Abstract
We explored associations between the common protozoan parasite Toxoplasma gondii and brain cancers in human populations. We predicted that T. gondii could increase the risk of brain cancer because it is a long-lived parasite that encysts in the brain, where it provokes inflammation and inhibits apoptosis. We used a medical geography approach based on the national incidence of brain cancers and seroprevalence of T. gondii. We corrected reports of incidence for national gross domestic product because wealth probably increases the ability to detect cancer. We also included gender, cell phone use and latitude as variables in our initial models. Prevalence of T. gondii explained 19 per cent of the residual variance in brain cancer incidence after controlling for the positive effects of gross domestic product and latitude among nations. Infection with T. gondii was associated with a 1.8-fold increase in the risk of brain cancers across the range of T. gondii prevalence in our dataset (4–67%). These results, though correlational, suggest that T. gondii should be investigated further as a possible oncogenic pathogen of humans.
Keywords: Toxoplasma gondii, brain cancer, medical geography
1. Introduction
Despite considerable research, the causes of brain cancers remain largely unknown. A few studies have found weak linkages between environmental factors and primary brain cell-derived tumours or lymphomas. In particular, brain cancers have been linked to ionizing radiations, though some have hypothesized associations with pesticides, solvents, electromagnetic fields (including cell phones) and nitrosamines in processed meats [1–4].
Persistent infections may promote cancer because long-term host defensive responses induce inflammation, which increases mutation rates [5]. In addition, intracellular pathogens may disrupt traditional cell barriers to cancer, allowing oncogenic mutations to accumulate through time [6]. Current evidence links Epstein–Barr virus, hepatitis B and C viruses, the bacterium Helicobacter pylori, human papilloma virus and the trematode Schistosoma haematobium to cancers of the lymph, liver, stomach, cervix and bladder, respectively [7]. Although several types of viruses (retroviruses, papovaviruses and adenovirus) cause brain tumours in experimental animals, few epidemiological studies have addressed the potential for pathogens to cause human brain cancers (see [2] for a review). Human cytomegalovirus is not currently causally implicated in human cancer, but emerging evidence suggests that infection and expression may be specifically associated with human malignancies, including malignant glioma [8].
Toxoplasma gondii is an apicomplexan parasite with a worldwide distribution. Cats are the final host and humans (and other warm-blooded vertebrates) are intermediate hosts. Following a period of asexual reproduction by tachyzoite forms (to which the host responds aggressively), the parasite enters a latent phase in a bradyzoite stage that persists for the host's lifetime in pseudocysts, macrophages and neurons of various tissues, notably in the brain [9]. About one-third of the world human population has antibodies to T. gondii [10]. Although conventional medicine considers latent toxoplasmosis asymptomatic, recent research has linked toxoplasmosis with a number of neurological pathologies [11,12]. In the latent phase, the cysts provoke the immune system (e.g. lymphocytes, plasma cells and macrophages), stimulating mild inflammation [13]. Also, they inhibit programmed cell death [14]. Toxoplasma gondii has been reported to cause gliomas in experimental animals [15,16]. One study by Schuman et al. [17] convincingly linked astrocytoma with antibodies to T. gondii. Ryan et al. [18] showed no association with adult glioma, but did report an association with meningioma.
An obvious test of the hypothesis that T. gondii promotes brain cancer would be to compare measures of T. gondii in cancer patients with controls. Schlehofer et al. [19] looked at the medical history of patients with brain tumours, finding that subjects with allergic conditions and a history of colds and flu had reduced risk of glioma. Unfortunately, this study had less than 2 per cent of cases where the individual had clinically reported toxoplasmosis, and the much more prevalent condition of latent toxoplasmosis was not assessed. Here, we approached the question using medical geography (see [20]). The variation in prevalence of subclinical infection with T. gondii from nation to nation provides an opportunity for a comparative study using data from human populations throughout the world.
2. Material and methods
We asked whether the prevalence of T. gondii in a nation was positively associated with the incidence of brain cancers in that nation. Because a variety of other factors could influence brain cancer, brain cancer detection and T. gondii, we considered possible effects of gender, wealth and environment.
(a). Data sources
International statistics on brain cancers in men and women were obtained from the International Agency for Research on Cancer (IARC GLOBOCAN project, year 2008, http://globocan.iarc.fr/). These data concern only malignant tumours, those classified C70-72 in the International Classification of Disease (ICD, 10th revision, available at http://apps.who.int/classifications/apps/icd/icd10online/). We did not include mortality data in our analysis as this variable is influenced by access to therapies, a parameter that strongly varies between countries. Instead, we used incidence data (age-standardized rate) that derive from population-based cancer registries. Age-standardized international statistics on T. gondii seroprevalence were from Lafferty [21]. Data on the per capita gross domestic product (GDP) for each country are available at https://www.cia.gov/library/publications/the-world-factbook/rankorder/2004rank.html. We obtained data on cell phone use (year 2010) from the Population reference Bureau (http://www.prb.org/Publications/Datasheets/2010/2010wpds.aspx).
(b). Analyses
Some of the recent increases in the incidence of brain cancers appear to be related to increased ability to detect disease using new technology and greater awareness [22]. We were concerned that detection might also vary owing to wealthy nations having higher detection ability. For this reason, we controlled for national wealth in nations by regressing the incidence of brain cancers against per capita gross domestic product (GDP) and taking the residuals (for a GDP-corrected measure of incidence). Data on all variables and T. gondii prevalence were available for 37 countries.
We used general linear models for hypothesis testing. Preliminary analysis indicated no effect of gender, so the incidence of males and females was averaged. Cell phone use was a competing environmental factor that has been hypothesized to increase cancer risk [23], and we considered this and T. gondii as competing explanatory factors in an initial model. Finally, because latitude was correlated with the prevalence of T. gondii and the GDP-corrected incidence of brain cancers, we conducted a separate analysis in which we further corrected the incidence of brain cancers for a potential effect of latitude. Using residuals to correct for priority effects may lead to inflated degrees of freedom. For this reason, we conducted a sequential F-test that assessed the effect of T. gondii prevalence after the effects of GDP were considered in a single model. To meet assumptions of normality, GDP, incidence of brain cancer and prevalence were transformed with the natural log, and the logit transformation was applied to prevalence. Ideally, we would have used direct measures of detection and other measures of infectious diseases potentially associated with brain cancers, but these data were not available to us.
3. Results and discussion
Variation in the incidence of brain cancers among nations increased with GDP (r2 = 0.52, p < 0.0001), consistent with the hypothesis that detection rates increase with wealth [21], and/or that brain tumour risk increases with increasing socioeconomic status. After accounting for GDP (natural log-transformed), cell phone use and gender were not significant factors (when analysed concurrently with T. gondii). The residual incidence of brain cancers after correcting for GDP increased significantly with the age-standardized, natural log-transformed prevalence of antibodies to T. gondii (n = 37 nations, r2 = 0.15, p = 0.019). A similar significant effect of T. gondii occurred for the sequential F-test (p = 0.016). In other words, after controlling for detection effort, the only significant predictor of brain cancers was T. gondii prevalence.
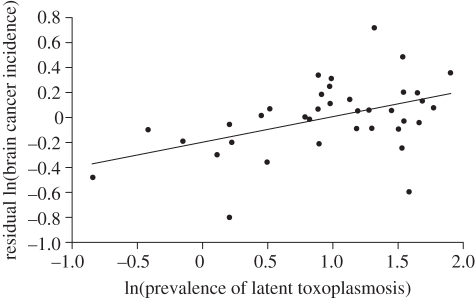
Like all medical geography studies, our results are correlational and explainable by alternative hypotheses. In addition, analyses of data aggregated at the population level may not pertain to individual risk [24,25]. This leaves open the possibility that brain cancers and T. gondii are both affected by a third correlated factor. For the environmental factors that we considered (latitude, longitude and cell phone use), only latitude was substantially associated with both T. gondii and the GDP-corrected incidence of brain cancers. After statistically controlling for the association between latitude and the incidence of brain cancers, the latitude-corrected incidence of brain cancers had a stronger positive association with T. gondii (r2 = 0.21, p = 0.005) than our initial analysis. In other words, prevalence of T. gondii explained a fifth of the residual variance of the incidence rate in brain cancers after controlling for the positive effects of GDP and latitude among nations (figure 1). Performing a similar analysis for the incidence of 46 cancers (and correcting for multiple comparisons) finds five significant associations with T. gondii: brain in men, brain in women, larynx in men, larynx in women and lung in men.
Figure 1.
The correlation between the incidence of brain cancers in adults from 37 countries and the prevalence of T. gondii (proportion of infected people, natural log-transformed). The residuals of incidence control for GDP (as a proxy of detection) and a positive (and unexplained) influence of latitude on brain cancer incidence. We averaged the incidence of brain cancers between males and females before plotting.
Although we do not currently have information at the level of infected and uninfected individuals, the slope of the relationship in figure 1 (intercept = 0.27 (±0.10), slope = 0.20 (±0.07)) suggests that across the range of prevalence in infection with T. gondii in our study (from 4 to 67%), there was a 1.8-fold increase in the incidence of brain cancers.
Other unmeasured variables could drive a spurious association with T. gondii. Furthermore, there are several types of brain cancers in our dataset, and these probably have diverse origins, suggesting that no one factor should be expected to explain a large amount of variation in the incidence of brain cancers among nations. Other infectious organisms might be carcinogenic (e.g. viruses, see [2]), but we did not have sufficient data to analyse these. Another limitation of this study is that we only had T. gondii data from a limited number of countries, so the conclusions would only be generalizable to other countries that are similar to those for which the prevalence values were available [26]. However, despite the limitations of a medical geography approach, we feel our results are sufficiently strong to propose that T. gondii potentially increases the risk of brain cancers in humans. Clearly, further research is necessary to determine the proximate links between T. gondii and different types of brain tumours and to investigate a mechanism of action. Other correlational studies are possible. For instance, the National Cancer Institute has an archive of blood from brain cancer patients that could be screened for antigens to T. gondii. Establishing a link between T. gondii and brain cancers could open the door to potential means to reduce cancer risk. Another interesting research direction would be to explore whether T. gondii has oncogenic effects when in synergy with other infections. Toxoplasma gondii might contribute to brain cancers in concert with viruses that have been associated with brain cancers (see §1), much as Plasmodium falciparum contributes to Burkitt's lymphoma in synergy with Epstein–Barr virus (see [6] for a review).
Acknowledgements
We thank A. Kuris, M. Sandoval and three anonymous referees for comments on the draft. This work was supported by the French consortium Evolution et Cancer (CNRS).
References
- 1.Preston-Martin S. 1996. Epidemiology of primary CNS neoplasms. Neurol. Clin. 14, 273–290 10.1016/S0733-8619(05)70256-5 (doi:10.1016/S0733-8619(05)70256-5) [DOI] [PubMed] [Google Scholar]
- 2.Wrensch M., Minn Y., Chew T., Bondy M., Berger M. S. 2002. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 4, 278–299 10.1215/S152285170200011X (doi:10.1215/S152285170200011X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher J. L., Schwartzbaum J. A., Wrensch M., Wiemels J. L. 2007. Epidemiology of brain tumors. Neurol. Clin. 25, 867–890 10.1016/j.ncl.2007.07.002 (doi:10.1016/j.ncl.2007.07.002) [DOI] [PubMed] [Google Scholar]
- 4.Nielsen S. S., McKean-Cowdin R., Farin F. M., Holly E. A., Preston-Martin S., Mueller B. A. 2009. Childhood brain tumors, residential insecticide exposure, and pesticide metabolism. Genes. Environ. Health Perspect. 118, 144–149 10.1289/ehp.0901226 (doi:10.1289/ehp.0901226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick F. A. 2001. Inflammation, carcinogenesis and cancer. Int. Immunopharmacol. 11, 1651–1667 10.1016/S1567-5769(01)00102-3 (doi:10.1016/S1567-5769(01)00102-3) [DOI] [PubMed] [Google Scholar]
- 6.Ewald P. W. 2009. An evolutionary perspective on parasitism as a cause of cancer. Adv. Parasitol. 68, 21–43 10.1016/S0065-308X(08)00602-7 (doi:10.1016/S0065-308X(08)00602-7) [DOI] [PubMed] [Google Scholar]
- 7.De Martel C., Franceschi S. 2009. Infections and cancer: established associations and new hypotheses. Crit. Rev. Oncol./Hematol. 70, 183–194 10.1016/j.critrevonc.2008.07.021 (doi:10.1016/j.critrevonc.2008.07.021) [DOI] [PubMed] [Google Scholar]
- 8.Soroceanu L., Cobbs C. S. 2011. Is HCMV a tumor promoter? Virus Res. 157, 193–203 10.1016/j.virusres.2010.10.026 (doi:10.1016/j.virusres.2010.10.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey J. P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28, 1019–1024 10.1016/S0020-7519(98)00023-X (doi:10.1016/S0020-7519(98)00023-X) [DOI] [PubMed] [Google Scholar]
- 10.Montoya J. G., Liesenfeld O. 2004. Toxoplasmosis. Lancet 363, 1965–1976 10.1111/j.1751-0813.1971.tb14751.x (doi:10.1111/j.1751-0813.1971.tb14751.x) [DOI] [PubMed] [Google Scholar]
- 11.Torrey E. F., Bartko J. J., Lun Z.-R., Yolken R. H. 2007. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr. Bull. 33, 729–736 10.1093/schbul/sbl050 (doi:10.1093/schbul/sbl050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fekadu A., Shibre T., Cleare A. J. 2010. Toxoplasmosis as a cause for behaviour disorders—overview of evidence and mechanisms. Folia Parasitol. 57, 105–113 [DOI] [PubMed] [Google Scholar]
- 13.Hermes G., et al. 2008. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J. Neuroinflammation 5, 48. 10.1186/1742-2094-5-48 (doi:10.1186/1742-2094-5-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash P. B., Purner M. B., Leon R. P., Clarke P., Duke R. C., Turiel T. J. 1998. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 160, 1824–1830 [PubMed] [Google Scholar]
- 15.Berleur M. P., Cordier S. 1995. The role of chemical, physical, or viral exposures and health factors in neurocarcinigenesis: implications for epidemiologic studies of brain tumors. Cancer Causes Control 6, 240–256 10.1007/BF00051796 (doi:10.1007/BF00051796) [DOI] [PubMed] [Google Scholar]
- 16.Wrensch M., Bondy M. L., Wiencke J., Yost M. 1993. Environmental risk factors for primary malignant brain tumors: a review. J. Neurooncol. 17, 47–64 10.1007/BF01054274 (doi:10.1007/BF01054274) [DOI] [PubMed] [Google Scholar]
- 17.Schuman L. M., Choi N. W., Gullen W. H. 1967. Relationship of central nervous system neoplasms to Toxoplasma gondii infection. Am. J. Public Health Nations Health 57, 848–856 10.2105/AJPH.57.5.848 (doi:10.2105/AJPH.57.5.848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan P., Hurley S. F., Johnson A. M., Salzberg M., Lee M. W., North J. B., McNeil J. J., McMichael A. J. 1993. Tumours of the brain and presence of antibodies to Toxoplasma gondii. Int. J. Epidemiol. 22, 412–419 10.1093/ije/22.3.412 (doi:10.1093/ije/22.3.412) [DOI] [PubMed] [Google Scholar]
- 19.Schlehofer B., et al. 1999. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int. J. Cancer 82, 155–160 (doi:10.1002/(SICI)1097-0215(19990719)82:2<155::AID-IJC1>3.3.CO;2-G) [DOI] [PubMed] [Google Scholar]
- 20.Thomas F., Elguero E., Brodeur J., Le Goff J., Missé D. 2011. Herpes simplex virus type 2 and cancer: a medical geography approach. Infect. Genetic. Evol. (doi:10.1016/j.meegid.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 21.Lafferty K. D. 2006. Can the common brain parasite, Toxoplasma gondii, influence human culture? Proc. R. Soc. B 273, 2749–2755 10.1098/rspb.2006.3641 (doi:10.1098/rspb.2006.3641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmeules M., Mikkelsen T., Mao Y. 1992. Increasing incidence of primary malignant brain tumors: influence of diagnostic methods. J. Natl Cancer Inst. 84, 442–445 10.1093/jnci/84.6.442 (doi:10.1093/jnci/84.6.442) [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization 2010. Fact sheet number 193. See http://www.who.int/mediacentre/factsheets/fs193/en/index.html
- 24.Morganstern H. 1982. Uses of ecologic analysis in epidemiologic research. Am. J. Publ. Health 72, 1336–1344 10.2105/AJPH.72.12.1336 (doi:10.2105/AJPH.72.12.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland S., Morgenstern H. 1989. Ecological bias, confounding, and effect modification. Int. J. Epidemiol. 18, 269–274 10.1093/ije/18.1.269 (doi:10.1093/ije/18.1.269) [DOI] [PubMed] [Google Scholar]
- 26.Schafer J. L., Graham J. W. 2002. Missing data: our view of the state of the art. Psychol. Methods 7, 147–177 10.1037//1082-989X.7.2.147 (doi:10.1037//1082-989X.7.2.147) [DOI] [PubMed] [Google Scholar]