that can thus serve as a quantity cue. Spermatophores are used by both internal and external fertilizers, and for QE the question is whether rival males can detect them. If spermatophores are deposited internally, detection requires probing the female reproductive tract, thus making it, most likely, an amount cue. If spermatophores are deposited externally, then they can serve as a discrete quantity cue, facilitating counting. Additionally, spermatophore production time varies drastically across species and taxa, and can be remarkably swift [101], extremely slow [102] or impeding on remating interval [103]. Furthermore, there are data tying SC to spermatophore traits [42,104]. In several species, once a spermatophore is fully formed, males are committed to a fixed ejaculate expenditure [30]. This leads to several questions: When do these males form the decision leading to spermetophoregenesis? What is the quantity stimulus to which they respond, and what is the lag between formation and deposition? Will there be a deposition if there has been a change in the assessment of SCR/I in the interim between genesis and copula? Answering those questions could provide data discerning the role amount and quantity play in the estimation process, and might support the argument that QE is guiding the physiological changes in response to SC.
— Diapause. Multivoltine insects can have diapause larval development under harsh conditions, forcing differential energy allocation to hibernation and subsequent development. Consequently, individuals of different generations are expected to differ substantially in many traits, including spermatogenesis [65]. Comparing males of different diapause generations could elucidate the role of environmental amount/quantity cues on adults' SC performance.
9. Conclusion
Cognition can affect behaviour and physiology in a complex web of ecological and evolutionary parameters. Through this prism, I argue that males' response to SC is driven by their cognitive ability to gauge the magnitude of their competition. Thus, QE has improved males' sexual competitiveness and, reciprocally, SC has contributed to the complexification of QE. This discussion strengthens current models of sperm expenditure by highlighting the cognitive components shaping it. It also suggests that an evolutionary analysis of cognition within ecological and behavioural contexts may consolidate theories concerning phylogenetic complexification of cognitive systems.
Acknowledgements
I thank Eva Jablonka, Christine Schwab, Rachael Brown, Aida Gómez Robles, Jan Verpooten and the anonymous reviewers for commenting on earlier drafts.
References
- 1.Shifferman E. M. 2009. Its own reward: lessons to be drawn from the reversed-reward contingency paradigm. Anim. Cogn. 12, 547–558 10.1007/s10071-009-0215-2 (doi:10.1007/s10071-009-0215-2) [DOI] [PubMed] [Google Scholar]
- 2.Shifferman E. M. 2011. The evolution of quantity estimation in the animal kingdom. PhD Thesis, Tel Aviv University, Tel Aviv [Google Scholar]
- 3.Shima J. S. 2002. Mechanisms of density- and number-dependent population regulation of a coral-reef fish. Mar. Fresh Res. 53, 175–179 10.1071/MF01133 (doi:10.1071/MF01133) [DOI] [Google Scholar]
- 4.Parker G. A. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 5.Birkhead T. R., Møller A. P. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press [Google Scholar]
- 6.Simmons Leigh W. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Wedell N., Gage M. J. G., Parker G. A. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 10.1016/s0169-5347(02)02533-8 (doi:10.1016/s0169-5347(02)02533-8) [DOI] [Google Scholar]
- 8.Parker G. A. 1982. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294 10.1016/0022-5193(82)90225-9 (doi:10.1016/0022-5193(82)90225-9) [DOI] [PubMed] [Google Scholar]
- 9.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1996. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297 10.1098/rspb.1996.0189 (doi:10.1098/rspb.1996.0189) [DOI] [Google Scholar]
- 10.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1997. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B 264, 1793–1802 10.1098/rspb.1997.0249 (doi:10.1098/rspb.1997.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ball M. A., Parker G. A. 1998. Sperm-competition games: energy dependence and competitor numbers in the continuous-external-fertilization model. IMA J. Math. Appl. Med. Biol. 15, 87–96 10.1093/imammb/15.1.87 (doi:10.1093/imammb/15.1.87) [DOI] [Google Scholar]
- 12.Byrne P. G. 2004. Male sperm expenditure under sperm competition risk and intensity in quacking frogs. Behav. Ecol. 15, 857–863 10.1093/beheco/arh098 (doi:10.1093/beheco/arh098) [DOI] [Google Scholar]
- 13.Schleicherová D., Lorenzi M. C., Sella G., Michiels N. K. 2010. Gender expression and group size: a test in a hermaphroditic and a gonochoric congeneric species of Ophryotrocha (Polychaeta). J. Exp. Biol. 213, 1586–1590 10.1242/jeb.041814 (doi:10.1242/jeb.041814) [DOI] [PubMed] [Google Scholar]
- 14.Bonilla M. M., Zeh D. W., White A. M., Zeh J. A. 2011. Discriminating males and unpredictable females: males bias sperm allocation in favor of virgin females. Ethology 117, 740–748 10.1111/j.1439-0310.2011.01928.x (doi:10.1111/j.1439-0310.2011.01928.x) [DOI] [Google Scholar]
- 15.Thomas M. L., Simmons L. W. 2009. Male-derived cuticular hydrocarbons signal sperm competition intensity and affect ejaculate expenditure in crickets. Proc. R. Soc. B 276, 383–388 10.1098/rspb.2008.1206 (doi:10.1098/rspb.2008.1206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schärer L., Wedekind C. 2001. Social situation, sperm competition and sex allocation in a simultaneous hermaphrodite parasite, the cestode Schistocephalus solidus. J. Evol. Biol. 14, 942–953 10.1046/j.1420-9101.2001.00350.x (doi:10.1046/j.1420-9101.2001.00350.x) [DOI] [PubMed] [Google Scholar]
- 17.Janicke T., Schärer L. 2010. Sperm competition affects sex allocation but not sperm morphology in a flatworm. Behav. Ecol. Sociobiol. 64, 1367–1375 10.1007/s00265-010-0951-y (doi:10.1007/s00265-010-0951-y) [DOI] [Google Scholar]
- 18.Schärer L., Ladurner P. 2003. Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc. R. Soc. Lond. B 270, 935–941 10.1098/rspb.2002.2323 (doi:10.1098/rspb.2002.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenzi M. C., Sella G., Schleicherová D., Ramella L. 2005. Outcrossing hermaphroditic polychaete worms adjust their sex allocation to social conditions. J. Evol. Biol. 18, 1341–1347 10.1111/j.1420-9101.2005.00916.x (doi:10.1111/j.1420-9101.2005.00916.x) [DOI] [PubMed] [Google Scholar]
- 20.Tan G. N., Govedich F. R., Burd M. 2004. Social group size, potential sperm competition and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: Glossiphoniidae). J. Evol. Biol. 17, 574–580 10.1111/j.1420-9101.2004.00692.x (doi:10.1111/j.1420-9101.2004.00692.x) [DOI] [PubMed] [Google Scholar]
- 21.Schleicherová D., Lorenzi M. C., Sella G. 2006. How outcrossing hermaphrodites sense the presence of conspecifics and suppress female allocation. Behav. Ecol. 17, 1–5 10.1093/beheco/ari093 (doi:10.1093/beheco/ari093) [DOI] [Google Scholar]
- 22.Hosken D. J., Ward P. I. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 10.1046/j.1461-0248.2001.00198.x (doi:10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 23.Bretman A., Fricke C., Chapman T. 2009. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B 276, 1705–1711 10.1098/rspb.2008.1878 (doi:10.1098/rspb.2008.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedorka K. M., Winterhalter W. E., Ware B. 2011. Perceived sperm competition intensity influences seminal fluid protein production prior to courtship and mating. Evolution 65, 584–590 10.1111/j.1558-5646.2010.01141.x (doi:10.1111/j.1558-5646.2010.01141.x) [DOI] [PubMed] [Google Scholar]
- 25.García-González F., Gomendio M. 2004. Adjustment of copula duration and ejaculate size according to the risk of sperm competition in the golden egg bug (Phyllomorpha laciniata). Behav. Ecol. 15, 23–30 10.1093/beheco/arg095 (doi:10.1093/beheco/arg095) [DOI] [Google Scholar]
- 26.Flay C. D., He X. Z., Wang Q. 2009. Influence of male density on the courtship and mating duration of male rice weevils, Sitophilus oryzae. N. Z. Plant Prot. 62, 76–79 [Google Scholar]
- 27.Solensky M. J., Oberhauser K. S. 2009. Male monarch butterflies, Danaus plexippus, adjust ejaculates in response to intensity of sperm competition. Anim. Behav. 77, 465–472 10.1016/j.anbehav.2008.10.026 (doi:10.1016/j.anbehav.2008.10.026) [DOI] [Google Scholar]
- 28.Schaus J. M., Sakaluk S. K. 2001. Ejaculate expenditures of male crickets in response to varying risk and intensity of sperm competition: not all species play games. Behav. Ecol. 12, 740–745 10.1093/beheco/12.6.740 (doi:10.1093/beheco/12.6.740) [DOI] [Google Scholar]
- 29.Thomas M. L., Simmons Leigh W. 2007. Male crickets adjust the viability of their sperm in response to female mating status. Am. Nat. 170, 190–195 10.1086/519404 (doi:10.1086/519404) [DOI] [PubMed] [Google Scholar]
- 30.Gage A. R., Barnard C. J. 1996. Male crickets increase sperm number in relation to competition and female size. Behav. Ecol. Sociobiol. 38, 349–353 10.1007/s002650050251 (doi:10.1007/s002650050251) [DOI] [Google Scholar]
- 31.Carazo P., Font E., Forteza-Behrendt E., Desfilis E. 2009. Quantity discrimination in Tenebrio molitor: evidence of numerosity discrimination in an invertebrate? Anim. Cogn. 12, 463–470 10.1007/s10071-008-0207-7 (doi:10.1007/s10071-008-0207-7) [DOI] [PubMed] [Google Scholar]
- 32.Baeza J. A. 2007. No effect of group size on sex allocation in a protandric-simultaneous hermaphroditic shrimp. J. Mar. Biol. Assoc. UK 87, 1169–1174 10.1017/S0025315407057542 (doi:10.1017/S0025315407057542) [DOI] [Google Scholar]
- 33.Aspbury A. S. 2007. Sperm competition effects on sperm production and expenditure in sailfin mollies, Poecilia latipinna. Behav. Ecol. 18, 776–780 10.1093/beheco/arm044 (doi:10.1093/beheco/arm044) [DOI] [Google Scholar]
- 34.Evans J. P. 2009. No evidence for sperm priming responses under varying sperm competition risk or intensity in guppies. Naturwissenschaften 96, 771–779 10.1007/s00114-009-0529-6 (doi:10.1007/s00114-009-0529-6) [DOI] [PubMed] [Google Scholar]
- 35.Candolin U., Reynolds J. D. 2002. Adjustments of ejaculation rates in response to risk of sperm competition in a fish, the bitterling (Rhodeus sericeus). Proc. R. Soc. Lond. B 269, 1549–1553 10.1098/rspb.2002.2055 (doi:10.1098/rspb.2002.2055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith C. H., Reichard M., Jurajda P. 2003. Assessment of sperm competition by European bitterling, Rhodeus sericeus. Behav. Ecol. Sociobiol. 53, 206–213 10.1007/s00265-002-0576-x (doi:10.1007/s00265-002-0576-x) [DOI] [Google Scholar]
- 37.Pilastro A., Scaggiante M., Rasotto M. B. 2002. Individual adjustment of sperm expenditure accords with sperm competition theory. Proc. Natl Acad. Sci. USA 99, 9913–9915 10.1073/pnas.152133499 (doi:10.1073/pnas.152133499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaggiante M., Rasotto M. B., Romualdi C., Pilastro A. 2005. Territorial male gobies respond aggressively to sneakers but do not adjust their sperm expenditure. Behav. Ecol. 16, 1001–1007 10.1093/beheco/ari081 (doi:10.1093/beheco/ari081) [DOI] [Google Scholar]
- 39.Fuller R. C. 1998. Sperm competition affects male behaviour and sperm output in the rainbow darter. Proc. R. Soc. Lond. B 265, 2365–2371 10.1098/rspb.1998.0585 (doi:10.1098/rspb.1998.0585) [DOI] [Google Scholar]
- 40.Galeotti P., Rubolini D., Pupin F., Sacchi R., Altobelli E., Nardi P. A., Fasola M. 2009. Presence of rivals reduces mating probability but does not affect ejaculate size in the freshwater crayfish Austropotamobius italicus. Behaviour 146, 45–68 10.1163/156853908x390922 (doi:10.1163/156853908x390922) [DOI] [Google Scholar]
- 41.Verrell P. A. 1983. The influence of the ambient sex ratio and intermale competition on the sexual behavior of the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Behav. Ecol. Sociobiol. 13, 307–313 10.1007/bf00299678 (doi:10.1007/bf00299678) [DOI] [Google Scholar]
- 42.McWilliams S. R. 1992. Courtship behavior of the small-mouthed salamander (Ambystoma texanum): the effects of conspecific males on male mating tactics. Behaviour 121, 1–19 10.1163/156853992X00417 (doi:10.1163/156853992X00417) [DOI] [Google Scholar]
- 43.Byrne P. G., Roberts J. D. 1999. Simultaneous mating with multiple males reduces fertilization success in the myobatrachid frog Crinia georgiana. Proc. R. Soc. Lond. B 266, 717–721 10.1098/rspb.1999.0695 (doi:10.1098/rspb.1999.0695) [DOI] [Google Scholar]
- 44.delBarco-Trillo J. H., Ferkin M. H. 2006. Male meadow voles respond differently to risk and intensity of sperm competition. Behav. Ecol. 17, 581–585 10.1093/beheco/ark001 (doi:10.1093/beheco/ark001) [DOI] [Google Scholar]
- 45.Lemaître J.-F., Ramm S. A., Hurst J. L., Stockley P. 2010. Social cues of sperm competition influence accessory reproductive gland size in a promiscuous mammal. Proc. R. Soc. B 278, 1171–1176 10.1098/rspb.2010.1828 (doi:10.1098/rspb.2010.1828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reznikova Z., Ryabko B. 2011. Numerical competence in animals, with an insight from ants. Behaviour 148, 405–434 10.1163/000579511x568562 (doi:10.1163/000579511x568562) [DOI] [Google Scholar]
- 47.Rondeau A., Sainte-Marie B. 2001. Variable mate-guarding time and sperm allocation by male snow crabs (Chionoecetes opilio) in response to sexual competition, and their impact on the mating success of females. Biol. Bull. 201, 204–217 10.2307/1543335 (doi:10.2307/1543335) [DOI] [PubMed] [Google Scholar]
- 48.Siva-Jothy M. T., Stutt A. D. 2003. A matter of taste: direct detection of female mating status in the bedbug. Proc. R. Soc. Lond. B 270, 649–652 10.1098/rspb.2002.2260 (doi:10.1098/rspb.2002.2260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.delBarco-Trillo J. H. 2011. Adjustment of sperm allocation under high risk of sperm competition across taxa: a meta-analysis. J. Evol. Biol. 24, 1706–1714 10.1111/j.1420-9101.2011.02293.x (doi:10.1111/j.1420-9101.2011.02293.x) [DOI] [PubMed] [Google Scholar]
- 50.Kelly C. D., Jennions M. D. 2011. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884 10.1111/j.1469-185X.2011.00175.x (doi:10.1111/j.1469-185X.2011.00175.x) [DOI] [PubMed] [Google Scholar]
- 51.Bode M., Marshall D. J. 2007. The quick and the dead? Sperm competition and sexual conflict in sea. Evolution 61, 2693–2700 10.1111/j.1558-5646.2007.00232.x (doi:10.1111/j.1558-5646.2007.00232.x) [DOI] [PubMed] [Google Scholar]
- 52.Engqvist L. 2007. Male scorpionflies assess the amount of rival sperm transferred by females' previous mates. Evolution 61, 1489–1494 10.1111/j.1558-5646.2007.00107.x (doi:10.1111/j.1558-5646.2007.00107.x) [DOI] [PubMed] [Google Scholar]
- 53.Gelman R., Gallistel C. R. 1978. The child's understanding of number. Cambridge, MA: Harvard University Press [Google Scholar]
- 54.Arnold K., Zuberbühler K. 2008. Meaningful call combinations in a non-human primate. Curr. Biol. 18, R202–R203 10.1016/j.cub.2008.01.040 (doi:10.1016/j.cub.2008.01.040) [DOI] [PubMed] [Google Scholar]
- 55.Sandner P., Schärer L. 2010. No plastic responses to experimental manipulation of sperm competition per se in a free-living flatworm. Ethology 116, 292–299 10.1111/j.1439-0310.2010.01746.x (doi:10.1111/j.1439-0310.2010.01746.x) [DOI] [Google Scholar]
- 56.Bierbach D., et al. 2011. Male fish use prior knowledge about rivals to adjust their mate choice. Biol. Lett. 7, 349–351 10.1098/rsbl.2010.0982 (doi:10.1098/rsbl.2010.0982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barry K. L., Kokko H. 2010. Male mate choice: why sequential choice can make its evolution difficult. Anim. Behav. 80, 163–169 10.1016/j.anbehav.2010.04.020 (doi:10.1016/j.anbehav.2010.04.020) [DOI] [Google Scholar]
- 58.Grant J. W. A., Bryant M. J., Soos C. E. 1995. Operational sex ratio, mediated by synchrony of female arrival, alters the variance of male mating success in Japanese medaka. Anim. Behav. 49, 367–375 10.1006/anbe.1995.9998 (doi:10.1006/anbe.1995.9998) [DOI] [Google Scholar]
- 59.Reinhardt K. 2001. Determinants of ejaculate size in a grasshopper (Chorthippus parallelus). Behav. Ecol. Sociobiol. 50, 503–510 10.1007/s002650100398 (doi:10.1007/s002650100398) [DOI] [Google Scholar]
- 60.Yamane T., Miyatake T. 2008. Strategic ejaculation and level of polyandry in Callosobruchus chinensis (Coleoptera: Bruchidae). J. Ethol. 26, 225–231 10.1007/s10164-007-0051-2 (doi:10.1007/s10164-007-0051-2) [DOI] [Google Scholar]
- 61.Martel V., Damiens D., Boivin G. 2008. Strategic ejaculation in the egg parasitoid Trichogramma turkestanica (Hymenoptera: Trichogrammatidae). Ecol. Entomol. 33, 357–361 10.1111/j.1365-2311.2007.00973.x (doi:10.1111/j.1365-2311.2007.00973.x) [DOI] [Google Scholar]
- 62.Loher W., Dambach M. 1989. Reproductive behavior. In Cricket behavior and neurobiology (eds Huber F., Moore T. E., Loher W.), pp. 43–82 Ithaca, NY: Comstock Pub. Associates [Google Scholar]
- 63.Immler S., Pryke S. R., Birkhead T. R., Griffith S. C. 2010. Pronounced within-individual plasticity in sperm morphometry across social environments. Evolution 64, 1634–1643 10.1111/j.1558-5646.2009.00924.x (doi:10.1111/j.1558-5646.2009.00924.x) [DOI] [PubMed] [Google Scholar]
- 64.McNamara K. B., Elgar M. A., Jones T. M. 2010. Adult responses to larval population size in the almond moth, Cadra cautella. Ethology 116, 39–46 10.1111/j.1439-0310.2009.01714.x (doi:10.1111/j.1439-0310.2009.01714.x) [DOI] [Google Scholar]
- 65.Vermeulen A., Engels S., Engqvist L., Sauer K. P. 2009. Phenotypic plasticity in sperm traits in scorpionflies (Mecoptera: Panorpidae): consequences of larval history and seasonality on sperm length and sperm transfer. Eur. J. Entomol. 106, 347–352 [Google Scholar]
- 66.Meck W. H., Church R. M. 1983. A mode control model of counting and timing processes. J. Exp. Psychol. Anim. Behav. Proc. 9, 320–334 10.1037/0097-7403.9.3.320 (doi:10.1037/0097-7403.9.3.320) [DOI] [PubMed] [Google Scholar]
- 67.Walsh V. 2003. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 7, 483–488 10.1016/j.tics.2003.09.002 (doi:10.1016/j.tics.2003.09.002) [DOI] [PubMed] [Google Scholar]
- 68.Ziege M., Mahlow K., Hennige-Schulz C., Kronmarck C., Tiedemann R., Streit B., Plath M. 2009. Audience effects in the Atlantic molly (Poecilia mexicana): prudent male mate choice in response to perceived sperm competition risk? Front. Zool. 6, 17. 10.1186/1742-9994-6-17 (doi:10.1186/1742-9994-6-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pettersson L. B., Ramnarine I. W., Becher S. A., Mahabir R., Magurran A. E. 2004. Sex ratio dynamics and fluctuating selection pressures in natural populations of the Trinidadian guppy, Poecilia reticulata. Behav. Ecol. Sociobiol. 55, 461–468 10.1007/s00265-003-0727-8 (doi:10.1007/s00265-003-0727-8) [DOI] [Google Scholar]
- 70.Montrose T. V., Edwin Harris W., Moore A. J., Moore P. J. 2008. Sperm competition within a dominance hierarchy: investment in social status vs. investment in ejaculates. J. Evol. Biol. 21, 1290–1296 10.1111/j.1420-9101.2008.01570.x (doi:10.1111/j.1420-9101.2008.01570.x) [DOI] [PubMed] [Google Scholar]
- 71.Oku K. 2009. Effects of density experience on mate guarding behavior by adult male Kanzawa spider mites. J. Ethol. 27, 279–283 10.1007/s10164-008-0117-9 (doi:10.1007/s10164-008-0117-9) [DOI] [Google Scholar]
- 72.Bretman A., Fricke C., Hetherington P., Stone R., Chapman T. 2010. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 21, 317–321 10.1093/beheco/arp189 (doi:10.1093/beheco/arp189) [DOI] [Google Scholar]
- 73.Stoltz J. A., Andrade M. C. B. 2009. Female's courtship threshold allows intruding males to mate with reduced effort. Proc. R. Soc. B 277, 585–592 10.1098/rspb.2009.1554 (doi:10.1098/rspb.2009.1554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edward D. A., Fricke C., Chapman T. 2010. Adaptations to sexual selection and sexual conflict: insights from experimental evolution and artificial selection. Phil. Trans. R. Soc. B 365, 2541–2548 10.1098/rstb.2010.0027 (doi:10.1098/rstb.2010.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Jong K., Wacker S., Amundsen T., Forsgren E. 2009. Do operational sex ratio and density affect mating behaviour? An experiment on the two-spotted goby. Anim. Behav. 78, 1229–1238 10.1016/j.anbehav.2009.08.006 (doi:10.1016/j.anbehav.2009.08.006) [DOI] [Google Scholar]
- 76.Price A. C., Helen Rodd F. 2006. The effect of social environment on male–male competition in guppies (Poecilia reticulata). Ethology 112, 22–32 10.1111/j.1439-0310.2006.01142.x (doi:10.1111/j.1439-0310.2006.01142.x) [DOI] [Google Scholar]
- 77.Mautz B. S., Jennions M. D. 2011. The effect of competitor presence and relative competitive ability on male mate choice. Behav. Ecol. 22, 769–775 10.1093/beheco/arr048 (doi:10.1093/beheco/arr048) [DOI] [Google Scholar]
- 78.Chapman M. R., Kramer D. L. 1996. Guarded resources: the effect of intruder number on the tactics and success of defenders and intruders. Anim. Behav. 52, 83–94 10.1006/anbe.1996.0154 (doi:10.1006/anbe.1996.0154) [DOI] [Google Scholar]
- 79.Keagy J., Savard J. F., Borgia G. 2011. Complex relationship between multiple measures of cognitive ability and male mating success in satin bowerbirds, Ptilonorhynchus violaceus. Anim. Behav. 81, 1063–1070 10.1016/j.anbehav.2011.02.018 (doi:10.1016/j.anbehav.2011.02.018) [DOI] [Google Scholar]
- 80.Shohet A. J., Watt P. J. 2009. Female guppies Poecilia reticulata prefer males that can learn fast. J. Fish Biol. 75, 1323–1330 10.1111/j.1095-8649.2009.02366.x (doi:10.1111/j.1095-8649.2009.02366.x) [DOI] [PubMed] [Google Scholar]
- 81.Lyons C., Barnard C. J. 2006. A learned response to sperm competition in the field cricket, Gryllus bimaculatus (de Geer). Anim. Behav. 72, 673–680 10.1016/j.anbehav.2005.12.006 (doi:10.1016/j.anbehav.2005.12.006) [DOI] [Google Scholar]
- 82.Matthews R. N., Domjan M., Ramsey M., Crews D. 2007. Learning effects on sperm competition and reproductive fitness. Psychol. Sci. 18, 758–762 10.1111/j.1467-9280.2007.01974.x (doi:10.1111/j.1467-9280.2007.01974.x) [DOI] [PubMed] [Google Scholar]
- 83.Boogert N. J., Fawcett T. W., Lefebvre L. 2011. Mate choice for cognitive traits: a review of the evidence in nonhuman vertebrates. Behav. Ecol. 22, 447–459 10.1093/beheco/arq173 (doi:10.1093/beheco/arq173) [DOI] [Google Scholar]
- 84.Clark A. G. 2002. Sperm competition and the maintenance of polymorphism. Heredity 88, 148–153 10.1038/sj.hdy.6800019 (doi:10.1038/sj.hdy.6800019) [DOI] [PubMed] [Google Scholar]
- 85.Simmons L. W., García-González F. 2008. Evolutionary reduction in testes size and competitive success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591 10.1111/j.1558-5646.2008.00479.x (doi:10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 86.Hosken D. J., Garner T. W. J., Ward P. I. 2001. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 11, 489–493 10.1016/s0960-9822(01)00146-4 (doi:10.1016/s0960-9822(01)00146-4) [DOI] [PubMed] [Google Scholar]
- 87.Holland B., Rice W. R. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088 10.1073/pnas.96.9.5083 (doi:10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lickliter R., Bahrick L. E., Markham R. G. 2006. Intersensory redundancy educates selective attention in bobwhite quail embryos. Dev. Sci. 9, 604–615 10.1111/j.1467-7687.2006.00539.x (doi:10.1111/j.1467-7687.2006.00539.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnstone R. A. 1996. Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Phil. Trans. R. Soc. B 351, 329–338 10.1098/rstb.1996.0026 (doi:10.1098/rstb.1996.0026) [DOI] [Google Scholar]
- 90.Smith C. C., Ryan M. J. 2010. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J. Evol. Biol. 23, 1759–1771 10.1111/j.1420-9101.2010.02041.x (doi:10.1111/j.1420-9101.2010.02041.x) [DOI] [PubMed] [Google Scholar]
- 91.Nakatsuru K., Kramer D. L. 1982. Is sperm cheap? Limited male fertility and female choice in the lemon Tetra (Pisces, Characidae). Science 216, 753–755 10.1126/science.216.4547.753 (doi:10.1126/science.216.4547.753) [DOI] [PubMed] [Google Scholar]
- 92.Pizzari T., Worley K., Burke T., Froman D. 2008. Sperm competition dynamics: ejaculate fertilising efficiency changes differentially with time. BMC Evol. Biol. 8, 332. 10.1186/1471-2148-8-332 (doi:10.1186/1471-2148-8-332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Awata S., Takeyama T., Makino Y., Kitamura Y., Kohda M. 2008. Cooperatively breeding cichlid fish adjust their testis size but not sperm traits in relation to sperm competition risk. Behav. Ecol. Sociobiol. 62, 1701–1710 10.1007/s00265-008-0598-0 (doi:10.1007/s00265-008-0598-0) [DOI] [Google Scholar]
- 94.Vaughn A. A., Ferkin M. H. 2011. The presence and number of male competitor's scent marks and female reproductive state affect the response of male meadow voles to female conspecifics' odours. Behaviour 148, 927–943 10.1163/000579511x584375 (doi:10.1163/000579511x584375) [DOI] [Google Scholar]
- 95.Vaughn A. A., delBarco-Trillo J. H., Ferkin M. H. 2010. Self-grooming by male meadow voles differs across copulation but is not affected by the risk and intensity of sperm competition. Behaviour 147, 259–274 10.1163/000579509x12523920754410 (doi:10.1163/000579509x12523920754410) [DOI] [Google Scholar]
- 96.delBarco-Trillo J. H., Ferkin M. H. 2007. Risk of sperm competition does not influence copulatory behavior in the promiscuous meadow vole (Microtus pennsylvanicus). J. Ethol. 25, 139–145 10.1007/s10164-006-0008-x (doi:10.1007/s10164-006-0008-x) [DOI] [Google Scholar]
- 97.LaMunyon C. W., Ward S. 1998. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc. R. Soc. Lond. B 265, 1997–2002 10.1098/rspb.1998.0531 (doi:10.1098/rspb.1998.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen C. W. 1991. Sex allocation in hermaphroditic sea basses. Am. Nat. 138, 650–667 10.1086/285240 (doi:10.1086/285240) [DOI] [Google Scholar]
- 99.Schärer L. 2009. Tests of sexual allocation theory in simultaneously hermaphroditic animals. Evolution 63, 1377–1405 10.1111/j.1558-5646.2009.00669.x (doi:10.1111/j.1558-5646.2009.00669.x) [DOI] [PubMed] [Google Scholar]
- 100.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 101.Ootsubo T., Sakai M. 1992. Initiation of spermatophore protrusion behavior in the male cricket Gryllus bimaculatus De Geer. Zoo. Sci. 9, 955–969 [Google Scholar]
- 102.Locher R., Baur B. 1999. Effects of intermating interval on spermatophore size and sperm number in the simultaneously hermaphroditic land snail Arianta arbustorum. Ethology 105, 839–849 10.1046/j.1439-0310.1999.00452.x (doi:10.1046/j.1439-0310.1999.00452.x) [DOI] [PubMed] [Google Scholar]
- 103.Reinhold K., Von Helversen D. 1997. Sperm number, spermatophore weight and remating in the bushcricket Poecilimon veluchianus. Ethology 103, 12–18 10.1111/j.1439-0310.1997.tb00002.x (doi:10.1111/j.1439-0310.1997.tb00002.x) [DOI] [Google Scholar]
- 104.Voight J. R. 2009. Differences in spermatophore availability among octopodid species (Cephalopoda: Octopoda). Malacologia 51, 143–153 10.4002/040.051.0110 (doi:10.4002/040.051.0110) [DOI] [Google Scholar]