Abstract
The titanic baleen whales (Cetacea, Mysticeti) have a bizarre skull morphology, including an elastic mandibular symphysis, which permits dynamic oral cavity expansion during bulk feeding. How this key innovation evolved from the sutured symphysis of archaeocetes has remained unclear. Now, mandibles of the Oligocene toothed mysticete Janjucetus hunderi show that basal mysticetes had an archaeocete-like sutured symphysis. This archaic morphology was paired with a wide rostrum typical of later-diverging baleen whales. This demonstrates that increased oral capacity via rostral widening preceded the evolution of mandibular innovations for filter feeding. Thus, the initial evolution of the mysticetes' unique cranial form and huge mouths was perhaps not linked to filtering plankton, but to enhancing suction feeding on individual prey.
Keywords: Cetacea, Mysticeti, Mammalodontidae, evolution, Australia, Oligocene
1. Introduction
Living baleen whales (Mysticeti) have bizarre skulls that enable dynamic oral cavity expansion and accommodation of vast volumes of sea water during filter feeding [1–3]. This is achieved with kinetic skull joints, including an elastic lower jaw joint (mandibular symphysis), which permits independent rotation of the jaws in two planes: on their long (α) and horizontal (Ω) axes [2,3]. This key innovation supported the evolution of titanic body sizes and the extraordinary success of mysticetes [1,4], but contrasts with the primitive rigid symphysis of early whales (archaeocetes) [5,6]. The transition from this morphology in raptorial feeding archaeocetes to the specialized jaws of baleen whales has been obscure: until now, all mysticetes were thought to possess an elastic symphysis [5,7]. Here, I report that the basal-toothed mysticete Janjucetus hunderi (Oligocene, Australia [7]) has an archaeocete-like sutured symphysis paired with a typically mysticete-like wide rostrum. Thus, rostral modifications for increased oral space preceded the evolution of mandibular innovations for filter feeding in early mysticetes. The initial shift to increasing oral capacity may represent skull-shape optimization for suction feeding, which was perhaps the first step towards the unique cranial form of baleen whales.
2. Material and methods
Museum Victoria, Melbourne (NMV) specimen P229455 and United States National Museum of Natural History, Washington, DC (USNM) specimen 534009 are casts of an incomplete pair of mandibles currently held in a private collection (figure 1a–d). The analysis, photographs and measurements (see the electronic supplementary material) are all based on the original fossils, which are from the Jan Juc Formation (Chattian, Late Oligocene, 24.2–27.9 Ma [8]) at Jan Juc Beach, southwest of Torquay, Victoria, Australia (near 38°21′ S, 144°17′ E). Additional institutional abbreviations: NMVC, Museum Victoria Mammalogy collection (Melbourne); UCMP, University of California Museum of Paleontology (Berkeley).
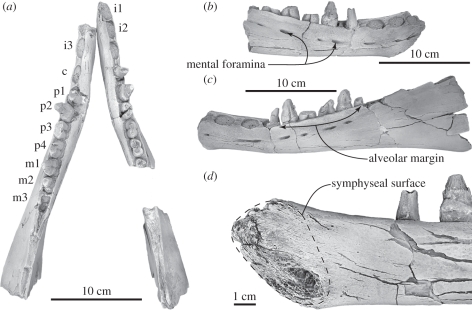
Figure 1.
Mandibles of Janjucetus hunderi. (a–d) Original fossil specimen represented by NMV P229455 and USNM 534009, whitened with ammonium chloride. (a) Left and right mandibles in dorsal view; (b) anterior part of right mandible in lateral view (c) left mandible in lateral view; and (d) symphyseal region of right mandible in medial view. Anatomical abbreviations: c, canine; i, incisor; m, molar; p, premolar.
3. Results
The pair of mandibles represented by NMV P229455 and USNM 534009 possess a suite of characters unique to the toothed mysticete clade Mammalodontidae: (i) short mandibular symphysis without a symphyseal groove (figure 1a,d); (ii) relatively large mental foramina; (iii) mandible has a salient lateral edge to the alveolar margin such that lower post-canine teeth are implanted within an alveolar groove (figure 1a–c); (iv) posterior half of the alveolar margin of the mandible forms an angle with the ventral margin of the mandible; (v) posterior half of the mandible with a straight ventral margin and no ventral expansion of the pan bone; (vi) teeth have salient longitudinal ridges developed on both the buccal and lingual surfaces of the crown enamel; (vii) posterior lower post-canines with two roots joined below the crown base by a transversely narrow isthmus; and (viii) lower post-canines closely spaced along the alveolar margin without elongated intervening diastemata or embrasure pits.
Within Mammalodontidae, the specimen represented by NMV P229455 and USNM 534009 differs from Mammalodon colliveri by having: (i) mandibles with externally concave profiles viewed dorsally (figure 1a); (ii) a robust alveolar portion of the mandible; (iii) seven lower post-canine teeth (Mammalodon has eight [6]); (iv) an anterior apex of the mandible that is not recurved medially; (v) a rugose symphyseal surface occupying the entire vertical diameter of the mandibular symphysis (figure 1d); and (vi) separate alveoli for i1 and i2. It unambiguously shares all but character (v) with the holotype specimen (NMV P216929) of J. hunderi (which is missing this region of the mandible [6,7]) supporting its referral to the latter species. Furthermore, measurements of the specimen represented by NMV P229455 and USNM 534009 closely approximate the equivalent measurements of NMV P216929 (see the electronic supplementary material).
4. Discussion
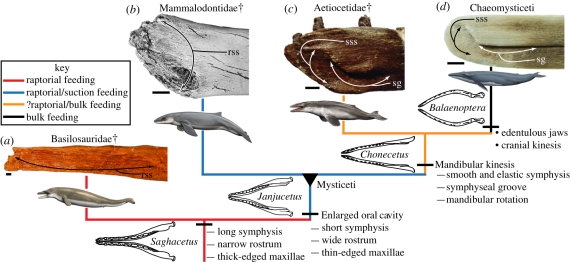
Until now, all living and fossil mysticetes have been shown to possess a mandibular symphysis characterized by a smooth symphyseal surface and longitudinal symphyseal groove (figure 2c,d) [2,3,9]. These features, respectively, accommodate a fibrocartilage annulus, which allows mandible rotation while feeding, and a thickened periosteum, which strengthens the symphysis by counteracting the torque exerted during oral cavity expansion [2,9]. Janjucetus lacks these derived features, resembling basilosaurid archaeocetes in having a rugose symphyseal surface (figure 2a,b). This shows that Janjucetus had a sutured symphysis and could not deploy α or Ω mandibular rotation during feeding. Yet, Janjucetus shares with all mysticetes a short symphyseal surface and a wide rostrum, specializations that in baleen whales increase palate surface area and oral cavity volume [2,6,7].
Figure 2.
Evolution of mysticete skull features linked to feeding, based on the phylogeny in the study of Fitzgerald [6]. (a–d) Mandibular symphyses in right medial view: (a) Zygorhiza kochii (USNM 11962); (b) Janjucetus hunderi (specimen represented by NMV P229455 and USNM 534009); (c) Aetiocetus weltoni (UCMP 122900) and (d) Balaenoptera acutorostrata (NMV C24936). Dagger symbol (†) denotes extinct clades. rss, rugose symphyseal surface; sg, symphyseal groove; sss, smooth symphyseal surface. Cetacean artwork by C. Buell.
Janjucetus bridges the gap in jaw morphology between archaeocetes and more advanced baleen whales, showing that the earliest mysticetes lacked mandibular kinesis (figure 2). A non-sutured and kinetic mandibular symphysis has until now been considered a key synapomorphy of Mysticeti [5,7]. The absence of this unique innovation in Janjucetus suggests that the earliest mysticetes could not engulf and expel large volumes of water, which is a crucial prerequisite for bulk filter feeding [1–3]. Thus, evolution of bulk planktivory was decoupled from initial enlargement of oral capacity via rostral widening—a feature present in Janjucetus and other basal toothed mysticetes. Odontocete species with a wide and blunt head shape are capable of generating significantly greater negative pressures during suction feeding than those with elongate and narrow jaws [10–12]. The increased rostral width in basal mysticetes may therefore represent optimization of head shape for suction feeding, which, in tandem with mandibular kinesis, was later co-opted for bulk feeding in edentulous mysticetes.
Acknowledgements
I thank R. Benson, T. Holland, F. Marx, J. Mead and T. Rich for comments; D. Bohaska, B. Crichton, W. Longmore, R. O'Brien and C. Potter for access to collections; Museum Victoria Preparation staff for casting; T. Deméré for the photograph in figure 2c; three anonymous referees and the editor for improvements to the manuscript; and C. Buell for permission to reproduce his artwork. This study was supported by the Harold Mitchell Foundation, Museum Victoria, and the Smithsonian Institution.
References
- 1.Goldbogen J. A. 2010. The ultimate mouthful: lunge feeding in rorqual whales. Am. Sci. 98, 124–131 [Google Scholar]
- 2.Lambertsen R. H., Ulrich N., Straley J. 1995. Frontomandibular stay of Balaenopteridae: a mechanism for momentum recapture during feeding. J. Mamm. 76, 877–899 10.2307/1382758 (doi:10.2307/1382758) [DOI] [Google Scholar]
- 3.Lambertsen R. H., Rasmussen K. J., Lancaster W. C., Hintz R. J. 2005. Functional morphology of the mouth of the bowhead whale and its implications for conservation. J. Mamm. 86, 342–352 10.1644/BER-123.1 (doi:10.1644/BER-123.1) [DOI] [Google Scholar]
- 4.Marx F. G., Uhen M. D. 2010. Climate, critters, and cetaceans: Cenozoic drivers of the evolution of modern whales. Science 327, 993–996 10.1126/science.1185581 (doi:10.1126/science.1185581) [DOI] [PubMed] [Google Scholar]
- 5.Deméré T. A., McGowen M. R., Berta A., Gatesy J. 2008. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 57, 15–37 10.1080/10635150701884632 (doi:10.1080/10635150701884632) [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald E. M. G. 2010. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool. J. Linn. Soc. 158, 367–476 10.1111/j.1096-3642.2009.00572.x (doi:10.1111/j.1096-3642.2009.00572.x) [DOI] [Google Scholar]
- 7.Fitzgerald E. M. G. 2006. A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales. Proc. R. Soc. B 273, 2955–2963 10.1098/rspb.2006.3664 (doi:10.1098/rspb.2006.3664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren S., Wallace M. W., Gallagher S. J., Dickinson J. A., McAllister A. 2009. Age constraints on Oligocene sedimentation in the Torquay Basin, southeastern Australia. Aus. J. Earth Sci. 56, 595–604 10.1080/08120090902806347 (doi:10.1080/08120090902806347) [DOI] [Google Scholar]
- 9.Johnston C., Deméré T. A., Berta A., Yonas J., Leger S. T. 2010. Observations on the musculoskeletal anatomy of the head of a neonate gray whale (Eschrichtius robustus). Mar. Mamm. Sci. 26, 186–194 10.1111/j.1748-7692.2009.00305.x (doi:10.1111/j.1748-7692.2009.00305.x) [DOI] [Google Scholar]
- 10.Werth A. J. 2006. Odontocete suction feeding: experimental analysis of water flow and head shape. J. Morphol. 267, 1415–1428 10.1002/jmor.10486 (doi:10.1002/jmor.10486) [DOI] [PubMed] [Google Scholar]
- 11.Werth A. J. 2006. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. J. Mamm. 87, 579–588 10.1644/05-MAMM-A-279R1.1 (doi:10.1644/05-MAMM-A-279R1.1) [DOI] [Google Scholar]
- 12.Johnston C., Berta A. 2011. Comparative anatomy and evolutionary history of suction feeding in cetaceans. Mar. Mamm. Sci. 27, 493–513 10.1111/j.1748-7692.2010.00420.x (doi:10.1111/j.1748-7692.2010.00420.x) [DOI] [Google Scholar]