Abstract
Elevated carbon dioxide (CO2) has recently been shown to affect chemosensory and auditory behaviour, and activity levels of larval reef fishes, increasing their risk of predation. However, the mechanisms underlying these changes are unknown. Behavioural lateralization is an expression of brain functional asymmetries, and thus provides a unique test of the hypothesis that elevated CO2 affects brain function in larval fishes. We tested the effect of near-future CO2 concentrations (880 µatm) on behavioural lateralization in the reef fish, Neopomacentrus azysron. Individuals exposed to current-day or elevated CO2 were observed in a detour test where they made repeated decisions about turning left or right. No preference for right or left turns was observed at the population level. However, individual control fish turned either left or right with greater frequency than expected by chance. Exposure to elevated-CO2 disrupted individual lateralization, with values that were not different from a random expectation. These results provide compelling evidence that elevated CO2 directly affects brain function in larval fishes. Given that lateralization enhances performance in a number of cognitive tasks and anti-predator behaviours, it is possible that a loss of lateralization could increase the vulnerability of larval fishes to predation in a future high-CO2 ocean.
Keywords: ocean acidification, climate change, brain function, lateralization, larval fish, coral reef
1. Introduction
Atmospheric carbon dioxide (CO2) concentrations, which have remained below 300 ppm for the past 850 000 years, are predicted to exceed 500 ppm by 2050 and could approach 1000 ppm by 2100 if anthropogenic CO2 emissions are not dramatically curtailed [1–3]. The concentration of CO2 in the shallow ocean is also increasing in line with the increases in atmospheric CO2 [4]. Recent studies have shown that CO2 concentrations predicted to occur in the ocean by the end of this century have a strong effect on olfactory [5,6] and auditory [7] mediated behaviour of larval reef fishes, causing them to become attracted or unresponsive to chemical cues and sounds that they normally avoid. Furthermore, juvenile fish exposed to elevated CO2 exhibit behavioural changes, such as higher activity levels and increased boldness, with significant consequences for survival in natural coral reef habitat [8]. These changes in a range of behaviours and sensory modalities suggest that elevated concentrations of CO2 may have a general effect on brain function and cognitive performance of reef fish larvae, although this hypothesis has not been conclusively tested.
The tendency to favour the left or right side during behavioural activities (i.e. behavioural lateralization) is an expression of brain functional asymmetries [9–11] that has been shown to confer advantages in a wide range of animal taxa, including humans and other primates, birds, reptiles, amphibians, fish and invertebrates [9]. In fishes, lateralized individuals show higher performance in cognitive tasks [12], schooling behaviour [13], spatial orientation [14] and escape reactivity [15]. Higher performance in such tasks is attributed to more efficient parallel processing leading to greater efficiency of neural computation and better cognitive performance in lateralized individuals when dealing with multiple tasks [16,17]. Therefore, lateralization is likely to represent a trade-off between high performance in such tasks and the ability to interact with the environment equally well regardless of the position of the stimulus [9,18].
Behavioural lateralization provides a powerful test of brain function for different decision-making tasks involving left versus right responses to environmental stimuli. Disruption in behavioural lateralization in CO2-treated individuals would be compelling evidence that exposure to elevated CO2 causes brain dysfunction in larval fishes. Here, we test the hypothesis that behavioural lateralization is affected by exposure to CO2 levels predicted to occur in the shallow ocean by the end of the century.
2. Material and methods
Settlement-stage larvae of Neopomacentrus azysron were collected overnight using light traps [19] moored in open water on the western side of Lizard Island (14°40′ S, 145°28′ E), in the northern Great Barrier Reef, Australia. Larvae were then maintained in identical rearing tanks at either control (440 µatm) or elevated CO2 (880 µatm). Larvae were maintained in the CO2 treatments for 4 consecutive days (electronic supplementary material). Behavioural lateralization was evaluated using a detour test [11]. Briefly, a single fish was introduced in a two-way T-maze runway (electronic supplementary material), and the direction of its turn (i.e. right or left) at the end of the runway was scored. Ten consecutive runs were observed for each fish, from which the score of the turning direction and the degree of lateralization were obtained. A total of 138 individuals (total length 12.09 ± 0.04 mm; mean ± s.e.) were used in the experiments (n = 70 for the control and n = 68 for the elevated-CO2 treatment). In addition, a random simulation (RS) was generated based on 10 random binary choices (i.e. left or right) per individual (n = 70). This simulation was generated in order to test if any of the samples yielded left–right proportions that were not different from that expected by random choice. Turning preference (i.e. bias in left or right turns) at the population level was assessed using the relative lateralization index (LR, from +100 to −100, indicating complete preference for left and right turning, respectively; see the electronic supplementary material). In this analysis, we compared all three groups (i.e. control, elevated-CO2, RS distribution). Furthermore, the distributions of the control and the elevated-CO2 individuals were compared with a theoretical binomial distribution (see the electronic supplementary material). The strength of lateralization (irrespective of its direction) at the individual-level was then assessed using the absolute lateralization index LA (ranging from 0 (an individual that turned in equal proportion to the right and to the left) to 100 (an individual that turned right or left on all 10 trials)). Comparison between control and elevated-CO2 fish determined whether elevated CO2 had an effect on lateralization, and the comparison of these treatments with the RS distribution determined whether treatments differed from a random left or right choice.
3. Results
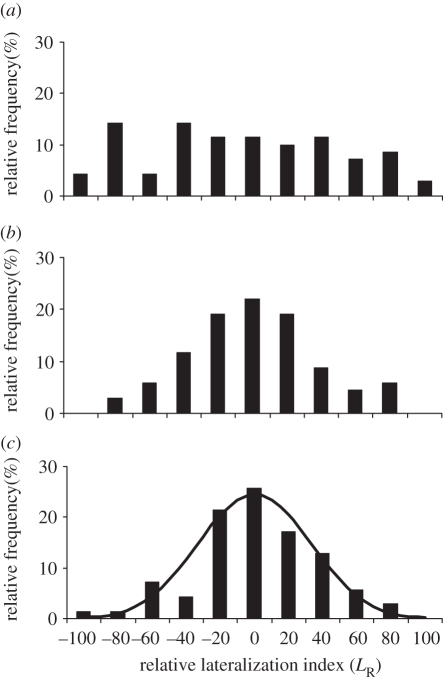
No preference for right or left turns was found at the group level (one sample t-tests; electronic supplementary material: control t(69) = −0.86, p > 0.5; elevated-CO2 t(67) = 0.06, p > 0.5; RS t(69) = 0.33, p > 0.5). Mean LR values ±s.e. were −5.71 ± 6.65, 0.29 ± 4.6 and 1.33 ± 4.35 for control, elevated CO2 and RS, respectively. A Kruskal–Wallis test indicated that there were no differences among the three groups (p > 0.5). The data distribution of LR was normal in all cases (Kolmogorov–Smirnov test, p > 0.1 in all cases; figure 1), whereas their variances differed (Bartlett test, p < 0.001). Multiple comparison among variances [20] showed significant differences in control versus elevated-CO2 and control versus RS (p < 0.005 in both cases), but not in elevated-CO2 versus RS (p > 0.5). Comparisons with a theoretical random (binomial) distribution [20] based on 50 per cent probability of right or left turns (electronic supplementary material) showed significant differences with the LR distribution of the control fish (G-test; G = 92.5; p < 0.0001) but not with the LR distribution of the elevated-CO2 fish (G-test; G = 10.9; p > 0.3; figure 1).
Figure 1.
(a) Relative frequency distributions of LR in control fish, (b) elevated-CO2 fish, (c) random simulation (bars) and random binomial distribution (curve). Positive and negative values indicate right and left turns, respectively. The extreme values of |100| indicate fish that turned in the same direction on all 10 trails.
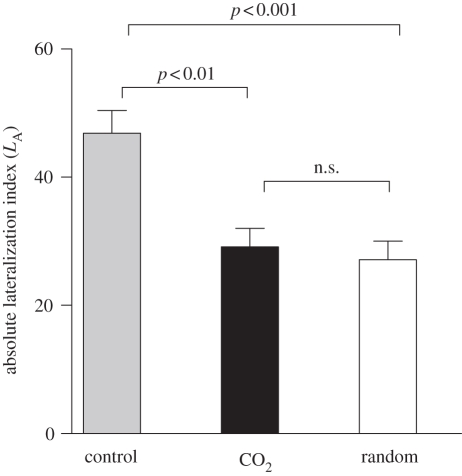
Absolute lateralization index differed markedly among groups (Kruskal–Wallis test, K = 19.38, p < 0.0001; figure 2). Dunn's multiple comparison tests found significant differences between elevated-CO2 and control (p < 0.01), RS and control (p < 0.001), but not between RS and elevated-CO2 (p > 0.05). This result is in accordance with the multiple comparison of variance in LR, as the significantly higher variance (see flatter distribution in figure 1) in the control LR when compared with the other groups corresponds to a higher mean value in the control LA (figure 2).
Figure 2.
LA (mean ± s.e.) in control fish (grey bar), elevated-CO2 fish (black bar) and random simulation (white bar). Significance levels for post hoc comparisons (Dunn's test) are shown in correspondence of each pair tested.
Therefore, while none of the groups were lateralized at the population level, individual-level lateralization occurred for the control group but not for the elevated-CO2 group, which did not differ from a random left–right choice.
4. Discussion
Our results show that exposure to CO2 levels that could occur in the shallow ocean by the end of this century disrupts the behavioural expression of cerebral lateralization in a coral reef fish. This demonstrates that elevated CO2 interferes with brain function in larval reef fishes. Specifically, a preference to turn right or left, as demonstrated by the control fish, is likely to be the result of the specialization of the visual system; fish are turning in such a way so as to assure a strong visual fix on their environment with a given eye [11]. The underlying basis of this behavioural lateralization may be related to the different specialization of contralateral brain structures [11]. Earlier work has shown that different types of detour tasks (e.g. using an opaque barrier versus a transparent barrier with a predator-model behind) result in different turning biases that may be related to the specific lateralization of visual tasks [11]. Therefore, it is possible that exposure to increased CO2 causes a malfunction in the expression of dominance of the brain hemisphere that controls eye preference in the visual task associated with the detour test.
Behavioural lateralization is thought to minimize the decision time and avoid simultaneous initiation of incompatible responses when facing tasks that require directional responses [9]. Furthermore, behavioural lateralization is suggested to increase cognitive performance [9,10] and has been shown to provide advantages when fish are faced with trading-off multiple cognitive tasks (e.g. feeding in the presence of a predator [16]). Therefore, it is possible that CO2 exposure may decrease the cognitive ability of fish larvae, especially within the context of multi-tasking [12,16]. Lateralization is a fundamental determinant of fish behaviour, especially within the contexts of predator–prey interactions, being related to higher performance in anti-predator responses [15], gregariousness [13] and spatial orientation [14]. Individuals from high-predation sites exhibit stronger lateralization than those from low-predation sites and these differences are heritable [21,22]. Consequently, loss of lateralization caused by elevated CO2 may affect the vulnerability of fish to predation, in addition to other effects observed in recent studies [5,8].
The degree of lateralization is likely to represent a trade-off between the high performance levels found in lateralized individuals and the ability to deal equally with stimuli from all sides as in non-lateralized individuals [9]. Not all control individuals were highly lateralized (figure 1), and therefore it is possible that the lateralization distribution found in nature (i.e. in the control fish) is driven by this trade-off at the population level. Exposure to elevated CO2 appears to disrupt this equilibrium, as lateralized individuals are no longer present, and the resultant LR distribution does not differ from that based on random left–right choice. Assuming that the LR distribution found in nature is the result of natural selection, any changes in that distribution as a result of brain dysfunction are likely to decrease the performance of the population. Similar to most previous studies on behavioural effects of elevated CO2, our experiments do not examine the potential for adaptation by selection of tolerant genotypes and this will be an important area for future research.
Our results provide strong evidence that elevated CO2 has a direct effect on brain function in larval reef fishes; however, the precise cellular mechanisms responsible are unknown. The fact that olfactory [5,6] and auditory function [7] are impaired, as well as changes in activity levels [8] and the effects on behavioural lateralization observed here, points to an effect of elevated CO2 on a highly conserved element of nervous system function. A likely scenario is that altered ion concentrations in the tissues owing to acid–base regulation in a high-CO2 environment [23] affect neural function in larval fishes, although this hypothesis remains to be tested.
Acknowledgements
Sue-Ann Watson conducted the sea water chemistry. Thanks to Marco Dadda for useful comments on an earlier draft of this manuscript. Funding was provided by the ARC Centre of Excellence for Coral Reef Studies. Research was carried out under approval from the Great Barrier Reef Marine Park Authority, and under James Cook University ethics guidelines.
References
- 1.Raupach M. R., Marland G., Ciais P., Le Quere C., Canadell J. G., Klepper G., Field C. B. 2007. Global and regional drivers of accelerating CO2 emissions. Proc. Natl Acad. Sci. USA 104, 10 288–10 293 10.1073/pnas.0700609104 (doi:10.1073/pnas.0700609104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoegh-Guldberg O., et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 10.1126/science.1152509 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 3.Meehl G. A., et al. 2007. Climate change 2007: the physical science basis. In Contribution of working group 1 to the fourth assessment report of the intergovernmental panel on climate change (eds Solomon S. D., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 747–845 Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Doney S. C. 2010. The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516 10.1126/science.1185198 (doi:10.1126/science.1185198) [DOI] [PubMed] [Google Scholar]
- 5.Dixson D. L., Munday P. L., Jones G. P. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 10.1111/j.1461-0248.2009.01400.x (doi:10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 6.Munday P. L., Dixson D. L., Donelson J. M., Jones G. P., Pratchett M. S., Devitsina G. V., Doving K. B. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 10.1073/pnas.0809996106 (doi:10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson S. D., Munday P. L., Wittenrich M. L., Manassa R., Dixson D. L., Gagliano M., Yan H. Y. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920 (doi:10.1098/rsbl.2011.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munday P. L., Dixson D. L., McCormick M. I., Meekan M., Ferrari M. C. O., Chivers D. P. 2010. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934 10.1073/pnas.1004519107 (doi:10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallortigara G., Rogers L. J. 2005. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–633 [DOI] [PubMed] [Google Scholar]
- 10.Bisazza A., Cantalupo C., Capocchiano M., Vallortigara G. 2000. Population lateralisation and social behaviour: a study with 16 species of fish. Laterality 5, 269–284 10.1080/135765000406111 (doi:10.1080/135765000406111) [DOI] [PubMed] [Google Scholar]
- 11.Bisazza A., Facchin L., Pignatti R., Vallortigara G. 1998. Lateralization of detour behaviour in poeciliid fish: the effect of species, gender and sexual motivation. Behav. Brain Res. 91, 157–164 10.1016/S0166-4328(97)00114-9 (doi:10.1016/S0166-4328(97)00114-9) [DOI] [PubMed] [Google Scholar]
- 12.Dadda M., Bisazza A. 2006. Lateralized female topminnows can forage and attend to a harassing male simultaneously. Behav. Ecol. 17, 358–363 10.1093/beheco/arj040 (doi:10.1093/beheco/arj040) [DOI] [Google Scholar]
- 13.Bisazza A., Dadda M. 2005. Enhanced schooling performance in lateralized fishes. Proc. R. Soc. B 272, 1677–1681 10.1098/rspb.2005.3145 (doi:10.1098/rspb.2005.3145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sovrano V. A., Dadda M., Bisazza A. 2005. Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav. Brain Res. 163, 122–127 10.1016/j.bbr.2005.04.012 (doi:10.1016/j.bbr.2005.04.012) [DOI] [PubMed] [Google Scholar]
- 15.Dadda M., Koolhaas W. H., Domenici P. 2010. Behavioural asymmetry affects escape performance in a teleost fish. Biol. Lett. 6, 414–417 10.1098/rsbl.2009.0904 (doi:10.1098/rsbl.2009.0904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadda M., Bisazza A. 2006. Does brain asymmetry allow efficient performance of simultaneous tasks? Anim. Behav. 72, 523–529 10.1016/j.anbehav.2005.10.019 (doi:10.1016/j.anbehav.2005.10.019) [DOI] [Google Scholar]
- 17.Rogers L. J. 2002. Advantages and disadvantages of lateralization. In Comparative vertebrate lateralization (eds Rogers L. J., Andrew R. J.), pp. 126–153 Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Dadda M., Zandona E., Agrillo C., Bisazza A. 2009. The costs of hemispheric specialization in a fish. Proc. R. Soc. B 276, 4399–4407 10.1098/rspb.2009.1406 (doi:10.1098/rspb.2009.1406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meekan M. G., Wilson S., Halford A., Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381 10.1007/s002270100577 (doi:10.1007/s002270100577) [DOI] [Google Scholar]
- 20.Zar J. 1984. Biostatistical analysis. Englewood Cliff, NJ: Prentice Hall [Google Scholar]
- 21.Brown C., Western J., Braithwaite V. A. 2007. The influence of early experience on and inheritance of, cerebral lateralization. Anim. Behav. 74, 231–238 10.1016/j.anbehav.2006.08.014 (doi:10.1016/j.anbehav.2006.08.014) [DOI] [Google Scholar]
- 22.Brown C., Gardner C., Braithwaite V. A. 2004. Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. Lond. B 271, S455–S457 10.1098/rsbl.2004.0222 (doi:10.1098/rsbl.2004.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portner H. O., Langenbuch M., Reipschlager A. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 10.1007/s10872-004-5763-0 (doi:10.1007/s10872-004-5763-0) [DOI] [Google Scholar]