Abstract
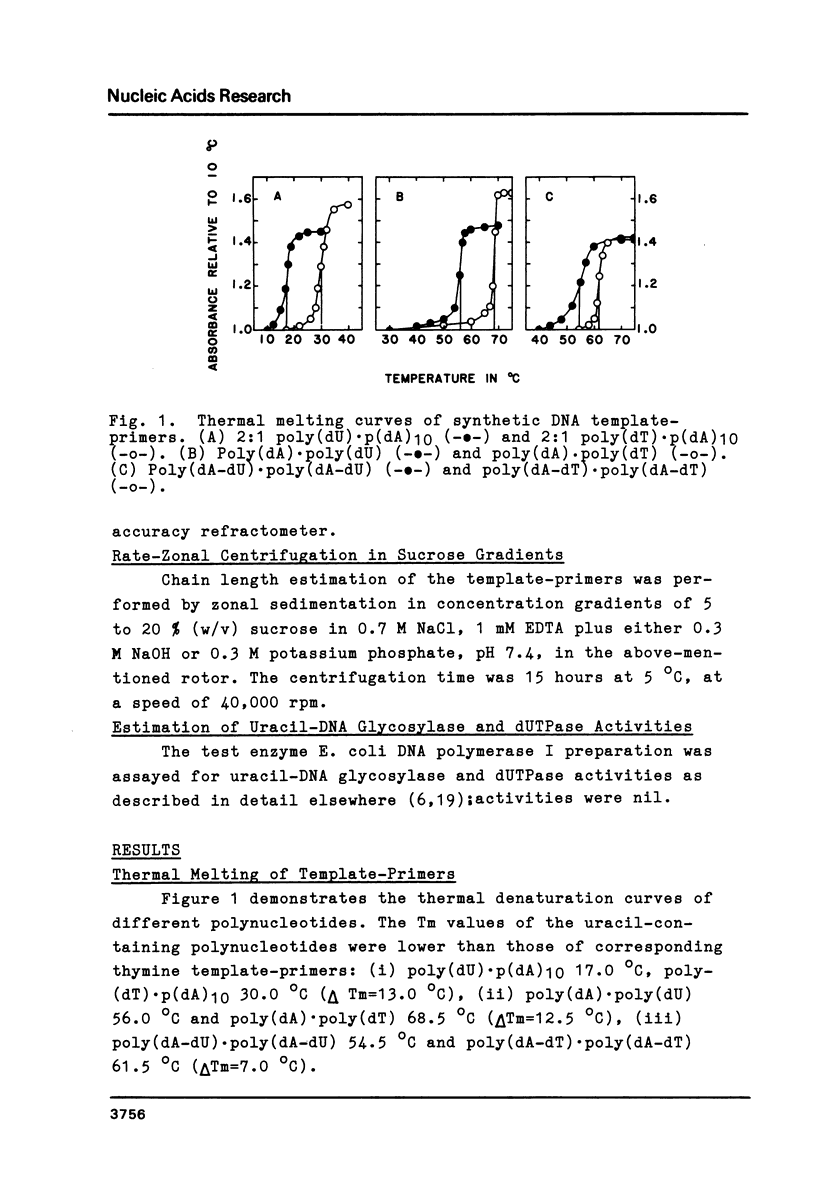
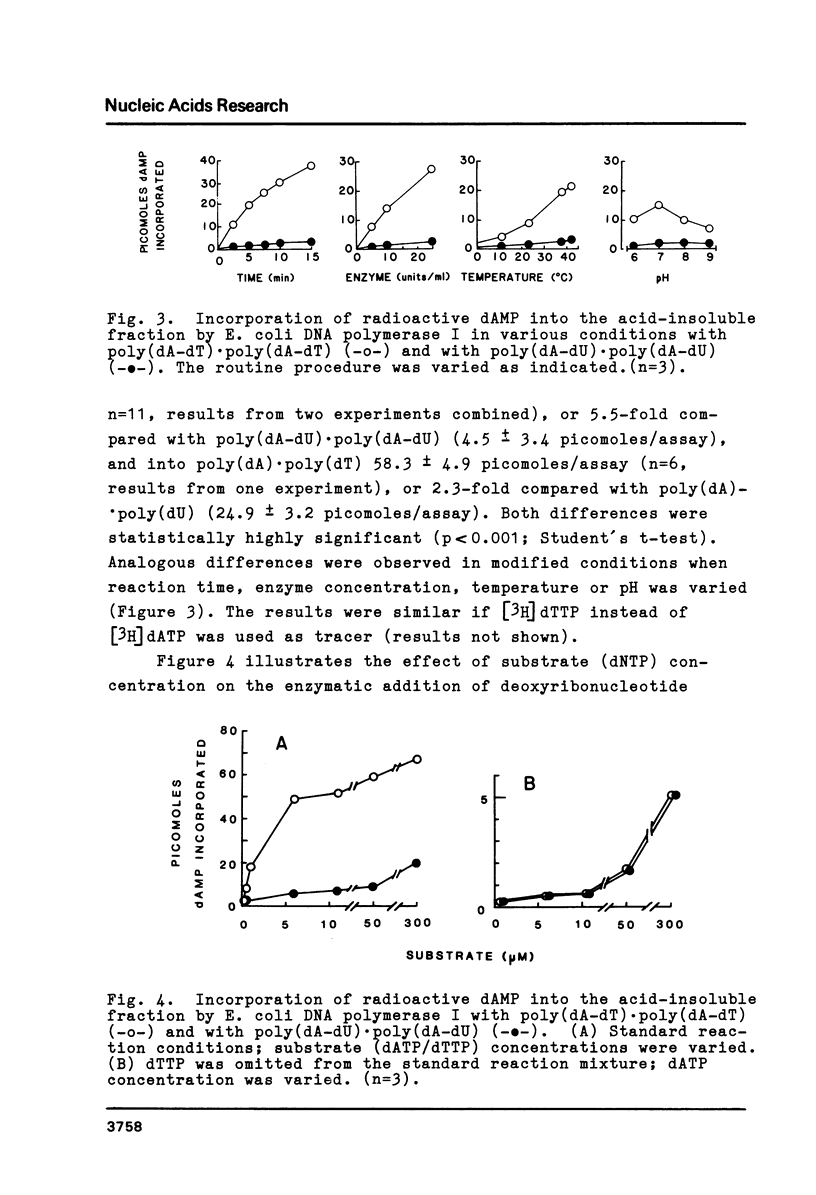
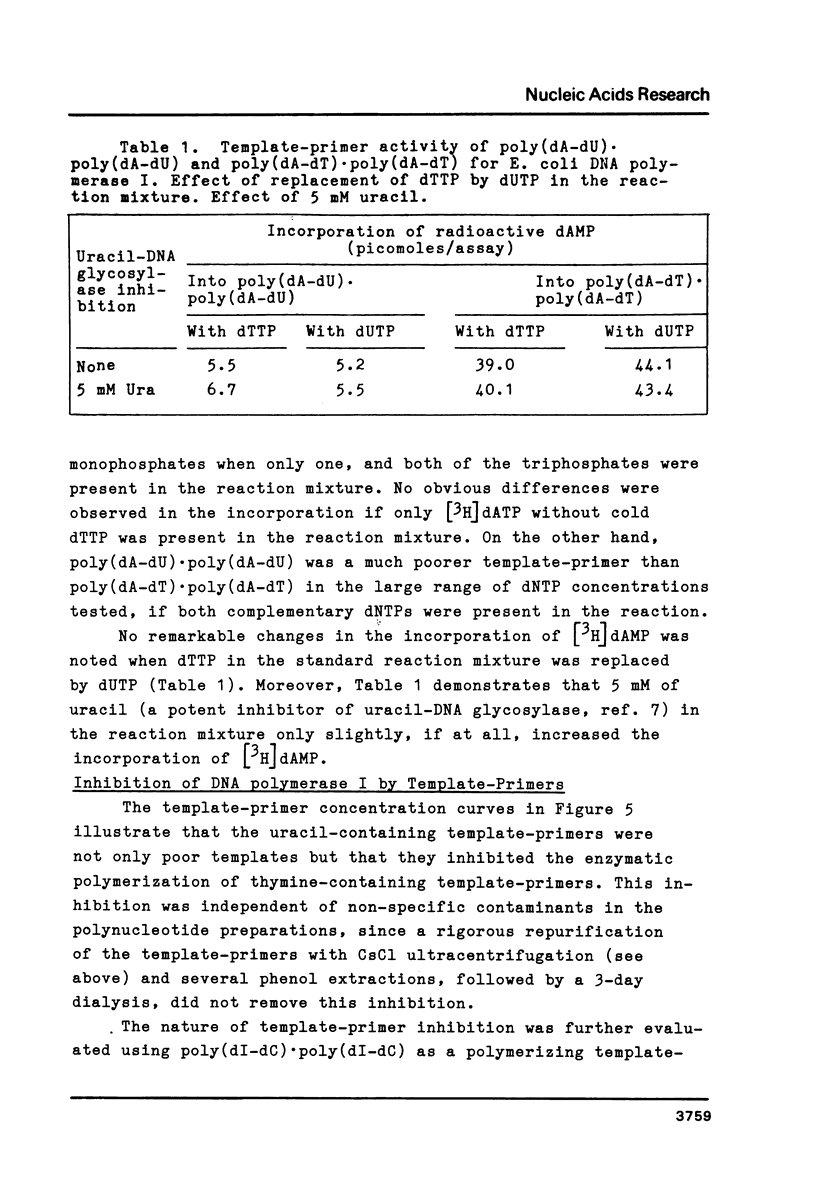
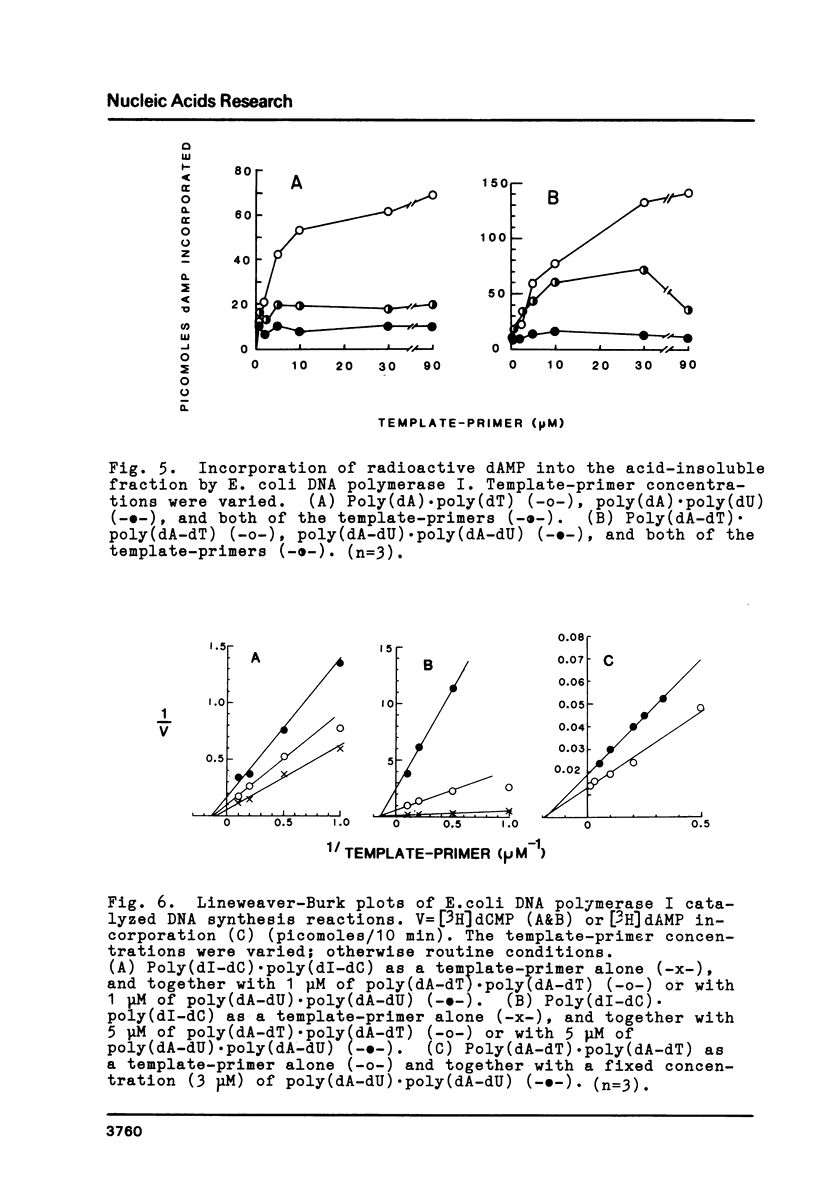
The physical and biochemical properties of two pairs of synthetic DNA template-primers were investigated. The copolymer poly(dA-dU) . poly(dA-dU) and the homopolymer duplex poly(dA). poly(dU) were characterized by a lower Tm and by a higher buoyant density value than the respective thymine polynucleotides poly(dA-dT) . poly(dA-dT) and poly(dA) . poly(dT). The polymerizing and the primer terminus adding reactions of a homogenous E. coli DNA polymerase I preparation, as measured by incorporation of [3H]dAMP into the acid-insoluble fraction, were significantly poorer with uracil-containing template-primers than with thymine templates. Moreover, the uracil-containing polynucleotides inhibited the polymerizing activity of DNA polymerase I to a greater extent than the thymine polynucleotides, when the enzymatic activity was investigated with a dATP/dTTP/dUTP-free incorporation system making use of poly(dI-dC) . poly(dI-dC) as the template-primer.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI L. E., HAEGGMARK A., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. II. FORMATION AND INTERCONVERSION OF DEOXYURIDINE PHOSPHATES. J Biol Chem. 1963 Oct;238:3407–3413. [PubMed] [Google Scholar]
- Bessman M. J., Lehman I. R., Adler J., Zimmerman S. B., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. III. THE INCORPORATION OF PYRIMIDINE AND PURINE ANALOGUES INTO DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. The role of deoxyuridine triphosphate nucleotidohydrolase, uracil-DNA glycosylase, and DNA polymerase alpha in the metabolism of FUdR in human tumor cells. Mol Pharmacol. 1980 Nov;18(3):513–520. [PubMed] [Google Scholar]
- Chamberlin M. J. Comparative properties of DNA, RNA, and hybrid homopolymer pairs. Fed Proc. 1965 Nov-Dec;24(6):1446–1457. [PubMed] [Google Scholar]
- Deutsch W. A., Spiering A. L. A new pathway expressed during a distinct stage of Drosophila development for the removal of dUMP residues in DNA. J Biol Chem. 1982 Apr 10;257(7):3366–3368. [PubMed] [Google Scholar]
- Duncan J., Hamilton L., Friedberg E. C. Enzymatic degradation of uracil-containing DNA. II. Evidence for N-glycosidase and nuclease activities in unfractionated extracts of Bacillus subtilis. J Virol. 1976 Aug;19(2):338–345. doi: 10.1128/jvi.19.2.338-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. S., Chalmers K., Murray N. E. Isolation and characterization of a lambdapolA transducing phage. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5632–5636. doi: 10.1073/pnas.74.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. S., Stump K. H. A rapid procedure for isolation of large quantities of Escherichia coli DNA polymerase I utilizing a lambdapolA transducing phage. J Biol Chem. 1979 May 10;254(9):3206–3210. [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Jentsch K. D., Jovin T. M. A simple and rapid purification method for Escherichia coli DNA polymerase I. J Biol Chem. 1979 Aug 25;254(16):7465–7467. [PubMed] [Google Scholar]
- SHUGAR D., SZER W. Secondary structure in poly-ribothymidylic acid. J Mol Biol. 1962 Nov;5:580–582. doi: 10.1016/s0022-2836(62)80134-x. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Sági J., Brahms S., Brahms J., Otvös L. Effect of 5-alkyl substitution of uracil on the thermal stability of poly [d(A-r5U)] copolymers. Nucleic Acids Res. 1979 Jun 25;6(8):2839–2848. doi: 10.1093/nar/6.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sági J., Czuppon A., Kajtár M., Szabolcs A., Szemzö A., Otvös L. Modified polynucleotides. VI. Properties of a synthetic DNA containing the anti-herpes agent (E)-5-(2-bromovinyl)-2'-deoxyuridine. Nucleic Acids Res. 1982 Oct 11;10(19):6051–6066. doi: 10.1093/nar/10.19.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sági J., Otvös L. Modified polynucleotides. IV. Template activity of 5-alkyluracil-containing poly [d(A-r5U)] copolymers for DNA and RNA polymerases. Nucleic Acids Res. 1979 Nov 24;7(6):1593–1601. doi: 10.1093/nar/7.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- Vilpo J. A. Mitogen induction of deoxyuridine triphosphatase activity in human T and B lymphocytes. Med Biol. 1983 Feb;61(1):54–58. [PubMed] [Google Scholar]
- Warner H. R., Duncan B. K., Garrett C., Neuhard J. Synthesis and metabolism of uracil-containing deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1981 Feb;145(2):687–695. doi: 10.1128/jb.145.2.687-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Masaki S. Utilization in vitro of deoxyuridine triphosphate in DNA synthesis by DNA polymerases alpha and beta from calf thymus. Biochim Biophys Acta. 1979 Feb 27;561(2):396–402. doi: 10.1016/0005-2787(79)90147-3. [DOI] [PubMed] [Google Scholar]



