Abstract
Rotator cuff tears represent the most common shoulder injuries in the United States. The debilitating effect of this degenerative condition coupled with the high incidence of failure associated with existing graft choices underscore the clinical need for alternative grafting solutions. The two critical design criteria for the ideal tendon graft would require the graft to not only exhibit physiologically relevant mechanical properties but also be able to facilitate functional graft integration by promoting the regeneration of the native tendon-to-bone interface. Centered on these design goals, this review will highlight current approaches to functional and integrative tendon repair. In particular, the application of biomimetic design principles through the use of nanofiber- and nanocomposite-based scaffolds for tendon tissue engineering will be discussed. This review will begin with nanofiber-based approaches to functional tendon repair, followed by a section highlighting the exciting research on tendon-to-bone interface regeneration, with an emphasis on implementation of strategic biomimicry in nanofiber scaffold design and the concomitant formation of graded multi-tissue systems for integrative soft tissue repair. This review will conclude with a summary and future directions section.
Keywords: tendon, bone, interface, insertion, nanofiber, hydroxyapatite, biomimetic, tissue engineering
A. Introduction
Rotator cuff tears are among the most common injuries afflicting the shoulder, and in the United States alone, over 250,000 cuff repairs are performed annually34. Clinical intervention is required as these tendon injuries do not heal, largely due to the complex anatomy and extended range of motion of the shoulder joint, as well as hypovascularization of the cuff tendons and relative weakening with degeneration13,18,34,93. Early primary anatomic repair followed by carefully controlled rehabilitation is currently the standard treatment for rotator cuff tears18. Advances in surgical techniques coupled with mechanical fixation methods have significantly improved biomechanical strength and graft stability post repair67. As such, failure rates between 20-90% have been reported after primary repair of chronic rotator cuff injuries26, attributed to factors such as degenerative and poorly vascularized tendons, muscle atrophy and lack of graft-to-bone integration28,30,56,75,76. These problems are exacerbated by the limited healing potential of the injured tissue, relative scarcity of autografts, and potential risks associated with allografts.
To improve healing, biological or synthetic polymer-based tendon grafts or augmentation devices65,70 have been explored to reconstruct large rotator cuff defects but with limited success. To date, extracellular matrix (ECM)-derived scaffolds have been the most commonly used grafts to augment rotator cuff repair19. Graft patches or tendon onlays based on decellularized allogeneic and xenogenic ECM2,14,18,20,80 provide both mechanical augmentation and the biological cues to improve healing, while also maintaining the ability to be remodeled by host cells. Small intestinal submucosa (SIS), containing a collagen nanofiber-based architecture and alignment, is commercially available as a graft patch for improving cuff repair. Although promising results have been reported in animal models, suboptimal outcomes were observed in human trials37,81, attributed to a mismatch in mechanical properties and rapid matrix remodeling experienced in the physiologically demanding and often diseased shoulder joint. A systematic comparison of four commercially available ECM scaffolds, specifically, Restore® made from porcine SIS, CuffPatch® made from porcine SIS, GraftJacket® made from human dermis, and TissueMend® made from bovine dermis was conducted by Derwin et al using a canine model. It was found that SIS scaffolds had significantly lower mechanical properties than the native tendon, also noted was a decrease in mechanical properties due to premature graft resportion20. Therefore, the debilitating effect of rotator cuff tears coupled with the high incidence of failure associated with existing graft choices underscore the clinical need for alternative grafting solution with physiologically-relevant mechanical properties.
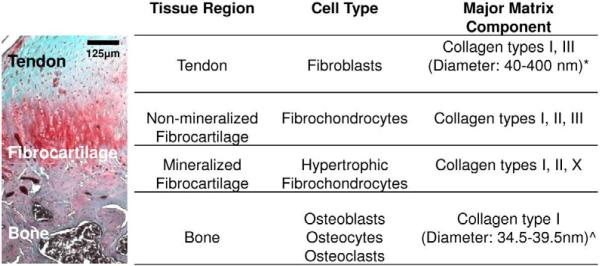
In addition to the aforementioned functional requirements for tendon grafting, another challenge in tendon repair arises from the need for biological fixation of the tendon graft. It has been observed that full-thickness rotator cuff tears most often result from avulsion of the supraspinatus tendon from the humeral head at the insertion site13, thereby requiring tendon-to-bone repair. The supraspinatus tendon inserts into the humeral head via a direct enthesis exhibiting region-dependent matrix heterogeneity and mineral content. Specifically, four distinct yet continuous tissue regions are observed at the tendon-bone interface (Fig. 1): tendon proper, non-mineralized fibrocartilage, mineralized fibrocartilage and bone6,7,91. The tendon proper consists of fibroblasts found between aligned collagen fibers in a matrix rich in type I collagen, with small amounts of type III collagen and proteoglycans10. The non-mineralized fibrocartilage region is composed of fibrochondrocytes in a matrix of types I, II, and III collagen with fibers oriented perpendicular to the calcified interface region43. The mineralized fibrocartilage region consists of hypertrophic fibrochondrocytes within a matrix of types I and II collagen43, as well as type X collagen87. The last region of the insertion site is bone which consists of osteoblasts, osteoclasts, and osteocytes in a mineralized matrix rich in type I collagen. This multi-tissue organization mediates load transfer between tendon and bone6,91, minimizes the formation of stress concentrations6,60,90, and supports the heterotypic cellular interactions necessary for interface function and homeostasis54. Published studies evaluating tendon-to-bone healing have demonstrated that the normal insertion site is not regenerated following cuff repair based on current mechanical fixation methods31,74, and this lack of tendon-bone integration remains a primary cause of repair failure. More recently, given the aforementioned limitations associated with available grafting options, by combining cells, growth factors, and/or biomaterials, the principles of tissue engineering46,58 have been applied to the formation of tendon-29,33,39,59 or bone-like58,95 tissues in vitro and in vivo, with promising results. As such, the critical barrier to their clinical application is in how to achieve biological fixation of these newly formed grafts either with each other and/or with the host environment54,61.
Figure 1.
Structure and composition of tendon-to-bone insertion site.
Masson's Trichrome (rat)62 *Liang et al. 2006; ^Tzaphlidou 2008
These observations collectively suggest that, for functional and integrative repair of rotator cuff injuries, the two critical design criteria for the ideal tendon graft would center on controlling scaffold design such that the graft will exhibit physiologically-relevant mechanical properties, in addition to facilitating the regeneration of tendon-to-bone interface. Focusing on these design considerations, the objective of this review is to provide an overview of current approaches to functional and integrative tendon repair. In particular, the application of biomimetic scaffold design, or more specifically, the use of nanofiber- and nanocomposite-based scaffolds for tendon tissue engineering will be reviewed. Scaffolds provide a framework for cells to attach, proliferate, and produce matrix, and can also serve as carriers for cells and the biomolecules necessary to guide and accelerate healing. To date, nanofibers have been widely investigated for the regeneration of a variety of connective tissues, such as bone27,97, meniscus3, intervertebral disk64, cartilage49, and ligament5,48. In addition to being biomimetic with respect to the collagenous matrix (Fig. 1), a distinct advantage of nanofiber scaffolds is that they can be engineered to resemble the native tendon extracellular matrix, exhibiting high aspect ratio, surface area, permeability and porosity12,50,55,63,69. Moreover, nanofiber organization and alignment can be modulated during fabrication63,68, which allows for scaffold structural and material properties to be readily tailored to meet the functional demands of the rotator cuff tendons. Matrix anisotropy can be incorporated into scaffold design with high fidelity by controlling nanofiber organization and alignment. This is especially desirable for functional and integrative tendon repair, as scaffolds with biomimetic anisotropy can be fabricated to recapitulate the inherent structure-function relationship of the rotator cuff tendons, as well as at the tendon-to-bone interface.
This paper will begin with a review of nanofiber-based approaches for functional tendon repair, followed by a section highlighting the exciting research on tendon-to-bone interface regeneration and biological fixation, with an emphasis on implementation of strategic biomimicry in nanofiber scaffold design and the concomitant formation of graded multi-tissue systems for integrative soft tissue repair. Finally, a summary will conclude the review and future directions will be discussed.
B. Current Approaches to Functional Tendon Repair
B.1. Nanofiber Scaffold Design and Modification for Tendon Tissue Engineering
The ideal scaffold for functional and integrative tendon repair must first meet the physiological demand of the native tendon by matching its mechanical properties, while simultaneously promoting host cell-mediated healing by mimicking the ultrastructural organization of the native tendon. Furthermore, the scaffold should be biodegradable in order to be gradually replaced by new tissue while maintaining its physiologically relevant mechanical properties. Lastly, the scaffold must integrate with the host tendon and surrounding bone tissue by promoting the regeneration of the native tendon-to-bone interface. It is well established that the highly organized nanoscale structure of tendons is characterized by closely-packed parallel collagen fiber bundles, varying in diameter and is composed of bundles of individual collagen fibrils approximately 1-2nm in diameter (Fig. 1). This structural arrangement is critical for the physiological function of tendons, which includes the stabilization and guidance of joint motion, transmission of physiological loads, and the maintenance of the anatomical alignment of the skeleton. Furthermore, the parallel alignment of collagen fibers along the direction of applied load results in one of the strongest tissues in the body42. The collagen fibers of tendons and ligaments typically exhibit a bimodal diameter distribution in the nanometer range (approximately 40 – 400nm) that varies according to the specific tissue type, as well as between individuals, and may also be altered during scar formation post injury52.
Several groups have explored tissue engineering methods for tendon or ligament repair1,15,21,53. Synthetic as well as biologically derived grafts have shown favorable results during in vitro culture trials, as well as in relevant in vivo models. It is common to use scaffolds composed of microfibers based on a variety of synthetic polymers, such as poly-L-lactic acid (PLLA), polylactide-co-glycolide (PLGA) and polyurethane15,53, as well as biological materials, such as collagen21 and silk1,36. While these approaches have shown promising results, the scaffold architecture differs significantly from that of the inherent nanoscale organization of tendons or ligaments. Given that scaffold fiber diameters have been shown to directly affect fibroblast phenotype and matrix production5, there is a significant interest in enhancing physiologically relevant soft tissue regeneration utilizing scaffolds that more closely mimic the native tissue nanostructure and mechanics.
The nanoscale architecture of the collagen-rich tendon matrix can be readily recapitulated with nanofiber scaffolds, which exhibits high surface area to volume ratio, low density, high porosity, variable pore size and mechanical properties approximating those of the native tissues. Nanofibers can be fabricated using a variety of methods44, such as drawing, template synthesis, temperature-induced phase separation, molecular self-assembly, and, most frequently, electrospinning57,72. Moffat et al was the first to report on the fabrication of PLGA nanofiber scaffolds with physiologically-relevant structural and mechanical properties for rotator cuff repair59. It was observed that human rotator cuff fibroblast morphology and growth on aligned (615nm mean fiber diameter) and unaligned (568nm mean fiber diameter) fiber matrices were dictated by fiber alignment, with distinct cell morphology and integrin expression profiles. Upregulation of α2 integrin, a key mediator of cellular attachment to collagenous matrices, was observed when the fibroblasts were cultured on aligned fibers, and upon which a types I and III collagen-rich matrix was deposited. More recently, Xie et al developed a single continuous PLGA nanofiber scaffold that transitioned from aligned to random orientation in order to examine the effects of this transitional region on rat tendon fibroblasts in vitro92. After seven days of culture, the study showed that cells proliferated on both aligned and random nanofiber orientations, but that a rounded morphology was found on unaligned nanofibers, while similar to what was seen by Moffat et al59, cells cultured on aligned nanofibers appeared long and spindle-like, and were aligned along the long axes of the fibers.
Biological response to polymeric nanofibers may also be enhanced by additional surface modifications. For example, Rho et al electrospun aligned type I collagen nanofiber scaffolds with a mean fiber diameter of 460nm and evaluated the response of human epidermal cells after coating the scaffolds with several adhesion proteins73. It was found that cell proliferation was enhanced by coating the scaffolds with both type I collagen and laminin. Recently, Park et al applied plasma treatment to polyglycolic acid (PGA), PLGA and PLLA nanofibers and grafted a surface layer of hydrophilic acrylic on these scaffolds66. It was found that NIH 3T3 fibroblasts seeded on these modified scaffolds spread and proliferated faster compared to those on unmodified controls. Another biomimetic method to enhance biological responses is to incorporate collagen into nanofiber scaffolds. Using co-electrospinning, Zhang et al added collagen to poly-ε-caprolactone (PCL) nanofibers as well as coated these nanofibers with collagen98. While these scaffolds promoted human dermal fibroblast growth independent of the incorporation method, cell migration into the scaffold was mainly observed in the co-electrospun PCL-collagen scaffolds. Similarly, Theisen et al seeded human tendon fibroblasts on a composite PLLA-type I collagen scaffold and found that, when compared to the PLLA control, the blended scaffold upregulated the expression of types I, III, X collagen and decorin85.
Nanofibers have also been used to improve existing scaffold design, resulting in a graft with a more biomimetic surface for eliciting desired cell response. For example, Sahoo et al electrospun PLGA nanofibers directly onto a woven microfiber PLGA scaffold in order to increase cell seeding efficiency while maintaining a scaffold that was mechanically competent78. The attachment, proliferation and differentiation of porcine bone marrow stromal cells was evaluated on these scaffolds and, when compared to scaffolds seeded via a fibrin gel delivery, it was found that seeding the cells onto nanofiber-coated scaffolds enhanced proliferation, collagen production and upregulated the gene expression of several tendon-related markers, namely decorin, biglycan, and type I collagen.
In an alternative strategy to enhance the mechanical properties of electrospun nanofibers, Barber et al fabricated braided nanofiber scaffolds by electrospinning bundles of aligned PLLA and braiding either 3, 4, or 5 bundles together to generate their final construct4. Human mesenchymal stem cells (hMSC) cultured on the braided scaffolds aligned parallel to the length of the nanofibers, and displayed realignment of actin filaments which progressed with culture time. Cells produced a matrix which bridged the gap between bundles, and when the hMSC were concurrently stimulated with cyclic tensile strain and cultured in tenogenic medium containing bone morphogenetic protein-2 (BMP-2), differentiation factor-5 (GDF-5), and fibroblast growth factor-2 (FGF-2) a significant upregulation of scleraxis was reported, indicative of hMSC differentiation into the tenogenic lineage.
The incorporation of bioactive molecules in the scaffold system to promote stem cell differentiation is another strategy adopted for tendon repair. Recently, Sahoo et al seeded rabbit mesenchymal progenitor cells on a hybrid scaffold for tendon repair77. The scaffold was fabricated by electrospinning basic fibroblast growth factor (bFGF) releasing PLGA nanofibers onto knitted silk microfibers. This novel scaffold mimicked the extracellular matrix in function, initially stimulating stem cell proliferation, and subsequently promoting tenogenic differentiation as indicated by an increase in both types I and III collagen expression after two weeks in vitro.
The recent identification of tendon stem cells (TSPCs)9 has provided another cell source for studying tendon development and repair. Human TSPCs typically reside in a matrix of parallel collagen fibers, thus fiber alignment is expected to play a role in regulating stem cells differentiation9,35. When Yin et al investigated the impact of PLLA nanofiber alignment on hTSPC differentiation96, the expression of the tendon-specific gene scleraxis, as well as the matrix gene collagen XIV, were significantly higher on aligned versus random scaffolds after 7 days of culturing in osteogenic media. On the other hand, both gene expression and histological staining indicated that the randomly orientated nanofibers stimulate hTSPC differentiation towards an osteogenic lineage, while this was not observed for the aligned nanofiber group, providing evidence that cell orientation induced by the scaffold nanotopography play an important role in cell differentiation. Finally, intramuscular evaluation of hTSPC-seeded nanofibers in an athymic mouse model showed that the aligned scaffold guided both cell organization and collagen bundle formation, while a random orientation of both cells and matrix were observed on the random fiber controls.
B.2. Mechanical Stimulation of Scaffolds
While limited results are available for mechanical loading on nanofiber-based scaffolds, the role of mechanical loading on tendon tissue engineering has been investigated extensively for collagen-based scaffolds. For example, Gilbert et al loaded NIH 3T3 fibroblasts cultured on porcine small intestinal submucosa (SIS-ECM) as a function of stretch (0%, 5%,10% and 15%) and loading frequencies (0.1, 0.3 and 0.5 Hz)32. It was found that in general, the expression of type I collagen increased while that of type III collagen decreased with increasing frequency, matching collagen expression profiles during late stage of remodeling during native tendon healing. In another study, Berry et al seeded human dermal fibroblasts in collagen gels and preloaded the gels (2 mN or 10mN static loading) before applying 10% cyclic strain (1 Hz) for 24 hours8. While cell proliferation increased with mechanical loading regardless of preloading regimen, elevated collagen synthesis was only seen in the 2mN group. Using a custom bioreactor, Garvin et al showed that avian flexor tendon cells seeded on the bioartifical tendon, when subjected to mechanical loading, upregulated types I, III, and XII collagen expression at levels consistent with those of cells found in the flexor tendon29.
Juncosa-Melvin et al investigated the potential of hMSCs seeded on collagen sponges for patellar tendon repair41. Mechanical loading was applied to the scaffold to a peak strain of 4% once every 5 minutes for up to 8 hours/day over two weeks of culture. It was observed that the stimulated constructs exhibited 2.5 times the linear stiffness of the non-stimulated controls. When tested in a rabbit patellar tendon defect model accompanied by normal cage activity after surgery, maximum force, linear stiffness, maximum stress and linear modulus for the stimulated scaffold group were found to approximate those of the native patellar tendon. In a follow-up study40, it was found that both types I and III collagen expression increased significantly in the stimulated group while no difference in decorin and fibronectin expression were evident with respect to the unloaded control.
Mechanical stimulation of nanofiber scaffolds with bioreactors has been used to promote cell infiltration through nanofiber scaffolds. Pham et al utilized a flow perfusion bioreactor and showed that rat MSC infiltration distance on bilayered constructs of unaligned PCL microfibers with PCL nanofibers on top was increased by a factor of 5 versus the static control68. Similarly, Srouji et al utilized a plug-flow bioreactor to culture hMSCs seeded on unaligned PCL and collagen nanofiber scaffolds83. After 6 weeks, cells were evident throughout the scaffold. The preconditioned constructs were implanted subcutaneously in nude mice and good integration with the surrounding tissues and neovascularization were found. These studies demonstrate the ability of media perfusion bioreactor systems to improve mass transport throughout 3D tissue engineered constructs and to promote the production of sufficient cell mass necessary for in vivo grafting and host integration.
C. Soft Tissue-to-Bone Interface Regeneration and Integrative Tendon Repair
The debilitating effect of rotator cuff tears coupled with the high incidence of failure associated with existing repair techniques14,20,37 underscore the clinical need for functional solutions for integrative tendon-to-bone repair. The supraspinatus tendon of the rotator cuff connects to bone via a direct insertion, a complex enthesis consisting of three distinct yet continuous regions of soft tissue, fibrocartilage, and bone6,16,89. The fibrocartilaginous interface region is further divided into non-calcified and calcified regions (Fig. 2). The insertion site serves several functions, including enabling the transfer of loads between distinct tissues6,91, minimizing the formation of stress concentrations6,60,90 and supporting the communication between multiple cell types necessary for interface function and homeostasis54. Therefore, regeneration of this multi-tissue transition is essential for biological fixation of tendon grafts.
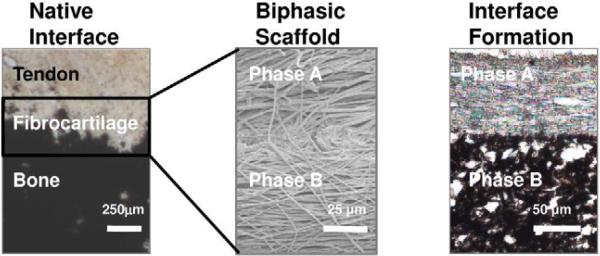
Figure 2.
Biomimetic Scaffold Design. Native tendon-to-bone interface with distinct yet continuous non-calcified and calcified matrix regions, (von Kossa, Lewis rat) inspired the design of the biphasic nanofiber scaffold (Phase A=PLGA and Phase B=PLGA-HA), which when tested in vivo, resulted in the formation of non-calcified and calcified matrix regions (subcutaneous, 3weeks).
To address this challenge, several groups have evaluated the feasibility of integrating tendon grafts with bone or biomaterials through the formation of anatomic insertion sites. Fujioka et al examined the effects of reattaching the bone and tendon in a rat model for Achilles tendon avulsion24. After four weeks, surgical reattachment of tendon to bone increased type X collagen deposition, and allowed tissue to maintain distinct regions of calcified and non-calcified fibrocartilage tissue. Additionally Inoue et al promoted supraspinatus tendon integration with a metallic implant using a bone marrow-infused bone graft38. Other approaches include reattaching the tendon to bone with the aid of natural materials such as periosteum or demineralized bone matrix. Specifically Chang et al sutured a periosteal flap from tendon to bone at the end of a rabbit infraspinatus tendon, and observed remodeling of tissue at the interface over 12 weeks11. At four weeks post-surgery, a fibrous layer with increased mechanical properties was seen at the interface region, which developed into a matrix similar to fibrocartilage after three months. This tissue layer possessed an increased failure load, proving improvement in integration at the interface. Similarly, Sundar et al attempted to augment interface healing post-surgery by implanting demineralized bone between tendon and bone in an ovine patellar tendon model84. It was found that bone enhanced deposition of both mineralized and non-mineralized fibrocartilage at the interface, with improved weight bearing. These pioneering studies collectively demonstrate the potential for regenerating the tendon-to-bone interface and delineate the need for functional grafting solutions that can promote biological fixation.
Current knowledge of the structure-function relationship at the tendon-bone insertion86,87 provides invaluable cues for biomimetic and integrative tendon scaffold design. Combining biomechanical testing with the quasi-linear viscoelastic model (QLV)25, Thomopoulos et al determined the mechanical properties of the rat supraspinatus tendon insertion sites and later related it to collagen orientation using a finite element model86,87. It was found that controlled collagen fiber alignment plays an important role in reducing stress concentration at the tendon-bone insertion86. Another hallmark of the tendon-to-bone interface is a region-dependent mineral distribution across the insertion site7,91. Calcium phosphate is a prime modulator of both the biochemical milieu and the nature of mechanical stimuli presented to cells. The presence of the non-calcified and calcified fibrocartilage regions at the interface is of functional significance, as higher matrix mineral content have been associated with greater mechanical properties in connective tissues17,23,71. Moffat et al correlated the aforementioned increase in compressive modulus across the interface to the onset of mineral presence in the calcified fibrocartilage region60. It is clear that both collagen alignment and mineral content are critical design parameters for functional and integrative tendon repair.
Based on these observations, the ideal scaffold for tendon-to-bone interface tissue engineering must exhibit a gradient of structural and mechanical properties mimicking those of the multi-tissue insertion. Compared to a homogenous structure, a stratified scaffold with pre-designed, tissue-specific matrix inhomogeneity can better sustain and transmit the distribution of complex loads inherent at the direct insertion site. A key criterion in stratified scaffold design is that the phases must be interconnected and pre-integrated with each other, thereby supporting the formation of distinct yet continuous multi-tissue regions. In other words, the scaffold would exhibit a gradient of physical properties in order to allow for the recapitulation of interface-like heterogeneity throughout the scaffold. It should also support growth and differentiation, as well as the interactions between heterotypic and homotypic cell populations to promote the formation and maintenance of multi-tissue interface. In addition, the scaffold phases should be biodegradable so it is gradually replaced by living tissue, and the degradation process must be balanced with respect to mechanical properties in order to permit physiological loading and neo-interface function. Finally, the interface scaffold must be compatible with existing tendon reconstruction grafts or pre-incorporated into tissue engineered graft design in order to achieve integrative and functional soft tissue repair.
To this end, a scaffold recapturing the nanoscale interface organization, with preferentially aligned nanofiber organization and region-dependent change in mineral content would be highly advantageous. Building on the functional PLGA nanofiber scaffold designed for tendon tissue engineering59, Moffat et al designed a biphasic scaffold, with the top layer consisted of nanofibers of PLGA, and the second layer of composite nanofibers of PLGA and hydroxyapatite nanoparticles. The biphasic design is aimed at regenerating both the non-mineralized and mineralized fibrocartilage regions of the tendon-to-bone insertion site, while promoting osteointegration with PLGA-HA nanofibers62. The response of tendon fibroblasts, osteoblasts and chondrocytes were evaluated on these nanocomposite scaffolds with promising results in vitro. When tested in vivo subcutaneously as well as in a rat rotator cuff repair model82, the biphasic scaffold supported regeneration of continuous non-calcified and calcified fibrocartilage regions (Fig. 2), demonstrating the potential of a biodegradable nanofiber-based scaffold system for integrative tendon-to-bone repair.
Controlling scaffold mineral distribution may be another promising approach for repairing the soft tissue-to-bone insertion site. Working with PCL nanofibers and utilizing a novel extrusion system coupled with electrospinning, Erisken et al incorporated calcium phosphate nanoparticles into non-woven nanofiber meshes, resulting in a gradient of mineral distribution across the depth of the PCL scaffold22. Within four weeks, culturing of MC3T3 cells on these nanofiber constructs led to the formation of a gradient of calcified matrix. Recently, using the simulated body fluid immersion method, Li et al formed a calcium phosphate coating on a nonwoven mat of gelatin-coated PCL and plasma-treated PLGA nanofibers in a graded manner51. It was observed that the gradient in mineral content resulted in spatial variations in the stiffness and affected the number of preosteoblastic MC3T3 cells that adhered to the substrate.
In addition to engineering the tendon-bone interface, the muscle-tendon junction is another critical research area for integrative tendon repair. As the tendon joins the muscle to bone, thus the myotendinous junction (MTJ), which connects muscle to tendon, acts as a bridge to distribute mechanical loads94. This interface consists of a band of fibroblast-laden, interdigitating band of tissue that connects the dense collagen fibers of the tendon to the more elastic muscle fibers while displaying a gradient of structural properties88. Current tissue engineering approaches, as demonstrated by Saxena et al, include the incorporation of myoblasts on a composite scaffold of fibronectin hydrogel and PGA79. A muscle-like matrix was formed in vitro and was capable of responding to an electrical stimulus. Recently, Larkin et al co-cultured skeletal muscle constructs with engineered tendon constructs in order to regenerate the muscle-tendon interface47. Interestingly, upregulation of paxillin was observed at the neo-interface, and the MTJ formed was able to sustain tensile loading beyond the physiological strain range. These studies demonstrate the promise of the biodegradable nanofiber-based scaffold system for interface tissue engineering and the potential of harnessing cellular interaction for engineering both the tendon-to-bone and muscle-to-tendon interface and ultimately, functional and integrative tendon repair 45.
D. Summary and future directions
Interface tissue engineering focuses on the functional regeneration of the anatomic interface between distinct tissue types to accelerate the translation of tissue engineered technologies to the clinical setting. It aims to develop innovative technologies for the formation of complex tissue systems, with the broader goal of achieving the biological fixation of tissue engineered grafts with the host environment. In this regard, nanotechnology-based approaches to connective tissue repair offer several distinct advantages. Specifically, nanofiber-based scaffold systems are advantageous due to their inherent characteristics, with potential to mimic the native collagenous tendon, interface and bone matrix, and ultimately regulate cellular response. In addition, nanofiber substrates can be fabricated from a variety of synthetic as well as natural polymers, with controlled geometry, mechanical properties, porosity, permeability, degradation kinetics, and fiber diameter. The studies highlighted here and many others collectively demonstrate the promise and the excitement in the field regarding nanotechnology-based scaffolds for guided orthopedic tissue engineering and integrative soft tissue repair.
The critical research question in the emerging field of orthopedic interface tissue engineering centers on how the graded structures between different types of connective tissues are formed, reestablished post-injury, and maintained in the body. Moreover, the effects of biological, physical, and chemical stimulation on interface formation and regeneration also remain to be explored. It is anticipated that advances in biomimetic design of nanofiber-based scaffolds for integrative soft tissue repair will be guided by continued exploration of the structure-function relationship of the native tissue-to-tissue interfaces, and increased understanding of the mechanisms governing its repair and development.
In addition, scaffold fabrication and scaling issues must be overcome in order for the widespread clinical utilization of nanofiber-based systems for functional orthopedic tissue engineering and integrative repair to be realized. For example, recent advances in nanotechnology and in the delivery of bioactive agents that are immobilized within the carriers could provide additional methods to control or enhance the formation of single- or multi-tissue systems. However, the electrospinning process used to fabricate nanofibers requires that the polymer first be soluble in a variety of toxic solvents, which may have undesired effects on the incorporation of either biomolecules or cells. Thus incorporation of biomolecules into nanofiber-based systems, while keeping them structurally stable and biologically active, and controlling their subsequent release remains to be investigated. Additionally, high-throughput fabrication and delivery processes need to be developed for scaling up nanofiber scaffolds and enabling their commercial applicability. Moreover, further optimization of scaffold design strategies is anticipated for effective clinical translation and surgical implementation. For example, combining the optimal strategies devised from the tendon and the tendon-to-bone interface regeneration in this review may yield a graft system which can enable integration with both soft and hard tissue. Such a composite scaffold system would be optimal for treating massive rotator cuff tears. Finally, the development of physiologically-relevant in vivo soft tissue repair models, of both healthy and diseased, are needed in order to evaluate the clinical efficacy of these biomimetic scaffolds
Conclusion
In closing, the design of biomimetic, nanofiber-based scaffolds for tendon and tendon-bone interface regeneration described in this review offer a promising strategy for achieving functional and integrative tendon repair. It is anticipated that these efforts will lead to the development of a new generation of biological fixation devices for soft tissue repair, and will improve clinical outcome as well as quality of life for patients suffering from the debilitating effects of soft tissue injuries.
Table I.
Scaffold-based Approaches for Tendon Repair
| Study | Scaffold Composition | Scaffold Design | Cell Type/Evaluation | Observations |
|---|---|---|---|---|
| Gilbert et al. 200732 | Porcine small intestinal submucosa (SIS) | Homogeneous | NIH 3T3 fibroblasts in vitro | Col I increases and Col III decreases with increased frequency of stretch |
| Juncosa-Melvin et al. 200641 | Collagen sponge (type I) | Homogeneous | hMSCs in vitro and in vivo (rat patellar tendon defect model) | Mechanical stimulation improves linear stiffness |
| Sahoo et al 200678 | PLGA Nanofibers (300-900nm) vs. PLGA microfibers | Variable fiber diameter | Porcine MSC in vitro | Cell attachment comparable to fibrin gel-microfiber control; gene expression indicates capacity to differentiate towards tendon lineage |
| Barber et al. 20114 | Braided aligned PLGA nanofiber (702±205nm) | 3, 4, or 5 aligned nanofiber bundles | hMSC in vitro | Abundant matrix formation, upregulation of scleraxis indicating differentiation into tenogenic lineage |
| Sahoo et al. 201077 | PLGA nanofiber (200-700nm) with FGF incorporated + silk microfiber | Unaligned PLGA nanofibers with electrospun onto knitted silk fibers | Rabbit BMSC in vitro | Scaffolds stimulate MSC proliferation; gene expression indicates tenogenic differentiation |
| Yin et al. 201096 | PLLA aligned (430±170nm) vs. unaligned (450±110nm) nanofibers | Homogeneous | Human tendon stem cells (TSPCs) in vitro & in vivo (intramuscular implantation in mouse model) | Aligned scaffolds promote expression of tenogenic markers. In vivo, aligned scaffolds guide cell and matrix organization |
| Pham et al. 200669 | Micro(2-10μm) or Nano(615±152nm)-microfibrous PCL | Variable fiber diameter | Rat MSC in vitro | Culture in flow perfusion bioreactor enhances MSC infiltration distance through scaffold |
| Srouji et al. 200883 | Unaligned PCL and collagen (1:1) | Homogeneous | hMSC in vitro & in vivo (subcutaneous, nude mouse model) | Plug-flow bioreactor culture enhances cell proliferation and infiltration; integration with host tissue and neovasculartization in vivo |
| Moffatt et al. 200959 | PLGA aligned (615±152nm) vs. unaligned nanofiber (568±147nm) | Homogeneous | Human rotator cuff tendon fibroblasts in vitro | Cell morphology and matrix alignment governed by fiber alignment |
Table II.
Scaffold-based Approaches for Tendon-to-Bone Interface Regeneration
| Study | Scaffold Composition | Scaffold Design | Cell type/Evaluation | Observations |
|---|---|---|---|---|
| Erisken et al. 200822 | Unaligned, extruded/electrospun PCL nanofibers (200–2000 nm) | Gradient of scaffold mineral content | Mouse preosteoblast cells (MC3T3) in vitro | Formation of a gradient of calcified matrix |
| Li et al. 200951 | Gelatin-coated PCL nanofibers and plasma treated PLGA | Graded coating of calcium phosphate | Mouse preosteoblast cells (MC3T3) in vitro | Cells preferentially adhere and proliferate on areas with high CP content |
| Xie et al. 201051 | Aligned and unaligned PLGA nanofibers | Aligned-to-random fiber orientation | Rat tendon fibroblasts in vitro | Cells align/organize on aligned fibers; cells remain unorganized on random fibers |
| Moffat et al. 201162 | Aligned PLGA deposited over PLGA-HA nanofibers Phase A (615±152nm) Phase B (340±77nm) | Biphasic with contiguous layer of PLGA and PLGA-HA | Bovine chondrocytes in vitro Rat BMSC in vivo Rat rotator cuff model | Contiguous non-calcified and calcified fibrocartilage-like matrix were formed in vitro and in vivo |
Acknowledgments
Funding: The authors gratefully acknowledge the National Institutes of Health (NIH/NIAMS AR052402; AR056459; and AR055280), the New York Stem Cell Initiative (HHL) and the National Science Foundation Graduate Fellowship (DRB), GK-12 Fellowship (GK-12 0338329, NML) and National Science and Engineering Council of Canada (NSERC PGS-D3, XZX) for funding support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: None
IRB: N/A – Review Article
Reference
- 1.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131–4141. doi: 10.1016/s0142-9612(02)00156-4. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13(5):377–383. doi: 10.1016/s1084952102000940. doi:10.1016/S1084952102000940. [DOI] [PubMed] [Google Scholar]
- 3.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. doi: 10.1016/ j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber JG, Handorf AM, Allee TJ, Li WJ. Braided Nanofibrous Scaffold for Tendon and Ligament Tissue Engineering. Tissue Eng Part A. 2011 Sep 6; doi: 10.1089/ten.tea.2010.0538. doi:10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 5.Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lacticco-glycolic acid) meshes. Biomaterials. 2006;27(33):5681–5688. doi: 10.1016/j.biomaterials.2006.07.005. doi:10.1016/ j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):931–945. doi: 10.1016/s1095-6433(02)00138-1. doi:10.1016/S1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 8.Berry CC, Shelton JC, Bader DL, Lee DA. Influence of external uniaxial cyclic strain on oriented fibroblast-seeded collagen gels. Tissue Eng. 2003;9(4):613–624. doi: 10.1089/107632703768247313. doi:10.1089/107632703768247313. [DOI] [PubMed] [Google Scholar]
- 9.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. ISSN:1078-8956. [DOI] [PubMed] [Google Scholar]
- 10.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28(1):1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 11.Chang CH, Chen CH, Su CY, Liu HT, Yu CM. Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1447–1453. doi: 10.1007/s00167-009-0809-x. doi:10.1007/ s00167-009-0809-x. [DOI] [PubMed] [Google Scholar]
- 12.Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25(1):11–22. doi: 10.1002/jor.20305. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 13.Codman EA. The Shoulder, Rupture of the Supraspinatus Tendon and Other Lesions In or About the Subacromial Bursa. 1934 [Google Scholar]
- 14.Coons DA, Alan Barber F. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14(3):185–190. doi: 10.1097/00132585-200609000-00011. doi:10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JA, Jr., Sahota JS, Gorum WJ, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3049–3054. doi: 10.1073/pnas.0608837104. doi:10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970;52(1):1–20. [PubMed] [Google Scholar]
- 17.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21(2):131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 18.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29(2):175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 19.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. Journal of Shoulder and Elbow Surgery. 2010;19(3):467–476. doi: 10.1016/j.jse.2009.10.020. doi:10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88(12):2665–2672. doi: 10.2106/JBJS.E.01307. doi:10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 21.Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29(11):1363–1371. doi: 10.1002/jbm.820291107. [DOI] [PubMed] [Google Scholar]
- 22.Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29(30):4065–4073. doi: 10.1016/j.biomaterials.2008.06.022. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203(2):191–202. doi: 10.1046/j.1469-7580.2003.00193.x. doi:10.1046/j.1469-7580.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka H, Thakur R, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37(3-4):205–218. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 25.Fung YC. Stress-strain-history relations of soft tissues in simple elongation. 1972:181–208. [Google Scholar]
- 26.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. ISSN:0021-9355. [DOI] [PubMed] [Google Scholar]
- 27.Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomol Eng. 2007;24(1):75–80. doi: 10.1016/j.bioeng.2006.05.017. ISSN:1389-0344. [DOI] [PubMed] [Google Scholar]
- 28.Gartsman GM. Massive, irreparable tears of the rotator cuff. Results of operative debridement and subacromial decompression. J Bone Joint Surg Am. 1997;79(5):715–721. doi: 10.2106/00004623-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9(5):967–979. doi: 10.1089/107632703322495619. doi:10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 30.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43–53. [PubMed] [Google Scholar]
- 31.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert TW, Stewart-Akers AM, Sydeski J, Nguyen TD, Badylak SF, Woo SL. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13(6):1313–1323. doi: 10.1089/ten.2006.0318. doi: 10. 1089/ ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 33.Goh JC, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31–S44. doi: 10.1089/10763270360696969. doi:10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 34.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. doi: 10.1177/ 0363546509339582. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31(6):791–797. doi: 10.1007/s00264-007-0395-9. doi:10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horan RL, Toponarski I, Boepple HE, Weitzel PP, Richmond JC, Altman GH. Design and characterization of a scaffold for anterior cruciate ligament engineering. J Knee Surg. 2009;22(1):82–92. doi: 10.1055/s-0030-1247730. doi:10.1055/s-0030-1247730. [DOI] [PubMed] [Google Scholar]
- 37.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic twotendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238–1244. doi: 10.2106/JBJS.E.00524. ISSN:0021-9355. [DOI] [PubMed] [Google Scholar]
- 38.Inoue N, Ikeda K, Aro HT, Frassica FJ, Sim FH, Chao EY. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002;20(5):957–966. doi: 10.1016/S0736-0266(02)00037-2. ISSN:0736-0266. [DOI] [PubMed] [Google Scholar]
- 39.Juncosa N, West JR, Galloway MT, Boivin GP, Butler DL. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech. 2003;36(4):483–488. doi: 10.1016/s0021-9290(02)00459-1. ISSN:0021-9290. [DOI] [PubMed] [Google Scholar]
- 40.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13(6):1219–1226. doi: 10.1089/ten.2006.0339. doi:10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 41.Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12(8):2291–2300. doi: 10.1089/ten.2006.12.2291. doi:10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 42.Jung HJ, Fisher MB, Woo SL. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol. 2009;1(1):9. doi: 10.1186/1758-2555-1-9. doi:10.1186/1758-2555-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J Anat. 1994;185(Pt 2):279–284. [PMC free article] [PubMed] [Google Scholar]
- 44.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. doi:10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 45.Ladd MR, Lee SJ, Stitzel JD, Atala A, Yoo JJ. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials. 2011;32(6):1549–1559. doi: 10.1016/j.biomaterials.2010.10.038. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 46.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 47.Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12(11):3149–3158. doi: 10.1089/ten.2006.12.3149. doi:10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26(11):1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. doi:10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 49.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003 Dec 15;67(4):1105–1114. doi: 10.1002/jbm.a.10101. doi:10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 50.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40(8):1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li XR, Xie JW, Lipner J, Yuan XY, Thomopoulos S, Xia YN. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano Letters. 2009;9(7):2763–2768. doi: 10.1021/nl901582f. doi:10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang R, Woo SL, Takakura Y, Moon DK, Jia F, Abramowitch SD. Long-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: a functional tissue engineering study. J Orthop Res. 2006;24(4):811–819. doi: 10.1002/jor.20080. doi:10.1002/jor.20080. [DOI] [PubMed] [Google Scholar]
- 53.Lu HH, Cooper JA, Jr., Manuel S, Freeman JW, Attawia MA, Ko FK, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26(23):4805–4816. doi: 10.1016/j.biomaterials.2004.11.050. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 54.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. doi:10.1007/b138509. [PubMed] [Google Scholar]
- 55.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1-2):101–109. doi: 10.1089/ten.2005.11.101. doi:10.1089/ ten. 2005. 11.101. [DOI] [PubMed] [Google Scholar]
- 56.Mansat P, Cofield RH, Kersten TE, Rowland CM. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28(2):205–213. doi: 10.1016/s0030-5898(05)70280-7. [DOI] [PubMed] [Google Scholar]
- 57.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of Collagen Nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. doi:10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 58.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 59.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15(1):115–126. doi: 10.1089/ten.tea.2008.0014. doi:10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):7947–7952. doi: 10.1073/pnas.0712150105. doi:10.1073 /pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moffat KL, Wang IN, Rodeo SA, Lu HH. Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin Sports Med. 2009;28(1):157–176. doi: 10.1016/j.csm.2008.08.006. doi:10.1016/j.csm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moffat KL, Zhang X, Greco S, Boushell MK, Guo XE, Doty SB, et al. In vitro and in vivo evaluation of a bi-phasic nanofiber scaffold for integrative rotator cuff repair. Transactions of Orthopeadic Research Society. 2011 [Google Scholar]
- 63.Murugan R, Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007;13(8):1845–1866. doi: 10.1089/ten.2006.0078. doi:10.1089/ ten. 2006.0078. [DOI] [PubMed] [Google Scholar]
- 64.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. doi:10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 65.Ozaki J, Fujimoto S, Masuhara K, Tamai S, Yoshimoto S. Reconstruction of chronic massive rotator cuff tears with synthetic materials. Clin Orthop Relat Res. 1986;(202):173–183. [PubMed] [Google Scholar]
- 66.Park K, Ju YM, Son JS, Ahn KD, Han DK. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J Biomater Sci Polym Ed. 2007;18(4):369–382. doi: 10.1163/156856207780424997. doi:10.1163/156856207780424997. [DOI] [PubMed] [Google Scholar]
- 67.Park MC, Cadet ER, Levine WN, Bigliani LU, Ahmad CS. Tendon-to-bone pressure distributions at a repaired rotator cuff footprint using transosseous suture and suture anchor fixation techniques. Am J Sports Med. 2005;33(8):1154–1159. doi: 10.1177/0363546504273053. doi:10.1177/0363546504273053. [DOI] [PubMed] [Google Scholar]
- 68.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 69.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 70.Post M. Rotator cuff repair with carbon filament. A preliminary report of five cases. Clin Orthop Relat Res. 1985;(196):154–158. [PubMed] [Google Scholar]
- 71.Ramakrishnan N, Xia Y, Bidthanapally A. Fourier-transform infrared anisotropy in cross and parallel sections of tendon and articular cartilage. J Orthop Surg Res. 2008;348 doi: 10.1186/1749-799X-3-48. doi:10.1186/1749-799X-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 73.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27(8):1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 74.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16(5 Suppl):S191–S197. doi: 10.1016/j.jse.2007.03.012. doi:10.1007/s11999-007-0112-4. [DOI] [PubMed] [Google Scholar]
- 75.Rokito AS, Zuckerman JD, Gallagher MA, Cuomo F. Strength after surgical repair of the rotator cuff. J Shoulder Elbow Surg. 1996;5(1):12–17. doi: 10.1016/s1058-2746(96)80025-5. [DOI] [PubMed] [Google Scholar]
- 76.Romeo AA, Hang DW, Bach BR, Jr., Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243–255. [PubMed] [Google Scholar]
- 77.Sahoo S, Ang LT, Goh JC, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010 Jun 15;93(4):1539–1550. doi: 10.1002/jbm.a.32645. doi:10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 78.Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12(1):91–99. doi: 10.1089/ten.2006.12.91. doi:10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 79.Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed Mater Eng. 2001;11(4):275–281. ISSN:0959-2989. [PubMed] [Google Scholar]
- 80.Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34(2):275–280. doi: 10.1177/0363546505279912. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 81.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004;13(5):538–541. doi: 10.1016/j.jse.2004.03.005. ISSN:1058-2746. [DOI] [PubMed] [Google Scholar]
- 82.Soslowsky LJ, Carpenter JE, DeBano CM, Banes AJ, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 83.Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. Journal of Materials Science-Materials in Medicine. 2008;19(3):1249–1255. doi: 10.1007/s10856-007-3218-z. doi:10.1007/s10856-007-3218-z. [DOI] [PubMed] [Google Scholar]
- 84.Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115–122. doi: 10.1002/jbm.b.31157. doi:10.1007/s00167-009-0809-x. [DOI] [PubMed] [Google Scholar]
- 85.Theisen C, Fuchs-Winkelmann S, Knappstein K, Efe T, Schmitt J, Paletta JR, et al. Influence of nanofibers on growth and gene expression of human tendon derived fibroblast. Biomed Eng Online. 2010;99 doi: 10.1186/1475-925X-9-9. ISSN:1475-925X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2005 Jul 14; doi: 10.1016/j.jbiomech.2005.05.021. doi:10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variations of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21(3):413–419. doi: 10.1016/S0736-0266(03)00057-3. doi:10.1016/S0736-0266(03) 0057-3. [DOI] [PubMed] [Google Scholar]
- 88.Tidball JG. Myotendinous junction injury in relation to junction structure and molecular composition. Exerc Sport Sci Rev. 1991;19:419–445. [PubMed] [Google Scholar]
- 89.Wang IE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006 Jun 15;24(8):1745–1755. doi: 10.1002/jor.20149. doi:10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 90.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1(1):22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 91.Woo SL, Maynard J, Butler DL, Lyon RM, Torzilli PA, Akeson WH, et al. Ligament, Tendon, and Joint Capsule Insertions to Bone. 1988;(4):133–166. [Google Scholar]
- 92.Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, et al. “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendonto-bone insertion site. Nanoscale. 2010 Jun 9;2(6):923–926. doi: 10.1039/c0nr00192a. doi:10.1039/c0nr00192a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamanaka K, Matsumoto T. The joint side tear of the rotator cuff. A follow up study by arthrography. Clin Orthop Relat Res. 1994;(304):68–73. [PubMed] [Google Scholar]
- 94.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15(2):127–141. doi: 10.1089/ten.teb.2008.0371. doi:10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17(2):175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 96.Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. ISSN:0142-9612. [DOI] [PubMed] [Google Scholar]
- 97.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. doi:0142-9612. [DOI] [PubMed] [Google Scholar]
- 98.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6(5):2583–2589. doi: 10.1021/bm050314k. doi:10.1021 bm050314k. [DOI] [PubMed] [Google Scholar]