Abstract
In indigenous arctic reindeer and ptarmigan, circadian rhythms are not expressed during the constant light of summer or constant dark of winter, and it has been hypothesized that a seasonal absence of circadian rhythms is common to all vertebrate residents of polar regions. Here, we show that, while free-living arctic ground squirrels do not express circadian rhythms during the heterothermic and pre-emergent euthermic intervals of hibernation, they display entrained daily rhythms of body temperature (Tb) throughout their active season, which includes six weeks of constant sun. In winter, ground squirrels are arrhythmic and regulate core body temperatures to within ±0.2°C for up to 18 days during steady-state torpor. In spring, after the use of torpor ends, male but not female ground squirrels, resume euthermic levels of Tb in their dark burrows but remain arrhythmic for up to 27 days. However, once activity on the surface begins, both sexes exhibit robust 24 h cycles of body temperature. We suggest that persistence of nycthemeral rhythms through the polar summer enables ground squirrels to minimize thermoregulatory costs. However, the environmental cues (zeitgebers) used to entrain rhythms during the constant light of the arctic summer in these semi-fossorial rodents are unknown.
Keywords: circadian rhythms, zeitgeber, body temperature, torpor, arctic ground squirrel
1. Introduction
Year-round residents of polar environments regularly cope in summer and winter with prolonged absences of available light–dark (LD) cycles of day and night for entrainment of circadian rhythms with geophysical time. In mammals, daily biochemical, physiological and behavioural functions are organized by a circadian master clock, contained within the suprachiasmatic nucleus (SCN) of the hypothalamus, which transmits its rhythmic information to other brain regions and peripheral organs via a variety of outputs [1]. The adaptive value of endogenous circadian rhythms has been supported by their ubiquitous nature among organisms, although in reindeer and ptarmigan that live in the arctic islands of Svalbard, there is recent evidence that circadian rhythms in behaviour and physiology are not expressed when daily transitions of dusk and dawn are not present [2,3]. From these observations, it was postulated that seasonal absence of circadian organization may be common to all resident polar vertebrates [2]. Supporting this hypothesis, the molecular clockwork that drives circadian rhythmicity is also weak or absent within the fibroblast cells of Svalbard reindeer [4]. However, the recent finding that bumble-bees display persistent nycthemeral rhythms in activity throughout the arctic summer suggests that entrainment of circadian rhythms in the absence of an LD cycle is possible and may have adaptive value for other arctic species [5]. To test this hypothesis in a small mammalian arctic resident, we monitored patterns of body temperature (Tb) throughout the year in free-living arctic ground squirrels in northern Alaska. Measurements of Tb are meaningful in assessing persistence of circadian rhythms because Tb affects animal energetics and performance and entrains peripheral clocks present in almost all cells of the body (reviewed in Dibner et al. [1]). Arctic ground squirrels hibernate through winters of 180–250 days while remaining sequestered in frozen hibernacula and subsisting on endogenous energy reserves [6]. Thus, our approach also provides a means of evaluating the debated issue of whether circadian Tb rhythms persist during hibernation [7,8].
2. Material and methods
Arctic ground squirrels (Urocitellus parryii) were studied near the Toolik Field Station (68°38′ N, 149°38′ W) on the North Slope of Alaska. Over 2006 and 2007, we obtained data on patterns of Tb during hibernation and across the summer active season from seven adult (greater than 1 year old) male and 11 adult female free-living animals using Tb-loggers (modified TidBits, Onset Computer Corp, Pocasset, MA, USA) implanted in the abdomen and programmed to record core Tb (±0.2°C) at 20 min intervals for up to 18 months (details are in Long et al. [9]). Analysis of Tb data, including creation of actograms and periodograms and testing for the significance and periodicity of rhythms in Tb using F-periodogram analysis, was performed using Clocklab software (Actimetrics, Evanston, IL, USA). We determined the period (τ) of Tb rhythms for 10 day blocks of data using F-periodogram analysis based on the algorhythm of Dörrscheidt & Beck [10]. We used a 20 min sampling interval and, consequently, any period of xh could fall within the range of xh±20 min [2]. We, therefore, established critical ranges to distinguish diel rhythms (τ = 24 ± 0.33 h) from potential circadian rhythms where τ ≈ 24 h (i.e. τ less than 23.67 or greater than 24.33).
3. Results
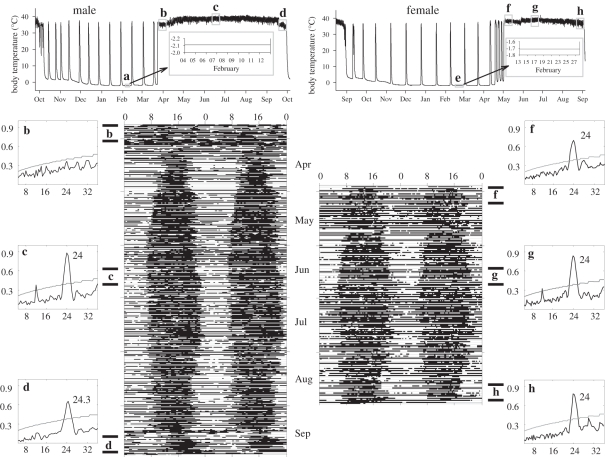
In hibernating males, Tb tracked soil temperature before soils froze but became constant (±0.2°C, within the resolution of the data logger) for up to 18 days during steady-state torpor when heat generation was required to prevent freezing (figure 1a). Following completion of their final torpor bout, when males become euthermic but remain sequestered in their burrows, males exhibited either an arrhythmic (six of seven) or ultradian (one of seven; τ = 5.67 h) Tb pattern (figure 1b). Robust and entrained Tb rhythms with a significant period of 24 h were established 10 to 27 days after males completed their final torpor bout, and these rhythms persisted throughout the active season in all individuals (figure 1c). In autumn, prior to initiating their first torpor bout of the hibernation season but while remaining in their burrows, three males exhibited low amplitude Tb rhythms for greater than or equal to 10 days. The period of these Tb rhythms during the first 10 days was 24.3 for two of the squirrels (i.e. not distinguishable from 24 h; one shown in figure 1d) and 24.6 (i.e. greater than 24 h) for the third ground squirrel. Similar to males, females exhibited constant Tb during steady-state torpor when soil temperature was substantially below freezing (figure 1e). Following their final torpor bout, all females rapidly developed a 24 h Tb rhythm that persisted throughout the active season (figure 1f–h).
Figure 1.
Annual patterns of core Tb of a representative male and female arctic ground squirrel together with double-plotted actograms of when Tb remained at euthermic levels (each row represents two consecutive days). For actograms, the x-axis is the time of day (hour) and black bars indicate intervals when Tb was above the mean. Blocks indicated by a and e during heterothermy of hibernation are torpor intervals that are expanded in the insert indicated by the arrow; b indicates the prolonged interval of euthermic Tb that occurs in males after heterothermy ends but before they emerge from their burrows; f indicates the post-emergence interval in females; g and c are mid active season intervals; d indicates when this male remained in the burrow prior to first torpor; h is the pre-immergence interval in this female. Box graphs b–d and f–h show the F—periodograms for rhythms of Tb that correspond to 10 day intervals indicated on the actograms and Tb traces. Black lines indicate the strength of rhythms (Q); curved grey lines indicate 95% confidence limit.
4. Discussion
We show that 24 h rhythms in Tb of arctic ground squirrels persist throughout the summer indicating that this species can probably entrain its master circadian clock to some external cue other than the LD cycle. These results are consistent with limited data indicating that caged ground squirrels exposed to ambient conditions at high latitude are more active during mid-day [11]. The Tb rhythms may not be driven by a circadian oscillator but instead reflect a direct behavioural response to 24 h fluctuations in ambient thermal conditions (i.e. masking). However, this appears unlikely given: (i) free-living squirrels spend their inactive periods below-ground and are therefore isolated to a great extent from the daily flux in environmental conditions to which they would need to respond, (ii) the initial increase in Tb does not result directly from activity but is anticipatory, occurring prior to daily emergence [9], and (iii) at least one of three males displayed Tb rhythms with a period greater than 24 h during the below-ground euthermic period prior to initiating torpor in autumn, which suggests that rhythmicity in Tb was controlled by a free-running circadian oscillator.
The ability of arctic ground squirrels to maintain 24 h Tb-rhythms in the absence of dusk and dawn is remarkable considering that, as semi-fossorial animals, they are not continuously exposed to parametric changes in light intensity, but rather expose themselves to discrete signal transitions from dark to light and vice-versa as they emerge and return to their dark burrows. This ability is not restricted to arctic species as the European ground squirrel (Spermophilus citellus), which occupies more temperate regions, remained entrained even though animals are not above-ground during the transition phases between light and dark [12]. Assuming ground squirrels use light as the primary cue to entrain their circadian rhythms, they must be sensitive to low-amplitude variation in parametric photic cues (such as intensity or spectral quality), yet insensitive to the larger variation in light induced by their own activity patterns. This could potentially be achieved through phase-dependent effects of light on the velocity of the pacemaker [12]. At the latitude our study was conducted, mean light intensity exhibits significant diel variation, although variability is high owing to the effects of cloud cover and fog [5]. Diel rhythms in the spectral composition of light at this latitude are generally more pronounced; Stelzer & Chittka [5] speculated that arctic bumble-bees, which display robust 24 h activity rhythms, might be entrained to rhythmicity in ultraviolet (UV) light. However, UV light does not penetrate the lens of the eye in European ground squirrels [13] and therefore, if this is also true in arctic ground squirrels, it should not play a role in their entrainment. Low-amplitude rhythms in ambient temperature are also unlikely to play a role because they are highly variable [9]. The position of the sun relative to local landmarks or its azimuth could theoretically serve as zeitgebers although their use would be limited during prolonged episodes of cloud cover.
Circadian clocks have adaptive value because they allow organisms to anticipate and exploit favourable environmental conditions that vary on a 24 h basis. We propose that maintenance of an entrained circadian clock with the centre of activity at solar noon during the arctic summer benefits arctic ground squirrels by allowing them to decrease their daily energy expenditure through timing their periods of above-ground activity to coincide with the warmer conditions that occur on a daily basis despite the 24 h daylight. Continuous Tb measures of arctic ground squirrel manikins placed in their natural habitat demonstrated that, through the active season, ambient thermal conditions are typically within the thermoneutral zone of ground squirrels during the daytime but always favour heat loss between 22.00 and 5.00 h [9]. Differences in the maintenance of rhythmicity between ground squirrels and similarly sized Svalbard ptarmigan, which do not exhibit diel rhythmicity during the arctic summer [3], may reflect that ground squirrels can exploit a thermal refuge of a burrow system and nest and thereby avoid exposure during the coldest time of day. Differences between these species may also be owing to latitudinal effects as ptarmigan were studied in the high arctic at 78°N. It is also possible that other ecological factors, such as predation pressure, influence differences in the persistence of daily rhythms among arctic species.
We found no evidence for low-amplitude Tb rhythms during prolonged deep torpor as has been reported in other species maintained in captivity (reviewed in Heller & Ruby [7]). It is possible that expression of circadian genes within the SCN of free-living arctic ground squirrels is arrested during the low Tb that accompanies deep torpor, as was found in captive hibernating European hamsters (Cricetus cricetus) held at 6°C [8]. Arrhythmicity following completion of the final torpor bout of the season in males is presumably associated with the period of below-ground euthermia that accompanies testicular recrudescence [14]. Such arrhythmicity might indicate that the circadian clock becomes internally desynchronized during hibernation. Alternatively, in the absence of photic stimuli, the SCN may remain uncoupled from the output pathways responsible for the generation of Tb rhythms. Expression of robust, entrained rhythms, however, begins as soon as animals are exposed to a daily light signal at emergence from hibernation [15].
Acknowledgements
All procedures were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee and work was conducted under appropriate state permits.
Funding was provided by the National Science Foundation to B.M.B. (EF-0732755) and C.L.B. (EF-0732763) and awards from the US Army Medical Research and Material Command (no. 05178001) to B.M.B.
References
- 1.Dibner C., Schibler U., Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 10.1146/annurev-physiol-021909-135821 (doi:10.1146/annurev-physiol-021909-135821) [DOI] [PubMed] [Google Scholar]
- 2.Van Oort B. E. H., Tyler N. J. C., Gerkema M. P., Folkow L., Blix A. S., Stokkan K. A. 2005. Circadian organization in reindeer. Nature 438, 1095–1096 10.1038/4381095a (doi:10.1038/4381095a) [DOI] [PubMed] [Google Scholar]
- 3.Reierth E., Stokkan K. A. 1998. Activity rhythm in high Arctic Svalbard ptarmigan (Lagopus mutus hyperboreus). Can. J. Zool. 76, 2031–2039 [Google Scholar]
- 4.Lu W., Meng Q. J., Tyler N. J. C., Stokkan K. A., Loudon A. S. 2010. A circadian clock is not required in an arctic mammal. Curr. Biol. 20, 533–537 10.1016/j.cub.2010.01.042 (doi:10.1016/j.cub.2010.01.042) [DOI] [PubMed] [Google Scholar]
- 5.Stelzer R. J., Chittka L. 2010. Bumblebee foraging rhythms under the midnight sun measured with radiofrequency identification. BMC Biol. 8, 93. 10.1186/1741-7007-8-93 (doi:10.1186/1741-7007-8-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck C. L., Barnes B. M. 1999. Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J. Mamm. 80, 430–442 10.2307/1383291 (doi:10.2307/1383291) [DOI] [Google Scholar]
- 7.Heller H. C., Ruby N. F. 2004. Sleep and circadian rhythms in mammalian torpor. Annu. Rev. Physiol. 66, 275–289 10.1146/annurev.physiol.66.032102.115313 (doi:10.1146/annurev.physiol.66.032102.115313) [DOI] [PubMed] [Google Scholar]
- 8.Revel F. G., Herwig A., Garidoo M. L., Dardente H., Menet J. S., Masson-Pévet M., Simonneaux V., Saboureau M., Pévet P. 2007. The circadian clock stops ticking during deep hibernation in the European hamster. Proc. Natl Acad. Sci. USA 104, 13 816–13 820 10.1073/pnas.0704699104 (doi:10.1073/pnas.0704699104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long R. A., Martin T. J., Barnes B. M. 2005. Body temperature and activity patterns in free-living arctic ground squirrels. J. Mamm. 86, 314–322 10.1644/BRG-224.1 (doi:10.1644/BRG-224.1) [DOI] [Google Scholar]
- 10.Dörrscheidt G. L., Beck L. 1975. Advanced methods for evaluating characteristic parameters (α, τ, ρ) of circadian rhythms. J. Math. Biol. 2, 107–121 [Google Scholar]
- 11.Swade R. H., Pittendrigh C. S. 1967. Circadian locomotor activity of rodents in the arctic. Am. Nat. 101, 433–466 10.1086/282510 (doi:10.1086/282510) [DOI] [Google Scholar]
- 12.Hut R., Van Oort B. E. H., Daan S. 1999. Natural entrainment without dawn or dusk: the case of the European ground squirrel. J. Biol. Rhythms 14, 290–299 10.1177/074873099129000704 (doi:10.1177/074873099129000704) [DOI] [PubMed] [Google Scholar]
- 13.Hut R. A., Shepper A., Daan S. 2000. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J. Comp. Physiol. A 186, 707–715 10.1007/s003590000124 (doi:10.1007/s003590000124) [DOI] [PubMed] [Google Scholar]
- 14.Barnes B. M. 1996. Relationships between hibernation and reproduction in male ground squirrels. In Adaptations to the cold (eds Geiser F., Hulbert A. J., Nicol S. C.), pp. 71–80 Armidale, Australia: University of New England Press [Google Scholar]
- 15.Williams C. T., Sheriff M. J., Schmutz J. A., Kohl F., Tøien Ø., Buck C. L., Barnes B. M. In press Data logging of body temperatures provides precise information on phenology of reproductive events in a free-living arctic hibernator. J. Comp. Physiol. B (doi:10.1007/s00360-011-0593-z) [DOI] [PubMed] [Google Scholar]