Abstract
Empirical evidence has shown that stressful conditions experienced during development may exert long-term negative effects on life-history traits. Although it has been suggested that oxidative stress has long-term effects, little is known about delayed consequences of oxidative stress experienced early in life in fitness-related traits. Here, we tested whether oxidative stress during development has long-term effects on a life-history trait directly related to fitness in three colonies of European shags Phalacrocorax aristotelis. Our results revealed that recruitment probability decreased with oxidative damage during the nestling period; oxidative damage, in turn, was related to the level of antioxidant capacity. Our results suggest a link between oxidative stress during development and survival to adulthood, a key element of population dynamics.
Keywords: oxidative damage, nestling stage, antioxidants, fitness, recruitment, European shag
1. Introduction
Empirical evidence has shown that stressful conditions experienced during early development can influence physiological and life-history traits during later life (reviewed in [1]), such as survival to adulthood [2], body condition at sexual maturity [3] and thereby fitness in animals including humans [3–5]. However, our knowledge of long-lasting consequences of stressful development remains limited, particularly in wild animals.
Oxidative stress, the imbalance between production of reactive oxygen species (ROS) and antioxidant defences leading to oxidative damages, is a potentially important physiological cost implicated in life-history trade-offs [6] and senescence [7]. During early development, organisms are prone to experience high levels of oxidative stress [8] and stressful conditions in very early life may alter the balance between ROS production and antioxidant defences and repair capacity [9,10]. In vertebrates, young may experience a high metabolic activity during rapid growth that in turn causes oxidative damage to the organism [11,12]. It has been proposed that oxidative damage during development may potentially constrain organisms' fitness in adulthood [13]. Nevertheless, evidence for negative associations between oxidative damage during early life and subsequent performance in wild animals is scarce.
Here, we tested whether recruitment probability was related to pre-fledging oxidative damage in a long-lived seabird, the European shag Phalacrocorax aristotelis. The European shag is a large altricial bird. Shag nestlings exhibit high growth and metabolic rates [14,15], and hence they presumably experience high levels of oxidative stress. In this species, early conditions influence telomere erosion, and it has been suggested that oxidative stress is the proximal cause of this relationship [16]. In three breeding colonies of shags, we first examined whether oxidative damage is related to circulatory antioxidant levels in full-grown nestlings. Then, using extensive monitoring data of marked birds during a four-year period, we examined if oxidative damage of shag nestlings influences their recruitment probability.
2. Material and methods
This study was carried out in three breeding colonies of the European shag (Illas Cíes, Illa de Ons and Sagres) at the Parque Nacional das Illas Atlánticas (Galicia, Spain; see the electronic supplementary material). Between April and June 2006, 107 nestlings from 77 nests (Cíes: 34 nests (n = 46 chicks); Ons: 35 (n = 51 chicks) and Sagres: 8 (n = 10 chicks)) were blood sampled and several morphometries, including bill, wing and tarsus length, were measured (electronic supplementary material). Blood samples were stored in the cool until they were centrifuged on the same day, and plasma samples were stored frozen at −80°C. Each nestling was marked with a numbered metal ring and a coloured plastic ring with an individual two-digit combination to facilitate identification from a distance.
We determined the level of plasma antioxidant capacity for each sample by Trolox equivalent method (see the electronic supplementary material). The level of lipid peroxidation in plasma (i.e. oxidative damage in lipids) was assessed by quantifying malondialdehydes using high-performance liquid chromatography (see Noguera et al. [17] and the electronic supplementary material).
During the period 2007–2010, we collected resighting data through intensive field monitoring (all colonies were visited three to five times per year) of marked shags in the Parque Nacional das Illas Atlánticas. European shags with colour rings are easily detected and have a high probability of being resighted (91%, with 95% confidence interval 0.83–0.99; [18]). Juvenile shags are philopatric, and most breeders (greater than 99%) recruit to the island where they hatched [18]. We also consulted other resighting schemes from elsewhere in Spain, with more than 1000 resightings of shags between 2007 and 2010, but none of the study birds recruited to a site other than the natal colony. Average age of first reproduction in our study population is 2.53 years [18] and most shags recruit within age 3 years [19].
We evaluated variables affecting lipid peroxidation level (log transformed) during development using a linear mixed model (LMM) with nest identity and colony of origin as random terms (nest identity nested within the natal colony). In the model, sex (see the electronic supplementary material) was included as a fixed factor, and chick age, hatching date, brood size and chick body condition (see the electronic supplementary material) were included as covariates. Antioxidant compounds prevent oxidative damage [20], so we also included plasma antioxidant level as a covariate in the model. Recruitment probability was analysed using a generalized linear mixed model (GLMM) with a binomial error distribution and a logit link function. In this model, sex was included as a fixed factor, and lipid peroxidation, chick age, hatching date, brood size and body condition as covariates. Nest identity nested within the colony of origin was included as a random term. In 2009, invasive American minks (Neovision vison) were detected for the first time at shag breeding areas on Illas Cíes. Thus, to be confident that results were not affected by the presence of this exotic predator, we ran the same model but excluding this colony. In all models, Satterthwaite's approximation for degrees of freedom was used [21]. All models were simplified by removing non-significant terms (backward deletion) and the significance level was set at 0.05.
3. Results
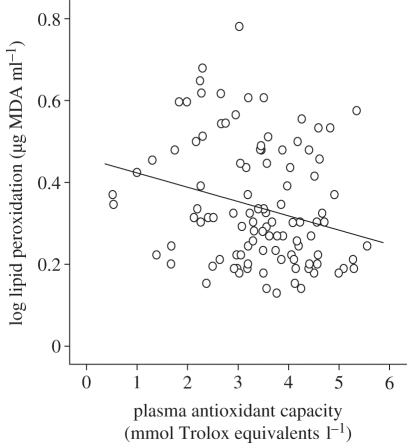
Mixed model analysis revealed that plasma antioxidant capacity was negatively related to lipid peroxidation in shag nestlings (table 1); plasma antioxidants explained 7.62 per cent of the variation in lipid peroxidation levels (figure 1). Sex, age, hatching date, brood size and body condition did not affect lipid peroxidation level (p > 0.23 in all cases), so they were excluded in the final model.
Table 1.
Summary of the minimal adequate models. (Model 1: LMM on lipid peroxidation and model 2: GLMM on recruitment of European shag chicks.)
| dependent variable | independent variables | estimate (±s.e.) | F | d.f. | p |
|---|---|---|---|---|---|
| model 1 | |||||
| lipid peroxidation | intercept | 0.04554 (0.004) | |||
| plasma antioxidant capacity | −0.00326 (0.001) | 5.88 | 1,102 | 0.017 | |
| model 2 | |||||
| recruitment | intercept | 0.5095 (0.297) | |||
| lipid peroxidation | −121.30 (42.802) | 8.03 | 1,98.2 | 0.005 | |
Figure 1.
Relationship between plasma antioxidant capacity and lipid peroxidation (malondialdehyde, MDA) levels in European shag chicks (solid line shows a linear regression).
A total of 11 nestlings out of 107 recruited into the breeding population (5, 5 and 1 in Sagres, Ons and Cíes, respectively). GLMM on recruitment revealed that lipid peroxidation level was the only variable that significantly predicted recruitment: recruitment probability decreased as nestling oxidative damage increased (table 1 and the electronic supplementary material). On average, recruiting shags had lower level of lipid peroxidation as nestlings (1.074 ± 0.007 µg ml–1) than non-recruits (1.085 ± 0.004 µg ml–1). Similar results were achieved when the analysis was restricted to predator-free colonies (lipid peroxidation: F1,51.11 = 4.93, p = 0.030). Recruitment probability was not affected by sex, age, hatching date, brood size and body condition during the nestling period (p > 0.10 in all cases), so they were excluded in the final model.
4. Discussion
In this study, we found that shag nestlings with stronger antioxidant status had lower levels of lipid peroxidation, suggesting a link between antioxidants and oxidative damage during development. Most importantly, recruitment probability correlated with oxidative damage of nestlings. Because recruitment is closely related to post-fledgling survival in this study population [18], our results suggest a link between oxidative stress during development and survival to adulthood in a natural population of a long-lived bird.
Previous studies have shown that elevated growth rates may increase oxidative stress levels during early life [10,12]. Nevertheless, in our study, body condition, sex, hatching date and brood size (variables affecting growth rates [18]) did not influence lipid peroxidation in full-grown nestlings. Shag chicks with higher antioxidant capacity also had a lower level of lipid peroxidation, suggesting that availability of antioxidant reserves could play an important role in preventing oxidative damage during early development [20]. Shag nestlings often face unfavourable conditions during growth owing to, for example, parasitism [22], food shortage and cold stress during adverse weather ([23]; shags have delayed homeothermy [15]). Oxidative damage during development can be a consequence of stressful conditions, such as pollutants [24], thermal stress [25], compensatory growth [10] and nutritional deficiencies and diseases [26].
In our study population, most chick mortality occurs during the first two weeks after hatching [23], and natal dispersal to other colonies is very rare ([18]; in this study, all birds recruited to the natal colony). Although our analysis did not allow us to separate recruitment and survival, in our study site failure to be seen in the colony during the 3 years of life presumably reflects mortality during the first years after independence [18]. Our results highlight the possibility that lipid peroxidation level, during the nestling stage, may be a direct or indirect factor that anticipates future recruitment in seabird populations. Although no other predictor variable than lipid peroxidation was related to recruitment probability, we cannot exclude the possibility that this might be owing to the lack of statistical power.
In summary, our study provides empirical evidence for a link between oxidative damage during development and later performance in a vertebrate. This study suggests that oxidative stress (or related traits) may shape life-history traits of free-living organisms [27]. Future studies should experimentally address whether factors governing oxidative status during the nestling period may have a delayed effect on recruitment and thereby on population dynamics.
Acknowledgements
This project was conducted with permissions from Xunta de Galicia ethics board, permit number 2006/190.
We thank C. Perez and I. Munilla, A. Sampedro and J. M. Sánchez for their help during fieldwork, A. Tato for her help in laboratory work and three anonymous reviewers for comments on the manuscript. We also thank D. Alvarez and J. Hidalgo for their help with resighting data, and J. A. Bouzas and the staff of the P. N. Illas Atlánticas for logistic support. J.C.N. was supported by a grant from MICINN (BES-2007-16432) and S.Y.K. by an Isidro Parga Pondal fellowship (Xunta de Galicia). Finance was provided by the Spanish Ministerio de Ciencia e Innovación (CGL2009-10883-C02-01) and Spanish Ministerio de Medio Ambiente (Organismo Autónomo Parques Nacionales, 48/2005). All permissions were provided by Servicio de Medio Ambiente, Xunta de Galicia.
References
- 1.Metcalfe N. B., Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 2.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Alvarez C., Bertrand S., Devevey G., Prost J., Faivre B., Chastel O., Sorci G. 2006. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution 60, 1913–1924 10.1111/j.0014-3820.2006.tb00534.x (doi:10.1111/j.0014-3820.2006.tb00534.x) [DOI] [PubMed] [Google Scholar]
- 4.Luo Z. C., Fraser W. D., Julien P., Deal C. L., Audibert F., Smith G. N., Xiong X., Walker M. 2006. Tracing the origins of ‘fetal origins’ of adult diseases: programming by oxidative stress? Med. Hypotheses 66, 38–44 10.1016/j.mehy.2005.08.020 (doi:10.1016/j.mehy.2005.08.020) [DOI] [PubMed] [Google Scholar]
- 5.Blas J., Bortolotti G. R., Tella J. L., Baos R., Marchant T. A. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 10.1073/pnas.0700232104 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monaghan P., Metcalfe N. B., Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurement and interpretation. Ecol. Lett. 12, 75–92 10.1111/j.1461-0248.2008.01258.x (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 7.Finkel T., Holbrook N. J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 10.1038/35041687 (doi:10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe N. B., Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of oxygen species in shaping phenotypes from conception to death. Funct. Ecol 24, 984–996 10.1111/j.1365-2435.2010.01750.x (doi:10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 9.Nussey D. H., Pemberton J. M., Pilkington J. G., Blount J. D. 2009. Life history correlates of oxidative damage in a free-living mammal population. Funct. Ecol. 23, 809–817 10.1111/j.1365-2435.2009.01555.x (doi:10.1111/j.1365-2435.2009.01555.x) [DOI] [Google Scholar]
- 10.Hall M. E., Blount J. D., Forbes S., Royle N. J. 2010. Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct. Ecol. 32, 365–373 10.1111/j.1365-2435.2009.01635.x (doi:10.1111/j.1365-2435.2009.01635.x) [DOI] [Google Scholar]
- 11.Rollo C. D. 2002. Growth negatively impacts the life span of mammals. Evol. Dev. 4, 55–61 10.1046/j.1525-142x.2002.01053.x (doi:10.1046/j.1525-142x.2002.01053.x) [DOI] [PubMed] [Google Scholar]
- 12.Kim S. Y., Noguera J. C., Morales J., Velando A. 2011. Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol. Ecol. 25, 461–472 10.1007/s10682-010-9426-x (doi:10.1007/s10682-010-9426-x) [DOI] [Google Scholar]
- 13.Mangel M., Munch S. B. 2005. A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 166, 155–176 10.1086/444439 (doi:10.1086/444439) [DOI] [PubMed] [Google Scholar]
- 14.Velando A., Graves J., Freire J. 2000. Sex-specific growth in the European shag Stictocarbo aristotelis, a seabird with size dimorphism. Ardea 88, 127–136 [Google Scholar]
- 15.Moe B., Brunvoll S., Mork D., Brobakk T. E., Bech C. 2005. Does food shortage delay development of homeothermy in European shag nestlings (Phalacrocorax aristotelis). J. Comp. Physiol. B 175, 21–30 10.1007/s00360-004-0458-9 (doi:10.1007/s00360-004-0458-9) [DOI] [PubMed] [Google Scholar]
- 16.Hall M. E., Nasir L., Daunt F., Gault E. A., Croxall J. P., Wanless S., Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B 271, 1571–1576 10.1098/rspb.2004.2768 (doi:10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguera J. C., Alonso-Alvarez C., Kim S. Y., Morales J., Velando A. 2011. Yolk testosterone reduces levels of oxidative damages during postnatal development. Biol. Lett. 7, 93–95 10.1098/rsbl.2010.0421 (doi:10.1098/rsbl.2010.0421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velando A., Freire J. 2002. Population modelling of European shag at their southern limit: conservation implications. Biol. Conserv. 107, 59–69 10.1016/S0006-3207(02)00044-7 (doi:10.1016/S0006-3207(02)00044-7) [DOI] [Google Scholar]
- 19.Aebischer N. J. 1986. Retrospective investigation of an ecological disaster in the shag, Phalacrocorax aristotelis: a general method based on long term marking. J. Anim. Ecol. 55, 613–619 10.2307/4743 (doi:10.2307/4743) [DOI] [Google Scholar]
- 20.Surai P. F. 2002. Natural antioxidants in avian nutrition and reproduction. Nottingham, UK: Nottingham University Press [Google Scholar]
- 21.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., Schabenberger O. 2006. SAS for mixed models, 2nd edn. Cary, NC: SAS Institute Inc [Google Scholar]
- 22.Reed T. E., Daunt F., Hall M. E., Phillips R. A., Wanless S., Cunningham E. J. A. 2008. Parasite treatment affects maternal investment in sons. Science 321, 1681–1682 10.1126/science.1159466 (doi:10.1126/science.1159466) [DOI] [PubMed] [Google Scholar]
- 23.Velando A., Ortega-Ruano J. E., Freire J. 1999. Chick mortality in European shag Stictocarbo aristotelis related to food limitations during adverse weather events. Ardea 87, 51–59 [Google Scholar]
- 24.Berglund A. M. M., Sturve J., Förlin L., Nyholm N. E. I. 2007. Oxidative stress in pied flycatcher (Ficedula hypoleuca) nestlings from metal contaminated environments in northern Sweden. Environ. Res. 105, 330–339 10.1016/j.envres.2007.06.002 (doi:10.1016/j.envres.2007.06.002) [DOI] [PubMed] [Google Scholar]
- 25.Lin H., Decuypere E., Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A 144, 11–17 [DOI] [PubMed] [Google Scholar]
- 26.Young P. B., Kennedy S., Molloy A. M., Scott J. M., Weir D. G., Kennedy D. G. 1997. Lipid peroxidation induced in vivo by hyperhomocysteinaemia in pigs. Atherosclerosis 129, 67–71 10.1016/S0021-9150(96)06016-9 (doi:10.1016/S0021-9150(96)06016-9) [DOI] [PubMed] [Google Scholar]
- 27.Bize P., Devevey G., Monaghan P., Doligez B., Christe P. 2008. Fecundity and survival in relation to resistance to oxidative stress in a free living bird. Ecology 89, 2584–2593 10.1890/07-1135.1 (doi:10.1890/07-1135.1) [DOI] [PubMed] [Google Scholar]