Abstract
The four-eyed fish, Anableps anableps, has eyes with unusual morphological adaptations for simultaneous vision above and below water. The retina, for example, is divided such that one region receives light from the aerial field and the other from the aquatic field. To understand better the adaptive value of this partitioned retina, we characterized photoreceptor distribution using in situ hybridization. Cones expressing sws1, sws2b and rh2-2 (i.e. UV, and short wavelength-sensitive) opsins were found throughout the retina, whereas cones expressing rh2-1 (middle wavelength-sensitive) were largely limited to the ventral retina and those expressing lws (long wavelength-sensitive) opsins were only expressed in the dorsal retina. We next asked when this pattern evolved relative to the ‘four-eyed’ morphology. We characterized opsin expression in Jenynsia onca, a member of the sister genus to Anableps with typical teleost eye morphology. In J. onca, sws1, sws2b, rh2-2 and rh2-1 opsins were expressed throughout the retina; while lws opsins were not expressed in the ventral retina. Thus, the change that coincides with the evolution of unusual anablepid eye morphology is the loss of rh2-1 expression in the dorsal retina, probably to accommodate increased lws opsin expression. The retinal area that samples aerial light appears not to have changed with respect to photoreceptor transcription.
Keywords: fish, visual pigment, wavelength sensitivity, intraretinal variability, spectral tuning, retinal topography
1. Introduction
The four-eyed fish, genus Anableps, presents a unique opportunity for studying how visual pigments play a role in adaptation to heterogeneous spectral environments and diverse visual tasks. Anableps anableps, typical of its genus, has large eyes extending above its head, allowing it to see above the waterline while keeping the rest of its body, and half of each eye, underwater. A pigmented band across the midline of the cornea creates distinct dorsal and ventral pupils, while an ovoid lens has evolved from the typical spherical lens of the fish to optimize simultaneous focusing of aerial and aquatic light [1]. The dorsal region of the retina receives upwelling light filtered through water and dissolved solutes that alter the spectral content. The ventral retina receives aerial light unfiltered by water. Thus, the eyes receive both broad-spectrum light and dimmer, narrow-spectrum light, but in separate regions of the retina (figure 1). This represents an exaggerated example of the spectral heterogeneity common to surface-dwelling fish, as upwelling light is filtered to a greater degree by water than downwelling light in near-surface aquatic environments. Previous work in fish has shown differences in sensitivity or opsin expression between regions of the retina, but the functional significance of intraretinal variability in fishes remains unclear ([2,3] but see [4]).
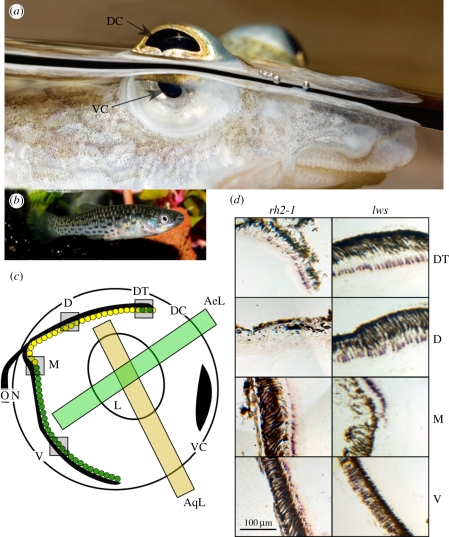
Figure 1.
Comparison of anablepid eye morphology. (a) A. anableps eye at the water surface. The dorsal cornea receives light from the aerial environment, while the ventral cornea receives light from the aquatic environment. DC, dorsal cornea; VC, ventral cornea. Photo by Andreas Werth. (b) Jenynsia onca female. The eye is morphologically normal in this species. Photo by Leo van der Meer. (c) A schematic of a sagittal section of an A. anableps eye. Light paths are indicated. Green and yellow dots indicate rh2-1 and lws gene expression, respectively. AeL, aerial line-of-sight; AqL, aquatic line-of-sight; L, lens; ON, optic nerve; DT, dorsal tip of the retina; D, dorsal retina; M, medial retina; V, ventral retina. (d) In situ hybridization images of adult A. anableps labelled with the rh2-1 or lws riboprobe. Cone cells expressing the gene of interest are purple. The brown area is retinal pigment epithelium.
Vertebrate vision is dependent upon five families of G-protein-coupled receptors collectively called visual opsins that are expressed in retinal rods and cones. The common ancestor of all vertebrates possessed two short wavelength-sensitive (sws1 and sws2), one middle wavelength-sensitive (rh2) and one long wavelength-sensitive (lws) cone opsin subfamilies [5]. Owing to the readily measurable connection between genotype (opsin gene sequence) and phenotype (wavelength of maximum sensitivity), much work has been done to correlate opsin gene repertoire, sequence and expression patterns with ecological factors [6]. One of the more consistent correlates identified is the spectral composition of ambient light [7,8]. However, so far, these associations have not been observed in the same species and at the same time in development. With the four-eyed fish, we can examine opsin expression in regions of the retina exposed to different spectral environments in the same individual at the same time.
We used in situ hybridization with opsin riboprobes to map cone opsin expression in the retina of A. anableps. This was repeated for Jenynsia onca, a species with normal eye morphology in the sister genus to Anableps. Recently, both species have had their cone opsin repertoires characterized, revealing nine genes in A. anableps, (one sws1, two sws2, two rh2 and four lws genes), and eight in J. onca (one less lws gene) [9,10]. By studying J. onca, we have inferred ancestral expression patterns and identified changes that have evolved in concert with the unique eye morphology of A. anableps.
2. Material and methods
The in situ hybridization procedure was adapted from a previously described protocol ([11,12] and see methods in the electronic supplementary material). Briefly, unique digoxigenin-labelled riboprobes, 352–792 bp in length, were designed from the A. anableps genes: sws1, sws2a, sws2b, rh2-1 and rh2-2. Both A. anableps and J. onca have recent duplications in the lws subfamily. Thus, owing to the high degree of sequence similarity among the lws paralogues, unique riboprobes could not be produced. Rather, in this study we used one riboprobe designed to bind all lws paralogues. Both eyes from A. anableps (two adults and two juveniles) and J. onca (three adults) were prepared and sectioned in the sagittal or transverse plane. Riboprobes were used individually on serial sections and overall expression patterns were inferred by comparing sections of both orientations and making use of the optic nerve as a landmark. Additional experiments on a third adult A. anableps were performed to confirm results for lws and rh2-1 riboprobes. Riboprobe specificity was confirmed with a riboprobe-RNA dot blot assay (data not shown).
3. Results
In situ hybridization was used to characterize the topography of cone photoreceptor subtypes. Anableps anableps and J. onca exhibited uniform distributions of cones expressing sws1, sws2b and rh2-2 (figure 2 and figures in the electronic supplementary material). Neither species exhibited detectable sws2a expression in the retina. Species-specific differences were noted for the rh2-1-expressing cones. In J. onca, rh2-1 had uniform expression across the retina in all sections, whereas adult A. anableps had rh2-1 in a large number of ventral cone cells and in a small patch of cones at the dorsal tip of the retina. This pattern was observed in all adults. In juveniles, rh2-1 expression was confined to a smaller number of cells in the ventral half of the eye, or was entirely absent. The lws riboprobe, which was designed to bind all lws paralogues, revealed inter-individual and interspecific differences in lws cone photoreceptor distributions. In J. onca, lws-expressing cones were limited to the dorsal retina in two fish (a male and a female) and to a transverse streak in the middle of the eye in one male. In A. anableps, lws cones were detected only in the dorsal half of the retina. This pattern appears to be the inverse of the rh2-1 cone distribution (figure 1).
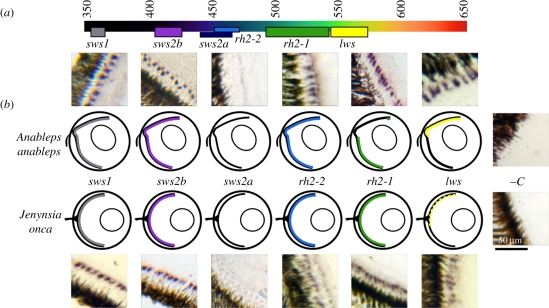
Figure 2.
Summary of expression domains for each gene tested in A. anableps and J. onca. (a) The visual spectrum as seen by humans with putative spectral sensitivity for each opsin tested. Numbers are in nanometres. (b) Schematic of eyes with dorsal on top, ventral on bottom. Coloured areas indicated expression, while dotted bars indicated areas of polymorphic expression. Cropped microscope images are retinal sections probed using each riboprobe. Cone cells expressing the gene of interest are purple.
4. Discussion
Anableps anableps' remarkable eyes simultaneously sample photons from terrestrial and aquatic habitats. In situ hybridization with six riboprobes demonstrated that A. anableps and the closely related J. onca express at least five cone opsins (sws1, sws2b, rh2-1, rh2-2 and lws), possibly more given the redundancy of our lws probe.
The wavelength of maximal sensitivity for a visual pigment (and consequently the photoreceptor expressing it) can be determined by microspectrophotometry (MSP) or in vitro protein reconstitution [13,14]. By comparing maximal sensitivity data from A. anableps and its relatives obtained using these techniques, we have endeavoured to assign specific opsin genes to cone cell spectral sensitivities: sws1 (356–365 nm, UV), sws2b (405–425 nm, violet), rh2-2 (452–472 nm, blue), rh2-1 (492–539 nm, green) and lws (543–576 nm, yellow) (figure 2a and discussed in supplementary materials). While these represent the value for individual opsin proteins, multiple proteins may be found co-expressed in a single cone, causing intermediate sensitivity values for the photoreceptor.
Early MSP work showed no difference in the prevalence of different cone cells between retinal halves [15]. However, our in situ hybridization experiments, which are better suited to assess photoreceptor distributions because they sampled more photoreceptors by several orders of magnitude, did detect differences: the dorsal retina, used for aquatic vision, has cone photoreceptors that express sws1, sws2b, rh2-2 and lws, while the ventral retina, used for aerial vision, expresses sws1, sws2b, rh2-2 and rh2-1 (figure 2). From these observations, we predict that wavelength sensitivity differs in the dorsal and ventral regions of the retina.
The pattern in A. anabelps of lws-positive cones only in the dorsal region of the retina and rh2-1-positive cones only in the ventral retina appears to have evolved at the same time as the unusual eye morphology. rh2-1 is expressed throughout the eye in J. onca and in another close relative, the guppy (Poecilia reticulata; D. J. Rennison 2011, personal communication). rh2 and lws genes are typically expressed in double cones, a two-cell complex characterized by a joint inner membrane and neighbouring outer segments. Thus, the reduction in rh2-1 transcripts in the dorsal region of the eye in A. anableps may be a trade-off that permits an increase in lws expression. Previous work has shown increases in long wavelength sensitivity in the dorsal retina through differences in chromophore use in frogs [16]. Here, we show evidence for a convergent adaptation mediated by changes in gene expression.
The reduction of rh2-1 expression and retention of lws expression in the dorsal retina suggest that A. anableps has enhanced sensitivity to long wavelength light (543–576 nm) in the aquatic field of view. This enhanced sensitivity could be advantageous in the brackish waters of the mangrove forests and river deltas that A. anableps inhabits, as these often contain dissolved organic matter that shifts light abundance to longer wavelengths [17]. Light measurements taken in similar mangrove habitat showed that downwelling light is most prevalent at approximately 500 nm, while upwelling light peaks at approximately 580 nm [4]. We propose that, by the differential expression of rh2-1 and lws, A. anableps is better able to match its double cones to the background light in each field of view.
Archerfish (Toxotes chatareus) experience a similar light environment to A. anableps and have had their vision studied using MSP [4]. They were found to have double cones in the dorsal retina that were most sensitive to the most prevalent wavelengths of upwelling light. However, unlike A. anableps, double cones in the ventro-nasal retina in archerfish were shifted to even longer wavelengths, suggesting that the ventral exclusion of lws opsin expression seen in three surface-dwelling, cyprinodontiformes fish has not occurred. This implies that other features beyond light environment, such as phylogeny or the visual tasks required, may affect opsin expression.
While the guppy expresses rh2-1 throughout the retina, recent in situ experiments in this species show many more lws-expressing cone cells in the dorsal retina than in the ventral retina, similar to J. onca (D. J. Rennison 2011, personal communication). Thus, it appears that the pattern we observe in A. anableps (non-overlapping lws and rh2-1 opsin expression domains) represents an exaggeration and the fine-tuning of what may be a common trend in surface-dwelling fish (although see [4]). Interestingly, it appears that the distinct opsin expression domains are driven primarily by the muddy water environment in which A. anableps lives. Opsin expression in the ventral retina, which in A. anableps is exposed to aerial light, is the same in the near surface-dwelling J. onca and P. reticulata. Thus, evolution of morphology has allowed for aerial vision, while gene expression has permitted fine-tuning of aquatic vision in this remarkable fish eye. Behavioural tests on wavelength sensitivity and discrimination in the aerial and aquatic fields of view may shed light on how these differences in expression domains influence vision.
Acknowledgements
We thank three anonymous reviewers for helpful suggestions. We were supported by NSERC Discovery grants (J.S.T.) and graduate scholarship (G.L.O.), as well as University of Victoria graduate fellowships (G.L.O. and D.J.R). We thank the University of Victoria advanced imaging centre for their assistance.
References
- 1.Klinkowstrøm A. 1895. Beitrage zur Kenntnis das Auge von Anableps tetrophthalmus Skan. Arck. Physiol. 5, 67–69 [Google Scholar]
- 2.Levine J. S., MacNichol E. F., Jr, Kraft T., Collins B. A. 1979. Intraretinal distribution of cone pigments in certain teleost fishes. Science 204, 523–526 10.1126/science.432658 (doi:10.1126/science.432658) [DOI] [PubMed] [Google Scholar]
- 3.Takechi M., Kawamura S. 2005. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. J. Exp. Biol. 208, 1337–1345 10.1242/jeb.01532 (doi:10.1242/jeb.01532) [DOI] [PubMed] [Google Scholar]
- 4.Temple S., Hart N. S., Marshall N. J., Collin S. P. 2010. A spitting image: specialization in archerfish eyes for vision at the interface between air and water. Proc. R. Soc. B 277, 2607–2615 10.1098/rspb.2010.0345 (doi:10.1098/rspb.2010.0345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies W. L., Cowing J. A., Carvalho L. S., Potter I. C., Trezise A. E. O., Hunt D. M., Collin S. P. 2007. Functional characterization, tuning, and regulation of visual pigment gene expression in an anadromous lamprey. FASEB J. 21, 2713–2724 10.1096/fj.06-8057com (doi:10.1096/fj.06-8057com) [DOI] [PubMed] [Google Scholar]
- 6.Bowmaker J. K. 1999. The ecology of visual pigments. Novartis Found. Symp. 224, 21–31 10.1002/9780470515693.ch3 (doi:10.1002/9780470515693.ch3) [DOI] [PubMed] [Google Scholar]
- 7.Hope A. J., Partridge J. C., Hayes P. K. 1998. Switch in rod opsin gene expression in the European eel, anguilla anguilla (L.). Proc. R. Soc. Lond. B 265, 869–874 10.1098/rspb.1998.0372 (doi:10.1098/rspb.1998.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lythgoe J. N. 1984. Visual pigments and environmental light. Vision Res. 24, 1539–1550 10.1016/S0042-6989(84)80003-6 (doi:10.1016/S0042-6989(84)80003-6) [DOI] [PubMed] [Google Scholar]
- 9.Windsor D. J., Owens G. L. 2009. The opsin repertoire of Jenynsia onca: a new perspective on gene duplication and divergence in livebearers. BMC Res. Notes 2, 159. 10.1186/1756-0500-2-159 (doi:10.1186/1756-0500-2-159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens G. L., Windsor D. J., Mui J., Taylor J. S. 2009. A fish eye out of water: ten visual opsins in the four-eyed fish, Anableps anableps. PLoS ONE 4, e5970. 10.1371/journal.pone.0005970 (doi:10.1371/journal.pone.0005970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison W. T., Dann S. G., Helvik J. V., Bradley C., Moyer H. D., Hawryshyn C. W. 2003. Ontogeny of ultraviolet-sensitive cones in the retina of rainbow trout (Oncorhynchus mykiss). J. Comp. Neurol. 461, 294–306 10.1002/cne.10682 (doi:10.1002/cne.10682) [DOI] [PubMed] [Google Scholar]
- 12.Barthel L. K., Raymond P. A. 2000. In situ hybridization studies of retinal neurons. Method Enzymol. 316, 579–586 10.1016/S0076-6879(00)16751-5 (doi:10.1016/S0076-6879(00)16751-5) [DOI] [PubMed] [Google Scholar]
- 13.Bowmaker J. K. 1984. Microspectrophotometry of vertebrate photoreceptors: a brief review. Vision Res. 24, 1641–1650 10.1016/0042-6989(84)90322-5 (doi:10.1016/0042-6989(84)90322-5) [DOI] [PubMed] [Google Scholar]
- 14.Oprian D. D., Molday R. S., Kaufman R. J., Khorana H. G. 1987. Expression of a synthetic bovine rhodopsin gene in monkey kidney cells. Proc. Natl Acad. Sci. USA 84, 8874–8878 10.1073/pnas.84.24.8874 (doi:10.1073/pnas.84.24.8874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery J. A., Bowmaker J. K. 1982. Visual pigments in the four-eyed fish, Anableps anableps. Nature 298, 62–63 10.1038/298062a0 (doi:10.1038/298062a0) [DOI] [Google Scholar]
- 16.Reuter T. E., White R. H., Wald G. 1971. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J. Gen. Physiol. 58, 351–371 10.1085/jgp.58.4.351 (doi:10.1085/jgp.58.4.351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller R. R. 1979. Ecology, habits and relationships of the middle american cuatro ojos, anableps dowi (pisces: Anablepidae). Copeia 1, 82–91 10.2307/1443732 (doi:10.2307/1443732) [DOI] [Google Scholar]