Abstract
Parental effects play a vital role in shaping offspring phenotype. In birds, incubation behaviour is a critical parental effect because it influences the early developmental environment and can therefore have lifelong consequences for offspring phenotype. Recent studies that manipulated incubation temperature found effects on hatchling body composition, condition and growth, suggesting that incubation temperature could also affect energetically costly physiological processes of young birds that are important to survival (e.g. immune responses). We artificially incubated wood duck (Aix sponsa) eggs at three biologically relevant temperatures. Following incubation, we used two immunoassays to measure acquired immune responses of ducklings. Ducklings incubated at the lowest temperature had reduced growth, body condition and responses to both of our immune challenges, compared with those from the higher temperatures. Our results show that incubation temperatures can be an important driver of phenotypic variation in avian populations.
Keywords: maternal effects, phytohemattagluttinin, sheep red blood cells
1. Introduction
Parental behaviour and physiology can have enormous non-genomic influences on offspring development and phenotype [1,2]. These non-genomic contributions, or parental effects, can shape an organism's life-history trajectory by influencing growth rates, age at maturity, survival and reproduction [2]. Non-genomic contributions may also serve as key links between changing environmental conditions, such as climate, and expression of adaptive phenotypes [1,3].
In birds, parental incubation influences both the humidity and temperature under which eggs develop [4]. Incubation behaviour by avian parents consists of on–off bouts that largely keep eggs within a narrow temperature range [4], but incubation temperatures become compromised when parents spend time away from the nest, resulting in longer incubation periods and lower hatching success of eggs [5]. However, studies have only recently revealed the consequences of variation in incubation temperature for avian phenotype. In wood ducks (Aix sponsa), slight differences (less than 1°C) in incubation temperature, reflecting variation of average temperatures of naturally incubated wood duck nests [6], influence duckling body composition, growth, condition, locomotor performance and stress hormone concentrations [6–8]. These results, along with several studies that detected correlations between avian immune parameters and incubation period among species [9,10], suggest that incubation temperature also might influence duckling immune responses.
In this study, we sought to determine whether incubation temperature influences the immunocompetence, growth and condition of hatchling wood ducks. Because another study demonstrated that duck embryos incubated at lower temperatures expended more energy during incubation, and potentially hatched with fewer energy reserves [11], we predicted that ducklings incubated at lower temperatures would grow more slowly, be in lower condition, and have less robust immune responses because immunity is energetically demanding [12]. In order to isolate temperature as the key environmental factor affecting duckling immunity, we experimentally incubated wood duck eggs in the laboratory at three biologically relevant temperatures.
2. Material and methods
(a). Egg collection and incubation
We monitored 201 nest-boxes in west-central South Carolina daily for wood duck eggs. We collected fresh unincubated eggs and transported them to Virginia Tech every 4 days, where they were artificially incubated in Grumbach incubators (model BSS 160) at one of three temperatures (35.0°C, 35.9°C and 37.0°C) at 60–65% humidity. These temperatures were chosen because they fall within the range of naturally incubated wood duck nests [6]. Incubators were programmed to allow two cool-down periods each day (approx. 3°C reduction in mean temperature for 75 min at 0815 and 1830) to simulate natural daily feeding recesses taken by mothers during incubation [13]. To avoid pseudoreplication and potential bias attributed to parental effects on immune parameters, we only measured immune function of one duckling per clutch per incubation temperature treatment.
(b). Duckling husbandry
After hatching, we maintained ducklings communally in plastic cages (2–3 ducklings/cage) in an environmental chamber (28°C, 14 L : 10 D photoperiod). Ducklings were randomly assigned to cages, and cages were oriented in the room in a stratified, random manner. A 50 W infrared light bulb suspended above each cage provided additional warmth to ducklings. Ducklings were allowed constant access to food (Dumor Chick Starter/Grower 20%) and water. All experiments were approved by IACUC.
(c). Duckling growth and immunity
We monitored growth by weighing ducklings daily and measuring tarsus length at 0, 2, 6, 10 and 20 days post hatch (dph).
We measured duckling immunocompetence using two commonly used novel antigens, which assay pro-inflammatory immune responsiveness (requires aspects of both innate and acquired immunity [14]), and humoral immunity (a branch of acquired immunity). At 6 dph, we measured pro-inflammatory immune responsiveness in 46 ducklings using phytohemattagluttinin (PHA) and humoral immunity in 58 ducklings using sheep red blood cells (SRBC) [15]. We quantified PHA responses by having a blind observer measure swelling at the site of injection (subtracting pre-injection values) and SRBC responses by measuring antibody titres in the plasma 6 days after SRBC injection (subtracting pre-injection values). Blood sampling and injections with novel antigens occurred between 1200 and 1600 h during March–August 2009.
(d). Data analysis
All statistical analyses were performed in SAS v. 9.1 (SAS Institute Inc., Cary, NC, USA) or Microsoft Excel. Where appropriate, we tested for normality and homoscedasticity. We used raw data values in statistical analyses except in our analysis of PHA response; fold increase in swelling was log10-transformed to meet parametric requirements. We tested for differences in body condition among incubation treatments using a repeated measures ANCOVA with mass as the dependent variable and tarsus as the covariate. Body condition is presented as a graph of average residuals of mass against tarsus length for visual purposes. We compared immune responses using two separate ANOVAs (SAS PROC mixed); in both models, we tested for the effects of duckling sex, incubation temperature and their interaction. The cage in which an individual was housed was included as a random effect. Body mass was initially included as a covariate in our immune models but was dropped from the final models owing to insignificance (p ≥ 0.29).
3. Results
Incubation temperature did not significantly influence (p ≥ 0.54) hatching success (35.0°C: 61%, n = 89; 35.9°C: 65%, n = 88 and 37.0 °C: 57%, n = 85) or post hatch survival (35.0°C: 80%, n = 50; 35.9°C: 82%, n = 51 and 37.0°C: 86%; n = 44). Incubation temperature significantly affected the duration of incubation (F2,129 = 160.89; p < 0.001) with higher temperatures inducing more rapid development (35.0°C: 37.2 ± 0.24; 35.9°C: 34.4 ± 0.18d; 37.0°C: 32.1 ± 0.18d).
There was a strong positive relationship between egg mass and hatchling mass (F1,100 = 241.48; p < 0.001) and egg mass and hatchling tarsus length (F1,100 = 26.80; p < 0.001). Incubation temperature did not influence hatchling mass (p = 0.992) but did influence tarsus length (F2,100 = 3.70; p = 0.028) Ducklings incubated at the lowest temperature had 1.9–2.8% longer tarsus than ducklings from the higher incubation temperatures.
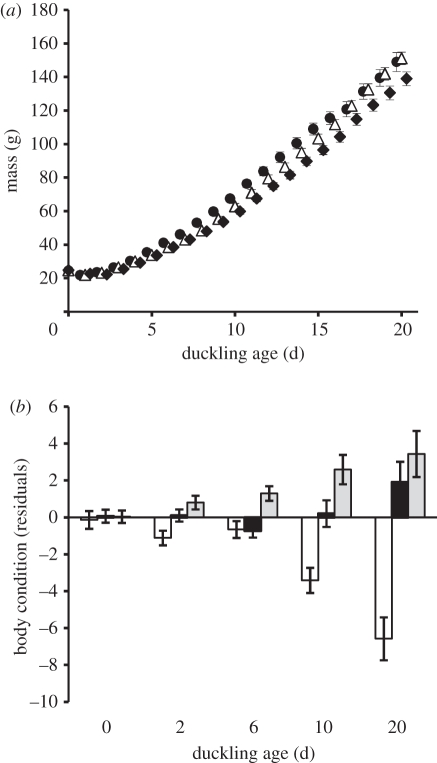
Incubation temperature significantly influenced duckling growth (temperature × age: F30,1380 = 3.54; p = 0.016; figure 1a). We detected a positive relationship between duckling tarsus length and body mass (F1,657 = 99.84; p < 0.001), but this differed over time based on incubation temperature indicating differences in body condition among treatments (temperature × age: F14,657 = 1.87; p = 0.027; figure 1b). Ducklings were similar in mass and condition during the first few days post-hatching; however, by 20 dph ducklings from the lowest incubation temperature weighed 7–8% less, and were in poorer body condition than ducklings from the higher incubation temperatures.
Figure 1.
Average (±1 s.e.) (a) duckling growth. Filled circles, 37.0°C (n = 32); triangles, 35.9°C (n = 34); filled diamonds, 35.0°C (n = 29). and (b) body condition of wood ducks (Aix sponsa) that hatched from eggs incubated at different temperatures. White bars, 35.0°C (n = 28); black bars, 35.9°C (n = 34); light grey bars, 37.0°C (n = 35).
Ducklings from each incubation temperature exhibited a pronounced swelling response after injection with PHA (figure 2a). However, swelling responses differed among incubation temperatures (temperature: F2,14 = 5.47; p = 0.018). Peak swelling, which occurred 24 h after injection, was lower in ducklings incubated at the lowest temperature compared with ducklings incubated at the higher temperatures. Ducklings from all incubation temperatures produced antibodies in response to SRBC injection (figure 2b). Similar to our PHA results, ducklings incubated at the lowest temperature had lower antibody responses to SRBC injection than ducklings incubated at the two higher temperatures (temperature: F2,21 = 5.04; p = 0.016). We did not detect a significant effect of sex nor a sex × temperature interaction on either of our immune responses (in all cases p > 0.132).
Figure 2.
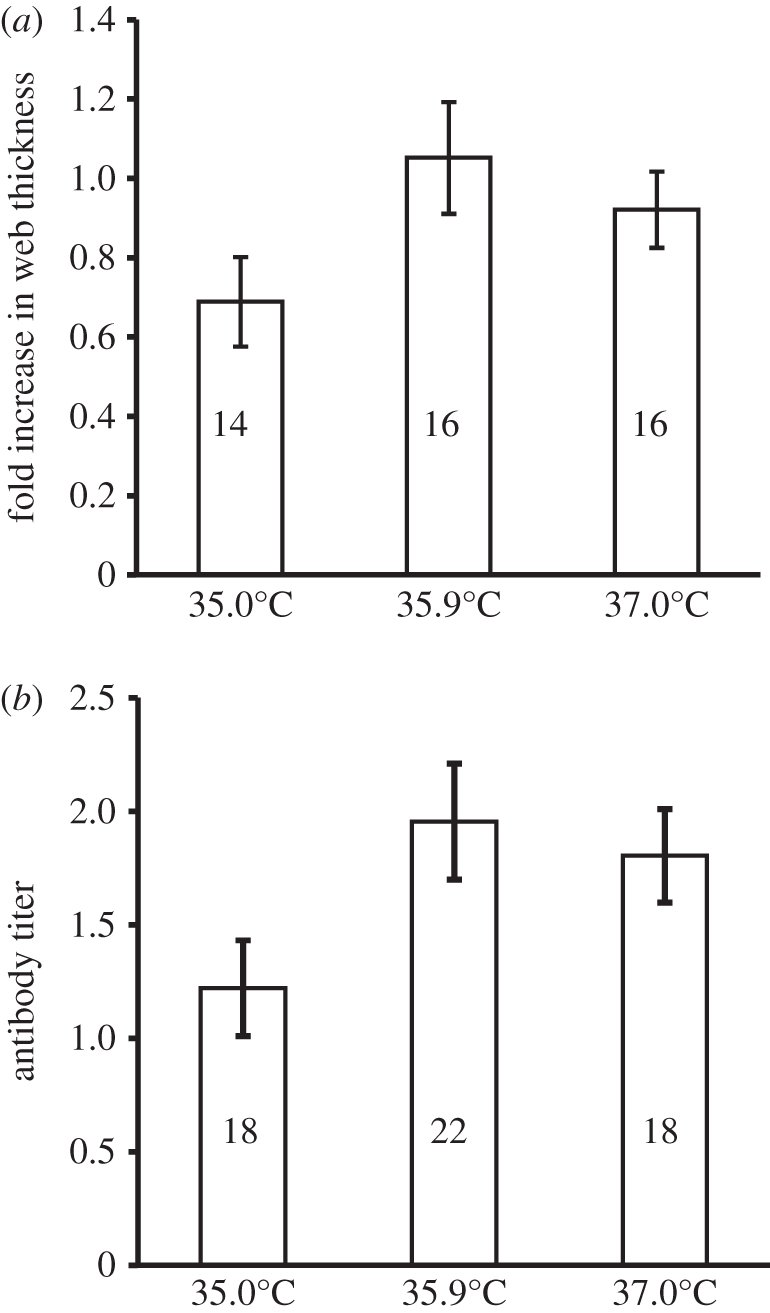
Immune responses (mean ± 1 s.e.) of wood ducks (Aix sponsa) that hatched from eggs incubated at different temperatures (35.0°C, 35.9°C or 37.0°C). (a) Fold increase in foot web thickness of ducklings 24 h after exposure to phytohemaggluttinin (b). Antibody titres of ducklings exposed to sheep red blood cells (SRBC). Samples sizes are on graph.
4. Discussion
Our results demonstrate that slight (approx. 1°C), biologically relevant [6], differences in incubation temperature affect the ability of ducklings to mount an immune response to novel antigens, a key response that influences vertebrate survival [16,17]. Specifically, ducklings from the low incubation temperature had 19–21% lower swelling in response to PHA injection and 32–38% lower SRBC responses than ducklings from the two higher incubation temperatures. Precocial species like the wood duck begin interacting with their environment shortly after hatching [7,8], so that compromised immunocompetence may make them more susceptible to pathogens and parasites they encounter while foraging.
Our findings are the first to demonstrate the importance of incubation temperature on the avian immune system, but they complement several others that suggest the duration of incubation, which negatively correlates with temperature, and nest microclimate can influence avian immunity. For instance, our results are consistent with a field study on an altricial species, the tree swallow (Tachycineta bicolor), which manipulated nest-box temperatures and detected lower innate immune responses in nestlings incubated in chilled nest-boxes compared with those incubated in control nest-boxes [18]. However, the causative factors influencing swallow immunity remain unresolved and could include changes in nest microclimate or changes in female behaviour. In contrast, our results pinpoint temperature as a key determinant of immunity that warrants further attention. Our findings are also consistent with an across-species comparison of response to PHA injection and length of incubation; birds with longer incubation periods had weaker swelling responses than birds with shorter incubation periods [9]. However, our results are inconsistent with the detected negative relationship between malaria prevalence and incubation period across 36 altricial bird species [10], and thus do not support the hypothesis that longer incubation periods increase immunocompetence because of greater development of the immune system [10].
In addition to altering immunity, we demonstrated that incubation temperature influences both duckling growth and body condition; low temperatures produced ducklings that grew slower and exhibited poorer body condition than higher temperatures. Because effects on growth and condition persisted until at least 20 dph, our results suggest that effects of incubation temperature on body size and mass may persist, or even amplify, throughout juvenile development. Body size is important for over winter survival and age at first breeding events [19,20]. Therefore, differences in condition and growth trajectories have implications for lifetime reproductive success.
The importance of maternal health for offspring phenotype is well documented [1], but we have only recently begun to appreciate the role that incubation conditions, which are largely determined by parental incubation behaviour, play in determining avian offspring phenotype. Here, we demonstrate that slight differences in incubation temperature produce variability in duckling immunocompetence, growth and condition. If less robust immune responses translate into greater disease susceptibility, then incubation conditions could influence disease dynamics in avian populations, especially if differences in immunocompetence persist into adulthood. For instance, slightly cooler incubation temperatures that arise when parents are less capable of maintaining nest temperatures—when nesting in sub-optimal habitats (e.g. areas of high disturbance or low resource availability) or sub-optimal environmental conditions (e.g. during droughts)—may produce a greater number of susceptible offspring, which could fuel disease outbreaks. Furthermore, as immune function, growth and body condition have important implications for survival there may be strong selection pressure on parents to maintain optimal nest temperatures. Perhaps most importantly, our findings suggest that if there is a genetic basis for variation in parental incubation behaviour, incubation conditions play an important role in producing phenotypic variation within avian populations which provides the variability upon which natural selection acts.
Acknowledgements
We thank B. Hopkins, Hopkins's Laboratory, Winkel's Laboratory, SREL field crew, B. Kennamer and L. Kirkpatrick. J. Willson, J. Walters and I. Moore reviewed the manuscript. Funding supplied by NSF grant IOB-0615361, Sigma Xi, SICB and Virginia Tech.
References
- 1.Mousseau T. A., Fox C. W. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press [Google Scholar]
- 2.Badyaev A. V., Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177 10.1016/j.semcdb.2008.11.013 (doi:10.1016/j.semcdb.2008.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayward L. S., Wingfield J. C. 2004. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 135, 365–371 10.1016/j.ygcen.2003.11.002 (doi:10.1016/j.ygcen.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 4.Deeming D. C. 2002. Avian incubation, behaviour, environment, and evolution. New York, NY: Oxford University Press [Google Scholar]
- 5.Reid J. M., Monaghan P., Nager R. G. 2002. Incubation and the costs of reproduction. In Avian incubation: behaviour, environment, and evolution (ed. Deeming D. C.), pp. 314–325 New York, NY: Oxford University Press [Google Scholar]
- 6.Hepp G. R., Kennamer R. A., Johnson M. H. 2006. Maternal effects in wood ducks: incubation temperature influences incubation period and neonate phenotype. Funct. Ecol. 20, 307–314 10.1111/j.1365-2435.2006.01108.x (doi:10.1111/j.1365-2435.2006.01108.x) [DOI] [Google Scholar]
- 7.DuRant S. E., Hepp G. R., Moore I. T., Hopkins B. C., Hopkins W. A. 2010. Slight differences in incubation temperature affect early growth and stress endocrinology of wood duck (Aix sponsa) ducklings. J. Exp. Biol. 213, 45–51 10.1242/jeb.o34488 (doi:10.1242/jeb.o34488) [DOI] [PubMed] [Google Scholar]
- 8.Hopkins B. C., DuRant S. E., Hepp G. R., Hopkins W. A. 2011. The early developmental environment influences locomotor performance in young wood ducks (Aix sponsa). J. Exp. Zool. 315, 274–279 10.1002/jez.673 (doi:10.1002/jez.673) [DOI] [PubMed] [Google Scholar]
- 9.Palacios M. G., Martin T. E. 2006. Incubation period and immune function: a comparative field study among coexisting birds. Oecologia 146, 505–512 10.1007/s00442-005-0220-3 (doi:10.1007/s00442-005-0220-3) [DOI] [PubMed] [Google Scholar]
- 10.Ricklefs R. E. 1992. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA 89, 4722–4725 10.1073/pnas.89.10.4722 (doi:10.1073/pnas.89.10.4722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuRant S. E. In press Embryonic developmental patterns and energy expenditure are affected by incubation temperature in wood ducks (Aix sponsa). Physiol. Biochem. Zool. [DOI] [PubMed] [Google Scholar]
- 12.Martin L. B., II, Scheuerlein A., Wikelski M. 2002. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. Lond. B 270, 153–158 10.1098/rspb.2002.2185 (doi:10.1098/rspb.2002.2185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manlove C. A., Hepp G. R. 2000. Patterns of nest attendance in female wood ducks. Condor 102, 286–291 10.1650/0010-5422(2000)102[0286:PONAIF]2.0.CO;2 (doi:10.1650/0010-5422(2000)102[0286:PONAIF]2.0.CO;2) [DOI] [Google Scholar]
- 14.Vinkler M., Bainova H., Albrecht T. 2010. Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct. Ecol. 24, 1081–1086 10.1111/j.1365-2435.2010.01711.x (doi:10.1111/j.1365-2435.2010.01711.x) [DOI] [Google Scholar]
- 15.Hanssen S. A., Hasselquist D., Folstad I., Erikstad K. E. 2004. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. Lond. B 271, 925–930 10.1098/rspb.2004.2678 (doi:10.1098/rspb.2004.2678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wobeser G. 2006. The essentials of disease in wild animals. Iowa: Blackwell Publishing [Google Scholar]
- 17.Moller A. P., Saino N. 2004. Immune response and survival. Oikos 104, 299–304 10.1111/j.0030-1299.2004.12844.x (doi:10.1111/j.0030-1299.2004.12844.x) [DOI] [Google Scholar]
- 18.Ardia D. R., Perez J. H., Clotfelter E. D. 2010. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc. R. Soc. B 277, 1881–1888 10.1098/rspb.2009.2138 (doi:10.1098/rspb.2009.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringsby T. H., Saether B., Solberg E. J. 1998. Factors affecting juvenile survival in House Sparrow (Passer domesticus). J. Avian Biol. 29, 241–247 10.2307/3677106 (doi:10.2307/3677106) [DOI] [Google Scholar]
- 20.Hepp G. R., Kennamer R. A., Harvey IV W. F. 1989. Recruitment and natal philopatry of wood ducks. Ecology 70, 897–903 10.2307/1941357 (doi:10.2307/1941357) [DOI] [Google Scholar]