Abstract
The fossil record presents palaeoecological patterns of rise and fall on multiple scales of time and biological organization. Here, we argue that the rise and fall of species can result from a tragedy of the commons, wherein the pursuit of self-interests by individual agents in a larger interactive system is detrimental to the overall performance or condition of the system. Species evolving within particular communities may conform to this situation, affecting the ecological robustness of their communities. Results from a trophic network model of Permian–Triassic terrestrial communities suggest that community performance on geological timescales may in turn constrain the evolutionary opportunities and histories of the species within them.
Keywords: tragedy of the commons, complexity, complex adaptive systems, palaeocommunity
1. Introduction
Waxing and waning of entities is a dominant feature of the palaeontological record. For example, species often increase in abundance and geographical range early in their histories, but undergo range contractions and become increasingly rare as they approach extinction [1]. Other taxonomic and non-genealogical entities, such as genera, so-called evolutionary faunas and types of ecological communities exhibit similar patterns [2–4]. Many of these macro-palaeoecological patterns result from the ecological histories of species, which themselves are the results of population dynamics that lie mostly below the resolution of the stratigraphic record. Individual species histories, however, are embedded in complex adaptive systems at a higher hierarchical level of biological organization, comprising biotic interactions among individually acting species. The feedback of species interactions and evolutionary change to both the abiotic contexts and the dynamics of the systems affect species histories.
Species evolution may therefore be constrained by the higher level systems that they create and in which they are embedded. Similarly, extinction would frequently be the result of an exhaustion of species' adaptive capacities in the face of a system's changing dynamics. From this, it is apparent that individual entities must balance selective pressures arising at various hierarchical levels, and that their waxings and wanings are the result of coupled dynamics at multiple scales of organization. Positive patterns or changes at one level need not be so at all levels, and species may evolve adaptations that do not benefit aspects of system performance, such as productivity or resistance to cascading or collateral extinctions; this is an evolutionary–ecological tragedy of the commons [5]. Here, we explore this argument by examining the manner in which the trophic breadths of species and relative guild species' richnesses determine community resistance to cascades of secondary extinction, and the top-down constraints subsequently exerted by communities on species. Specifically, certain combinations of species richness and ecological diversity may constrain the evolutionary histories of individual species because of their impact on community properties. We will first conduct a thought experiment and then provide an empirical example.
2. A thought experiment
Imagine a marine community in the Late Pre-Cambrian comprising many ancestors of modern animal phyla, into which we introduce a proficient or novel adaptation in a single species [6]. Proficient adaptations are a hallmark of the Cambrian Explosion; the ‘vacancy’ of morpho-ecological niches and simpler interspecific interactions would have made them highly probable. The result of the adaptation would be a rapid increase in both the relative abundance and spatial range of the species possessing it, but the increase would be unlikely to continue unabated for two reasons. First, in ecological time, a population cannot increase infinitely because of density-dependent effects of limited resources, even in the absence of interspecific biotic interactions. Second, antagonistic interactions are likely to evolve on evolutionary timescales because the species itself now represents a valuable resource for adapting predators, parasites and pathogens. The species would also be an agent of selection on any other species using a common resource, thereby driving the evolution of competitors. The result is the onset or acceleration of biotic interactions, hypothesized to be a major driver of the Explosion [7]. Biotic interactors are agents of selection, and the feedback of communities of interacting species into their own selective environments is a source of diversification and complexification [8].
Even an explosion of evolutionary ecological activity, either the Cambrian Explosion or any community early in its history, is not without constraint. Rapid inflation will be followed by a deceleration of the proliferation of new ecological types. In the case of the Cambrian Explosion, developmental and genetic constraints [9] and ecological saturation [10] or exhaustion [7] are probable decelerating mechanisms. Ecologically, there is, however, more than saturation or exhaustion at work. Theoretically, there may always be room enough for new adaptations [10]. Species evolution, however, is an exploration of fitness landscapes that respond dynamically to abiotic variation and are coupled dynamically to the landscapes of other species in the community. We hypothesize that the long-term success of a species depends on a balance between the effectiveness of its adaptations and responses to selection, and the manner in which those adaptations and responses affect the overall performance of its community. In particular, there is the need to avoid a tragedy of the commons, where the interests or actions of an individual species can be detrimental to the community [5]. Whether the balance is tipped in favour of selfish species adaptation or community performance in turn depends on two factors. First, at the species level, there is the extent to which the species is connected to the community; that is, the number and strengths of its biotic interactions. Adaptation can be viewed as a sum of responses to the biotic and abiotic environments, and the former factor is measured in terms of interactions. Species with fewer interspecific interactions probably have a lesser impact on the commons (i.e. community properties). Second, the relationship between the functional partitioning of species richness and community robustness constrains the ecological flexibility or variability of species on evolutionary timescales. The constraints are generally negligible, except when functional partitioning leads to significant increases in the number and strengths of biotic interactions. We explain these arguments with the following empirical example.
3. An empirical example
The Karoo Basin of South Africa preserves one of the best records of Middle Permian–Middle Triassic terrestrial communities [11], including the End-Permian mass extinction event [12]. A succession of communities ranging from the Wordian to the Changhsingian was dominated compositionally by therapsid tetrapods. The Earliest Triassic Lystrosaurus assemblage zone (LAZ), the community present in the immediate aftermath of the extinction, was comparable in richness to preceding communities, indicating a rapid diversification of at least some surviving species. It differed from its predecessors, however, in several aspects, notably an increased diversity of temnospondyl amphibians, greater richness of small body-sized amniote carnivores/insectivores, and the complete or near-complete extinction of amniote herbivores across a wide range of body sizes.
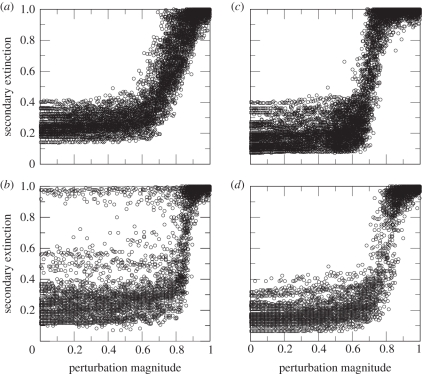
A dynamic trophic model of LAZ also exhibits significant differences. A model of the responses of LAZ and preceding communities to perturbations of primary productivity, a likely disruption during the extinction episode, measured the number of secondary extinctions predicted to result from perturbations of specific magnitudes [13]. The trophic model partitions observed taxon richness according to trophic functions and ecologies, and examines the ensemble of possible food web topologies that could be derived from that partitioning scheme [14,15]. All preceding communities exhibited a sterotyped response of high resistance to secondary extinction at low to moderate levels of perturbation, with a dramatic loss of resistance within a critical range of disruption (figure 1a). LAZ, however, exhibits a broad variance of resistance at even the lowest levels of disruption, with some topologies of the community being as resistant as preceding communities, but others having very low resistance (figure 1b). This implies that if the same rules for reconstructing patterns of biotic interactions are applied to all the communities, then LAZ communities would have been extremely vulnerable to environmental variation. The vulnerability is caused by the decreased herbivore richness and the increased richness of their predators.
Figure 1.
Modelled resistance of three Permian–Triassic palaeocommunities of the Karoo Basin. (a) Late Permian Dicynodon assemblage zone, (b) earliest Early Triassic Lystrosaurus assemblage zone (LAZ), (c) late Early Triassic Cynognathus Assemblage Zone (CAZ). The x-axis measures the magnitude of disruption to primary productivity, ranging from 0 to 1 (complete disruption). The y-axis measures community resistance as the number of species becoming secondarily extinct, after accounting for network topological and secondary demographic effects, again on a scale of 0 (no extinctions) to 1 (complete extinction). One hundred food webs were sampled per palaeocommunity, hence each level of primary productivity disruption has 100 corresponding responses. (d) the LAZ when very small body-sized amniote carnivores/insectivores were constrained to specialize on a single prey species only.
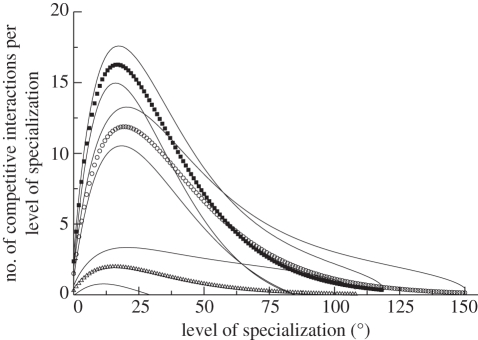
Maintaining trophic breadths equivalent to similar predators in preceding communities would result in intense competition among LAZ predators for a greatly reduced pool of prey species. This is demonstrated by estimating the number of direct competitors per prey species (figure 2; electronic supplementary material). The functional partitioning of species richness determines the food web topologies that are possible for a community, and the skewed partitioning of LAZ resulted in it sitting on a combinatorial cusp; some topologies are as resistant to perturbation as those of preceding communities, whereas others have very little resistance because of the feedback between competing predators. Confirmation of this is offered by an experimental manipulation. If predators in the LAZ system are all constrained to be extreme specialists, thereby both reducing the probability of inter-predator competition and the combinatorial space of food web topologies [15], the result is a dramatic increase of community resistance (figure 1d). We neither claim that specialization reduces the probability of a species' extinction, nor that specialization necessarily played a significant role in stabilizing Early Triassic Karoo communities (see below), but rather that under conditions of intensified interspecific interactions, specialization would have reduced the role of those interactions in system-wide cascades of secondary extinction. The apparent super-abundance of the herbivore Lystrosaurus [16] could also have diminished the intensity of some of the interactions inferred here, but not all the competing predatory guilds preyed on Lystrosaurus. Furthermore, simulations conducted where Lystrosaurus is given artificially infinite abundance fail to eliminate high secondary extinction (electronic supplementary material).
Figure 2.
Number of intra-guild competitive interactions expected to involve species of a given in-degree, or level of specialization, in the guild of very small body-sized amniote carnivores/insectivores. The competitive interactions include one-neighbour interactions only, where competitors share the same prey species. Derivation and calculation of the estimates are explained in the electronic supplementary material. Symbols are the expected (mean) values, whereas solid lines represent variation of 1 s.d. about the mean. Open circles, Dicynodon assemblage zone; closed squares, LAZ; open triangles, CAZ.
Herbivore richness declined most likely because of extinctions driven by plant die-offs and/or turnover during the End-Permian event [17]. More obscure is the reason for increases of predator richness in the immediate aftermath. Nevertheless, the short-term evolutionary success of the LAZ predators may have sown their own long-term decline as a tragedy of the commons. Their very richness decreased the resistance of the community to low magnitude and possibly frequent disruptions of primary productivity. In fact, succeeding LAZ was the Cynognathus assemblage zone (CAZ), a very resistant community (figure 1c) characterized by herbivore richness far exceeding predator richness, exactly the opposite of LAZ. In this case, we hypothesize that increased community resistance constrained the evolution of new predators and possibly increased their rates of extinction, while being neutral to or possibly favouring the addition of herbivore taxa to the system.
4. Summary
Species evolve in response to interactions with their abiotic and biotic environments. Changes to those environments almost always result in increased selective pressures. The acquisition of a new adaptation, while permitting the bearer to increase its abundance and range, likewise represents an environmental degradation to species with which it interacts. Successful individual adaptation of one or more of those species neither guarantees enhanced performance of their community, nor of any other higher levels of biological organization, hence our reference to the tragedy of the commons. Successful performance at those higher levels may be necessary, however, for species persistence, and success or failure is projected downwards to the level of the species. Patterns of rise and fall at higher ecological levels are therefore both driven by, and drive, similar patterns at lower evolutionary levels.
Acknowledgements
This paper is dedicated to Percy Roopnarine, who introduced the tragedy of the commons to P.D.R. many years ago.
References
- 1.Liow L. H., Stenseth N. C. 2007. The rise and fall of species: implications for macroevolutionary and macroecological studies. Proc. R. Soc. B 274, 2745–2752 10.1098/rspb.2007.1006 (doi:10.1098/rspb.2007.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enquist B. J., Jordan M. A., Brown J. H. 1995. Connections between ecology, biogeography, and paleobiology: relationship between local abundance and geographic distribution in fossil and recent molluscs. Evol. Ecol. 9, 586–604 10.1007/BF01237657 (doi:10.1007/BF01237657) [DOI] [Google Scholar]
- 3.Foote M. 2007. Symmetric waxing and waning of marine invertebrate genera. Paleobiology 33, 517–529 10.1666/06084.1 (doi:10.1666/06084.1) [DOI] [Google Scholar]
- 4.Alroy J. 2010. The shifting balance of diversity among major marine animal groups. Science 329, 1191–1194 10.1126/science.1189910 (doi:10.1126/science.1189910) [DOI] [PubMed] [Google Scholar]
- 5.Hardin G. 1968. The tragedy of the commons. Science 162, 1243–1248 10.1126/science.162.3859.1243 (doi:10.1126/science.162.3859.1243) [DOI] [PubMed] [Google Scholar]
- 6.Page S. E. 2010. Diversity and complexity. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Marshall C. R. 2006. Explaining the Cambrian ‘explosion’ of animals. Annu. Rev. Earth Planet Sci. 34, 355–384 10.1146/annurev.earth.33.031504.103001 (doi:10.1146/annurev.earth.33.031504.103001) [DOI] [Google Scholar]
- 8.Vermeij G. J. 2009. Comparative economics: evolution and the modern economy. J. Bioeconom. 11, 105–134 10.1007/s10818-009-9062-0 (doi:10.1007/s10818-009-9062-0) [DOI] [Google Scholar]
- 9.Valentine J. W., Jablonski D., Erwin D. H. 1999. Fossils, molecules and embryos: new perspectives on the Cambrian explosion. Development 126, 851–859 [DOI] [PubMed] [Google Scholar]
- 10.Valentine J. W. 1980. Determinants of diversity in higher taxonomic categories. Paleobiology 6, 444–450 [Google Scholar]
- 11.Rubidge B. S. 2005. Reuniting lost continents—fossil reptiles from the ancient Karoo and their wanderlust. S. Afr. J. Geol. 108, 135–172 10.2113/108.1.135 (doi:10.2113/108.1.135) [DOI] [Google Scholar]
- 12.Botha J., Smith R. M. H. 2006. Rapid vertebrate recuperation in the Karoo Basin of South Africa following the end-Permian extinction. J. Afr. Earth Sci. 45, 502–514 10.1016/j.jafrearsci.2006.04.006 (doi:10.1016/j.jafrearsci.2006.04.006) [DOI] [Google Scholar]
- 13.Roopnarine P. D., Angielczyk K. D., Wang S. C., Hertog R. 2007. Trophic network models explain instability of Early Triassic terrestrial communities. Proc. R. Soc. B 274, 2077–2086 10.1098/rspb.2007.0515 (doi:10.1098/rspb.2007.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roopnarine P. D. 2006. Extinction cascades and catastrophe in ancient food webs. Paleobiology 32, 1–19 [Google Scholar]
- 15.Roopnarine P. D. 2010. Networks, extinction, and palecommunity food webs. In Quantitative methods in paleobiology, vol. 16 (eds Alroy J., Hunt G.), pp. 143–161 The Paleontological Society Papers [Google Scholar]
- 16.Nicolas M., Rubidge B. S. 2010. Changes in Permo-Triassic tetrapod ecological representation in the Beaufort Group (Karoo Supergroup) of South Africa. Lethaia 43, 45–59 10.1111/j.1502-3931.2009.00171.x (doi:10.1111/j.1502-3931.2009.00171.x) [DOI] [Google Scholar]
- 17.Hochuli P. A., Hermann E., Vigran J. O., Bucher H., Weissert H. 2010. Rapid demise and recovery of plant ecosystems across the end-Permian extinction event. Global Planet Change 74, 144–155 10.1016/j.gloplacha.2010.10.004 (doi:10.1016/j.gloplacha.2010.10.004) [DOI] [Google Scholar]