Abstract
Adult hawksbill turtles (Eretmochelys imbricata) are typically described as open-coast, coral reef and hard substrate dwellers. Here, we report new satellite tracking data on female hawksbills from several countries in the eastern Pacific that revealed previously undocumented behaviour for adults of the species. In contrast to patterns of habitat use exhibited by their Caribbean and Indo-Pacific counterparts, eastern Pacific hawksbills generally occupied inshore estuaries, wherein they had strong associations with mangrove saltwater forests. The use of inshore habitats and affinities with mangrove saltwater forests presents a previously unknown life-history paradigm for adult hawksbill turtles and suggests a potentially unique evolutionary trajectory for the species. Our findings highlight the variability in life-history strategies that marine turtles and other wide-ranging marine wildlife may exhibit among ocean regions, and the importance of understanding such disparities from an ecological and management perspective.
Keywords: Hawksbill, habitat use, mangroves, estuary, eastern Pacific, life history
1. Introduction
Elucidating habitat use and movement behaviour is a central component of wildlife research, conservation and management [1]. However, understanding these characteristics for highly vagile, aquatic organisms such as marine turtles is complicated by the inherent logistical difficulties in studying this taxon [2]. Drastic declines in many marine turtle populations have further confounded characterizations of life-history strategies [3].
The advent of satellite telemetry technologies has provided unique insights into the plasticity in life-history strategies employed by marine species, including marine turtles (e.g. [4–6]). Despite many such findings, the breadth of strategies exhibited by species remains unclear and are often erroneously assumed to be invariant.
Hawksbill turtles (Eretmochelys imbricata) are critically endangered [7] and the majority of hawksbill habitat use and movement research to date has been centred in the Wider Caribbean and Indo-Pacific regions. Based on this research, adults have been characterized as inhabiting open-coast areas consisting of coral reefs and other hard-bottom substrates [8,9]. Although juvenile hawksbills have been described occasionally using peripheral habitats (e.g. [10,11]), such accounts are rare.
As recently as 2007, hawksbills were considered functionally extirpated in the eastern Pacific Ocean, based on scarce reports of their presence and the sparse coral reef distribution in the region (see review in Mortimer & Donnelly [7]). While the recent identification of several important nesting rookeries improved the conservation outlook for the species [12], hawksbill life history in this region remains poorly understood. To explore hawksbill habitat use and associations in the eastern Pacific, we equipped 12 adult female turtles with satellite transmitters along the Pacific coasts of El Salvador, Nicaragua and Ecuador. This study represents the first initiative to track individuals from this remnant hawksbill population and describes novel habitat use that will inform regional conservation efforts.
2. Material and methods
We used a combination of satellite tag models (Wildlife Computers and Telonics) varying in size, weight and design. Turtle position data were acquired through System Argos (Landover, MA, USA) using a newly developed Kalman geoprocessing algorithm [13]. We implemented a series of filters to raw location points, including travel speed (greater than 5 km h−1), turning angles (less than 25°) and a land-distance filter [14]. We defined non-migratory behaviours as post-nesting movements within the extent of normal interesting movements [15]. Foraging area destinations were classified as either ‘inshore’, if turtles settled within estuary canals or ‘near-shore’ if turtles settled in areas along the open-coast. We used the programme Maximum Entropy (MaxEnt) [16] to identify potential relationships between coastal features (e.g. mangroves, beach, rocky shoreline, urban areas, etc.; see electronic supplementary material, table S1 for complete list and definitions) and foraging phase turtle location points. Marine features were not included in the analysis owing to a lack of data on these attributes. Coastal habitat features were manually digitized from remotely sensed, high-resolution images available through the online Environmental Systems Research Institute (ESRI) Resource Centre (http://resources.arcgis.com). The importance of each coastal habitat feature on hawksbill distribution was evaluated using the Per cent Contribution model available in MaxEnt [16].
3. Results
Hawksbill turtles (mean curved carapace length = 84.4 cm; s.d. = 6.3 cm) were tagged at six sites between June 2008 and July 2010. We analysed a total of 2981 location points, during which turtles were tracked for an average of 148 days (s.d. = 106 days).
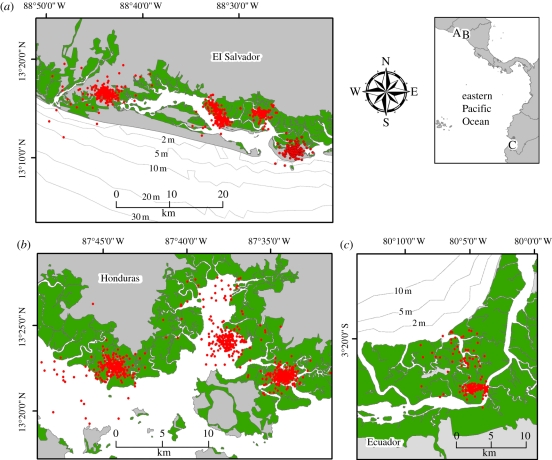
Turtles demonstrated two distinct patterns of habitat use, with 10 individuals (83.3%) inhabiting inshore waterways (figure 1) and two individuals (16.7%) settling in near-shore areas. Mean per cent Contribution values for the top three coastal habitat features were (presented in rank order) mangrove saltwater forest (mean = 62.9%, s.d. = 34.5%), cleared fields (mean = 15.8%, s.d. = 20.7%) and shrimp ponds (mean = 9.5%, s.d. = 18.9%).
Figure 1.
Inshore foraging areas used by 10 hawksbill turtles at Jiquilisco-Xiriualtique Bay, El Salvador (n = 4), Gulf of Fonseca, Honduras (n = 4) and Jambeli Canal, Ecuador (n = 2). Mangrove saltwater forest and shrimp ponds (i.e. converted mangrove saltwater forests) shown for reference. Red circles, turtle location points; green regions, mangroves/shrimp ponds.
4. Discussion
Our tracking efforts revealed radically novel habitat use for adult hawksbills. In contrast to other adult hawksbill populations that inhabit open-coast coral reef habitats, the majority (83%) of hawksbills in our study settled within confined inshore estuarine bays, often in canals less than 50 m wide. Within these areas, hawksbill habitat use was strongly associated with mangrove saltwater forests and shrimp ponds, the latter representing converted mangrove saltwater forests. Despite greater than 2000 km among study sites, these patterns were consistent across all inshore foraging areas. Thus, in the eastern Pacific, mangrove estuaries appear to be the principal habitat type used by adult hawksbills, which is a significant departure from the long-maintained life-history dogma that hawksbills are obligate coral reef dwellers [9,17]. In fact, seven of the turtles in our study were tagged at nesting sites adjacent to rock and coral reef habitats, but left these areas to move into mangrove estuaries. Whether the preferential use of mangrove estuaries by adult hawksbills is a behaviour exclusive to the eastern Pacific remains unclear, but is plausible considering this as the first-ever discovery of this behaviour despite decades of research in other ocean regions.
Our findings have important conservation implications both for hawksbills and their mangrove estuary habitat given the concentration of anthropogenic pressures in such areas. Known threats include small-scale fisheries bycatch, rampant (illegal) egg collection, unsustainable coastal development and poorly regulated protected areas [7,12,18].
Historical evidence suggests that hawksbills have always been less common than other sea turtle species in the eastern Pacific [12]. This is probably attributable to the species having evolved in association with habitat types (i.e. coral reefs) that are relatively scarce in the eastern Pacific. Considering that the eastern Pacific appears to be the most recently colonized ocean region for hawksbills [19], the exploitation of mangrove estuaries as preferred habitat probably represents a relatively recent adaptation and possibly a unique evolutionary trajectory for this population. Given that hawksbills were recently considered functionally extirpated in the eastern Pacific [12], our findings emphasize the importance of considering atypical habitats for rare species.
The eastern Pacific is characterized by substantial environmental forcing [20], which has led to the contraction and expansion of sea turtle populations over time [19,21] and such processes can play a major role in the development of new life-history strategies (see refs in Hewitt [22]). Other sea turtle species inhabiting the eastern Pacific, specifically leatherback (Dermochelys coriacea) [21] and green turtles (Chelonia mydas) [23], also show stark behavioural, morphological and reproductive differences to their conspecifics in other ocean basins, suggesting environmental variability in the area may lead to the development of novel biological traits.
Our findings highlight the complexities of life-history characterizations and suggest that other widely accepted generalizations for hawksbills (e.g. dietary habits, juvenile development patterns and ecological roles [1]) warrant further scrutiny. Furthermore, our research draws attention to the importance of identifying variation in life-history strategies of long-lived species with broad geographical distributions in order to inform robust conservation planning and to gain insight into broader ecological processes.
Acknowledgements
We thank Southwest Fisheries Science Centre of the National Ocean and Atmospheric Administration, National Fish and Wildlife Foundation, US Fish and Wildlife Service, Machalilla National Park, Paso Pacifico, Asociación para el Desarrollo Empresarial y Ambiental de Puerto Parada, Fundación para la Protección del Arrecife de Los Cóbanos, Cooperativa de Pescadores El Maculís, Ministry of the Environment and Natural Resources of El Salvador, Cooperativa Multisectorial de Jiquilillo, Los Zorros y Padre Ramos and The Ocean Foundation for financial/logisitical support. We recognize the following individuals: Rene Flores, Cristabel Flores, Georgina Mariona, Wilfredo Lopez, Tarla Peterson, Sarah Otterstrom, Liza Gonzalez, Salvador Sanchez, Perla Torres, Eduardo Altamirano Urbina, Eddy Maradiaga, Luís Manzanares, Micaela Peña, Juan Pablo Muñoz, Gabriela Anhalzer, Felipe Vallejo, Michelle Pico, Earl Possardt, Grover Jeane, Michael Carey, Philippe Gaspar, Remy Lopez, Michael Coyne and Harry Johnson. We acknowledge insightful reviewer comments by Graeme Hayes.
References
- 1.Bolten A. B. 2003. Variation in sea turtle life-history patterns: neritic vs. oceanic developmental stages. In The biology of sea turtles (eds Lutz P. L., Musick J. A., Wyneken J.), pp. 243–257 Boca Raton, FL: CRC Press [Google Scholar]
- 2.Plotkin P. 2003. Adult migrations and habitat use. In The biology of sea turtles, vol. 2 (eds Lutz P. L., Musick J. A., Wyneken J.), pp. 225–242 Boca Raton, FL: CRC Press [Google Scholar]
- 3.Allen M. S. 2007. Three millennia of human and sea turtle interactions in remote Oceania. Coral Reefs 26, 959–970 10.1007/s00338-007-0234-x (doi:10.1007/s00338-007-0234-x) [DOI] [Google Scholar]
- 4.Hawkes L. A., Broderick A. C., Coyne M. S., Godfrey M. H., Loez-Jurado L., Lopez-Suarez P., Merino S. E., Varo-Cruz N. 2006. Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Curr. Biol. 16, 990–995 10.1016/j.cub.2006.03.063 (doi:10.1016/j.cub.2006.03.063) [DOI] [PubMed] [Google Scholar]
- 5.McClellan C. M., Read A. J. 2007. Complexity and variation in loggerhead sea turtle life history. Biol. Lett. 3, 592–594 10.1111/j.1365-2664.2010.01817.x (doi:10.1111/j.1365-2664.2010.01817.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schofield G., Hobson V. J., Fossette S., Lilley M. K. S., Katselidis K. A., Hays G. C. 2010. Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers. Distrib. 16, 840–853 10.1111/j.1472-4642.2010.00694.x (doi:10.1111/j.1472-4642.2010.00694.x) [DOI] [Google Scholar]
- 7.Mortimer J. A., Donnelly M. 2008. Eretmochelys imbricata. In IUCN Red List of Threatened Species v. 2010.1. See http://www.iucnredlist.org [accessed 11 March 2011]
- 8.Leon Y. M., Bjorndal K. A. 2002. Selective feeding in the hawksbill turtle, an important predator in coral reef ecosystems. Mar. Ecol. Prog. Ser. 245, 249–258 10.3354/meps245249 (doi:10.3354/meps245249) [DOI] [Google Scholar]
- 9.Meylan A. 1988. Spongivory in hawksbill turtles: a diet of glass. Science 239, 393–395 10.1126/science.239.4838.393 (doi:10.1126/science.239.4838.393) [DOI] [PubMed] [Google Scholar]
- 10.Bjorndal K. A., Bolten A. B. 2010. Hawksbill sea turtles in seagrass pastures: success in a peripheral habitat. Mar. Biol. 157, 135–145 10.1007/S00227-009-1304-0 (doi:10.1007/S00227-009-1304-0) [DOI] [Google Scholar]
- 11.Hasbún C. R., Vasquez M., León M. 1998. Unusual record of a juvenile sea turtle in a mangrove estuary, El Salvador. Mar. Turtle Newslett. 81, 10 [Google Scholar]
- 12.Gaos A. R., et al. 2010. Signs of hope in the eastern Pacific: international collaboration reveals encouraging status for a severely depleted population of hawksbill turtles Eretmochelys imbricata. Oryx 44, 595–601 10.1017/S0030605310000773 (doi:10.1017/S0030605310000773) [DOI] [Google Scholar]
- 13.Lopez R., Malardé J. P. Collecte Localisation Satellites, France.: 2011. Improving ARGOS Doppler location using Kalman filtering. [Google Scholar]
- 14.Hays G. C., Luschi P., Papi F., del Seppia C., Marsh R. 1999. Changes in behaviour during the inter-nesting period and post-nesting migration for Ascension Island green turtles. Mar. Ecol. Prog. Ser. 189, 263–273 [Google Scholar]
- 15.Godley B. J., Blumenthal J. M., Broderick A. C., Coyne M. S., Godfrey M. H., Hawkes L. A., Witt M. J. 2008. Satellite tracking of sea turtles: where have we been and where do we go next? Endang. Species Res. 4, 3–22 [Google Scholar]
- 16.Phillips S. J., Anderson R. P., Schapire R. E. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 10.1016/j.ecolmodel.2005.03.026 (doi:10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 17.Limpus C. J. 1992. The hawksbill turtle, Eretmochelys imbricata, in Queensland: population structure within a southern Great Barrier Reef feeding ground. Wildl. Res. 19, 489–506 10.1071/WR9920489 (doi:10.1071/WR9920489) [DOI] [Google Scholar]
- 18.Liles M. J., Jandres M. V., López W. A., Mariona G. I., Hasbún C. R., Seminoff J. A. 2011. Hawksbill turtles (Eretmochelys imbricata) in El Salvador: nesting distribution and mortality at the largest remaining nesting aggregation in the eastern Pacific Ocean. Endang. Species Res. 14, 23–30 10.3354/esr00338 (doi:10.3354/esr00338) [DOI] [Google Scholar]
- 19.Bowen B. W., Karl S. A. 2007. Population genetics and phylogeography of sea turtles. Mol. Ecol. 16, 4886–4907 10.1111/j.1365-294X.2007.03542.x (doi:10.1111/j.1365-294X.2007.03542.x) [DOI] [PubMed] [Google Scholar]
- 20.Saba V. S., Spotila J. R., Chavez F. P., Musick J. A. 2008. Bottom-up and climatic forcing on the worldwide population of leatherback turtles. Ecology 89, 1414–1427 10.1890/07-0364.1 (doi:10.1890/07-0364.1) [DOI] [PubMed] [Google Scholar]
- 21.Wallace B. P., Saba V. S. 2009. Environmental and anthropogenic impacts on intra-specific variation in leatherback turtles: opportunities for targeted research and conservation. Endang. Species Res. 7, 1–11 10.3354/esr00177 (doi:10.3354/esr00177) [DOI] [Google Scholar]
- 22.Hewitt G. M. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 58, 247–276 10.1111/j.1095-8312.1996.tb01434.x (doi:10.1111/j.1095-8312.1996.tb01434.x) [DOI] [Google Scholar]
- 23.Pritchard P. C. H., Trebbau P. 1984. The turtles of Venezuela, vol. 2 Caracas, Venezuela: Fundacion de Internados Rurales; Society for the Study of Amphibians and Reptiles, Contributions to Herpetology [Google Scholar]