Abstract
Aggressive mimics are predatory species that resemble a ‘model’ species to gain access to food, mating opportunities or transportation at the expense of a signal receiver. Costs to the model may be variable, depending on the strength of the interaction between mimics and signal receivers. In the Indopacific, the bluestriped fangblenny Plagiotremus rhinorhynchos mimics juvenile cleaner wrasse Labroides dimidiatus. Instead of removing ectoparasites from larger coral reef fish, fangblennies attack fish to feed on scales and body tissue. In this study, juvenile cleaner wrasse suffered significant costs when associated with P. rhinorhynchos mimics in terms of reduced cleaning activity. Furthermore, the costs incurred by the model increased with heightened aggression by mimics towards signal receivers. This was apparently because of behavioural changes in signal receivers, as cleaning stations with mimics that attacked frequently were visited less. Variation in the costs incurred by the model may influence mimicry accuracy and avoidance learning by the signal receiver and thus affect the overall success and maintenance of the mimicry system.
Keywords: aggressive mimicry, cleaning symbioses, coral reef fish, Labroides dimidiatus, Plagiotremus rhinorhynchos
1. Introduction
Aggressive mimics are defined as predatory or parasitic species that resemble harmless or beneficial species in order to gain fitness benefits, including access to food, mating opportunities or transport [1,2]. Such systems usually involve three participants: a model, a mimic and a dupe (or signal receiver) [1]. Theory predicts that while aggressive mimics benefit from such an association, models incur costs in terms of reduced foraging or mating opportunities and increased energy expenditure [2–4]. However, costs to the model are predicted to vary depending on the strength of the interaction between mimics and signal receivers. This may directly affect the success and maintenance of the mimicry systems and subsequently the selective pressures that drive its evolution; signal receivers may avoid or attempt to discriminate between models and mimics more strongly when the costs of being attacked or frequency of attacks are high [3,5]. For example, Ophrys orchids mimic female wasps to attract males, which then carry pollen between flowers [6–8]. Costs to the signal receiver will depend on how much time and energy is spent visiting mimics and transferring pollen between plants. The amount of pollen produced may vary between season or geographical location. If pollen loads are high, selective pressures may drive male wasps to be more vigilant when discriminating between orchids and female wasps, and may also drive them to evolve better discriminating capabilities. There currently appears to be no empirical evidence that has quantified variation in costs to the model in relation to strength of interaction between mimic and signal receiver (in terms of aggression).
Perhaps, one of the most intriguing examples of mimicry exists on Indopacific coral reefs: juvenile bluestreaked cleaner wrasse Labroides dimidiatus are mimicked by the bluestriped fangblenny Plagiotremus rhinorhynchos [1,9–11] (figure 1). Instead of removing ectoparasites, cleaner mimics nip at passing reef fish to remove scales, pieces of fin or body tissue [9,11]. Plagiotremus rhinorhynchos benefit from associating with juvenile cleaner wrasse in terms of increased access to reef fish victims [12]. Conversely, juvenile cleaner wrasse incur costs of having an associated mimic in terms of reduced foraging (cleaning) activity. Cleaner wrasse with a mimic had 38 per cent fewer clients visiting the cleaning station and spent 29 per cent less time inspecting clients, compared with cleaner wrasse without a mimic [12]. In this study, I tested how intra-specific variation in aggression by mimic fangblennies towards coral reef fish (in terms of number of attacks on reef fish victims) influences the costs incurred by the cleaner wrasse models (in terms of reduced cleaning activity), to elucidate the mechanisms behind how aggressive mimics impose costs on their model. If reef fish clients use the mere presence of a mimic to avoid or cut short a cleaning interaction once a mimic is detected, then the costs to the model should be independent of the extent of aggression shown by the mimic. However, if clients modify their behaviour in response to the aggressive levels of mimics, then costs to the model should be correlated with increased aggression by mimics.
Figure 1.
The model and mimic: (a) juvenile Labroides dimidiatus and (b) Plagiotremus rhinorhynchos.
2. Material and methods
Fieldwork was conducted on coral reefs around Lizard Island (14°40′ S, 145°28′ E) and Heron Island (23°26′ S, 151°55′ E), Great Barrier Reef, Australia between July 2005 and February 2007. Focal observations were conducted for 20 min on SCUBA or snorkel at depths between 2 and 15 m on 25 P. rhinorhynchos. An observation was then conducted for 20 min on the juvenile cleaner wrasse with which the mimic was associated. To compare cleaning rates for juvenile cleaner wrasse with and without a mimic, a lone cleaner wrasse was then located on a similar section of the reef between 5 and 20 m away and a third 20 min observation was conducted.
For cleaner mimics, the following information was recorded: the number of attacks by the mimic, defined as a dart by the fangblenny towards the reef fish (successful attacks were recorded when visible contact was made with the reef fish), and the number of chases towards the mimic by other reef fish, defined as another fish species swimming rapidly and directly at the fangblenny (and whether this was in retaliation to a mimic attack). For cleaner wrasse, the following information was recorded: the time spent inspecting clients, defined as the cleaner hovering near to or touching the client while actively searching for food items, and is correlated with the number of bites taken on clients [13], time was recorded from when the cleaner approached the client fish until it departed; the identity of the client; and the number of cleaning interactions. Owing to the variability in cleaning time between reef site and location, the difference in inspection time between cleaners with and without a mimic was calculated as a percentage. The location of all cleaner wrasse and mimics were marked using flagging tape to prevent repeat observations being conducted on the same individual.
3. Results
(a). Mimic observations
Plagiotremus rhinorhynchos successfully attacked reef fish between 0 and 10 times per 20 min observation (mean ± s.d. = 2.5 ± 2.4). Reef fish victims included: Pomacentridae (e.g. Abudefduf abnormalis and Amblyglyphidodon curacao), Acanthuridae (e.g. Acanthurus nigrofuscus), Labridae (e.g. Hemigymnus melapterus and Cheilinus trilobatus), Scaridae (e.g. Scarus schlegeli) and Lutjanidae (e.g. Lutjanus fulviflamma). The number of chases towards P. rhinorhynchos from other coral reef fish ranged from 0 to 10 (median, interquartile range = 0, 3.5), and the majority were retaliatory chases after being attacked (40/43, 93%).
(b). Cleaner fish observations
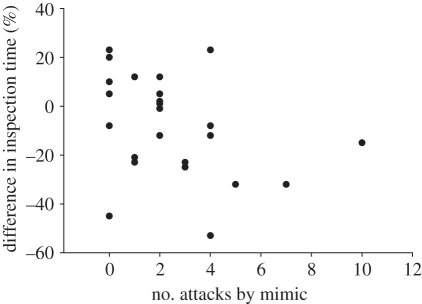
All juvenile cleaner wrasse (with and without mimics) spent between 152 and 576 s per 20 min (mean ± s.d. = 245 ± 134) inspecting between 5 and 38 clients (mean ± s.d. = 15.1 ± 4.5) from 5 to 24 species (mean ± s.d. = 13.4 ± 5.2). There was an overall reduction in inspection time (t24 = −2.20, p = 0.04) and in the total number of clients visiting cleaners (t24 = −2.03, p = 0.04) for juvenile cleaner wrasse with a mimic compared with juvenile cleaner wrasse without a mimic. There was no difference in the total number of species that visited juvenile cleaner wrasse with a mimic compared with juvenile cleaner wrasse without a mimic (t24 = −1.23, p = 0.12). The difference in total time spent inspecting clients by individual juvenile cleaner wrasse with and without cleaner mimics (cleaner with a mimic–cleaner without a mimic) was negatively correlated with the number of attacks by P. rhinorhynchos (Spearman rank rs = −3.20, n = 25, p = 0.05; figure 2), as was the difference in total number of individuals visiting cleaners (Spearman rank rs = −4.12, n = 25, p = 0.04). There was no correlation between number of attacks and the percentage difference in cleaning bout duration (Spearman rank rs = −1.50, n = 25, p = 0.29).
Figure 2.
The number of successful attacks by the aggressive mimic P. rhinorhynchos towards coral reef fish (20 min−1) in relation to the difference in total time (%) cleaner wrasse with and without an associated mimic spent inspecting coral reef fish clients (n = 25).
4. Discussion
Juvenile cleaner wrasse incurred more costs, in terms of a reduction in cleaning activity, when they were associated with the cleaner mimic P. rhinorhynchos (also shown in [12]), and cleaning activity was reduced in relation to the number of times that mimics attacked coral reef fish. Aggressive mimics, therefore, appear to drive behavioural changes in signal receivers, in response to the extent of aggression received. It appears that signal receivers are less likely to visit a cleaning station or they are less likely to return to the cleaning station after aggression from a mimic. There was no evidence that mimics affect the duration of individual cleaning interactions. Cleaner mimics very rarely attack fish being cleaned (this study; [12]), but instead attack passing reef fish or those in the immediate vicinity.
Aggressive behaviour exhibited by mimics may vary due to differences in size, sex, spawning behaviour, availability of other food sources, strength of competition between individuals and risk of predation. While populations of mimics may exhibit both temporal and spatial variation in each of these factors, it is perhaps the latter three (alternative food, competition and predation pressure) that could drive geographical variation and thus affect the success and the maintenance of the aggressive mimicry system between populations. If mimics are too aggressive towards signal receivers, and models suffer from a significant reduction in foraging costs, models may relocate to new sites, or attempt to chase mimics away. Conversely, if mimics are not aggressive enough, mimics may fail to gain access to an adequate food source. Therefore, the maintenance of aggressive mimicry systems may rely on a balance between learning and forgetting in signal receivers [14,15]. Indeed, reef fish learn to avoid the attacks of aggressive mimics both spatially and pre-emptively [5].
In Batesian and aggressive mimicry systems, the predators of mimics and victims of attack, respectively, should act as selective agents forcing mimics to accurately resemble their models [16,17]. However, this study shows that the selective pressures that drive mimics to accurately resemble their models, and signal receivers to discriminate between mimics and models, can vary within a mimicry system. Mimicry accuracy, discrimination and avoidance learning by signal receivers may be driven by the nature of the relationship between participants, including the extent of aggression exhibited by the mimic, which varies both within and between species. The status of mimetic relationships appears to be highly dynamic and is likely to vary both temporally and spatially.
Acknowledgements
Thank you to the staff at Lizard and Heron Island Research Stations for logistical support, and to L. Curtis, M. Eckes, P. Mansell and B. Cameron for their help in the field. This research was funded by the Australian Research Council with a grant and fellowship to K.L.C.
References
- 1.Wickler W. 1965. Mimicry and evolution of animal communication. Nature 208, 519–521 10.1038/208519a0 (doi:10.1038/208519a0) [DOI] [Google Scholar]
- 2.Wickler W. 1968. Mimicry in plants and animals. New York, NY: McGraw-Hill [Google Scholar]
- 3.Cheney K. L., Côté I. M. 2005. Frequency-dependent success of aggressive mimics in a cleaning symbiosis. Proc. R. Soc. B 272, 2635–2639 10.1098/rspb.2005.3256 (doi:10.1098/rspb.2005.3256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes K. F., Yeargan K. V. 1999. Exploitation of intraspecific communication systems: Illicit signalers and receivers. Ann. Entomol. Soc. Am. 92, 960–970 [Google Scholar]
- 5.Cheney K. L. 2008. The role of avoidance learning in an aggressive mimicry system. Behav. Ecol. 19, 583–588 10.1093/beheco/arn001 (doi:10.1093/beheco/arn001) [DOI] [Google Scholar]
- 6.Dafni A. 1987. Pollination in orchids and related genera: evolution from rewards to deception. In Orchid biology, reviews and perspectives (ed. Arditti J.), pp. 80–104 Ithaca, NY: Cornell University Press [Google Scholar]
- 7.Kullenberg B. 1961. Studies in Ophrys pollination. Zool. Bidrag Uppsala 34, 1–340 [Google Scholar]
- 8.Nilsson L. A. 1992. Orchid pollination biology. Trends Ecol. Evol. 7, 255–259 10.1016/0169-5347(92)90170-G (doi:10.1016/0169-5347(92)90170-G) [DOI] [PubMed] [Google Scholar]
- 9.Kuwamara T. 1981. Mimicry of the cleaner wrasse Labroides dimidiatus by the blennies Aspidontus taeniatus and Plagiotremus rhynorhynchos. Nanki Seibutu 23, 61–70 [Google Scholar]
- 10.Moland E., Eagle J. A., Jones G. P. 2005. Ecology and evolution of mimicry in coral reef fishes. Oceanogr. Mar. Biol. Annu. Rev. 43, 455–482 [Google Scholar]
- 11.Wickler W. 1966. Mimicry in tropical fishes. Phil. Trans. R. Soc. Lond. B 251, 473–474 10.1098/rstb.1966.0036 (doi:10.1098/rstb.1966.0036) [DOI] [Google Scholar]
- 12.Côté I. M., Cheney K. L. 2004. Distance-dependent costs and benefits of aggressive mimicry in a cleaning symbiosis. Proc. R. Soc. Lond. B 271, 2627–2630 10.1098/rspb.2004.2904 (doi:10.1098/rspb.2004.2904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grutter A. S. 1996. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Progr. Ser. 130, 61–70 10.3354/meps130061 (doi:10.3354/meps130061) [DOI] [Google Scholar]
- 14.Speed M. P., Turner J. R. G. 1999. Learning and memory in mimicry: II. Do we understand the mimicry spectrum? Biol. J. Linn. Soc 67, 281–312 10.1111/j.1095-8312.1999.tb01935.x (doi:10.1111/j.1095-8312.1999.tb01935.x) [DOI] [Google Scholar]
- 15.Turner J. R. G., Speed M. P. 1996. Learning and memory in mimicry. I. Simulations of laboratory experiments. Phil. Trans. R. Soc. Lond. B 351, 1157–1170 10.1098/rstb.1996.0100 (doi:10.1098/rstb.1996.0100) [DOI] [Google Scholar]
- 16.Huheey J. E. 1988. Mathematical models of mimicry. Am. Nat. 131, S22–S41 10.1086/284765 (doi:10.1086/284765) [DOI] [Google Scholar]
- 17.Sheppard P. M. 1958. Natural selection and heredity. London: Hutchinson [Google Scholar]