Abstract
We have previously shown that Interleukin-21, a pleiotropic C γ-chain signaling cytokine, induces the expression of the cytotoxic molecules granzyme B (GrB) and perforin in vitro in CD8 T cells and NK cells of chronically HIV infected individuals. In this pilot study, four chronically SIV infected Rhesus macaques (RM) in late- stage disease were given two doses of recombinant MamuIL-21, 50μg/kg, intravenously 7 days apart, followed by one subcutaneous dose, 100μg/kg, 23 days after the second dose. Three animals served as controls. After each dose of IL-21, increases were noted in frequency and mean fluorescence intensity of GrB and perforin expression in memory and effector subsets of CD8 T cells in peripheral blood (PB), in peripheral and mesenteric lymph node (LN) cells, in PB memory and effector CD4 T cells and in NK cells. Frequencies of SIV-gag specific CD107a+IFNγ+ CD8 increased 3.8 fold in PB and 1.8 fold in LN. In addition, PB CD27+ memory B cells were 2 fold higher and serum SIV antibodies increased significantly after IL-21 administration. No changes were observed in markers of T cell activation, T cell proliferation or plasma virus load. Thus, administration of IL-21 to chronically SIV infected viremic animals was safe, well tolerated and could augment the cytotoxic potential of T cells and NK cells, promote B cell differentiation with increases in SIV antibody titers without discernable increase in cellular activation. Further studies are warranted to elucidate the effects and potential benefit of IL-21 administration in the context of SIV/HIV infection and in HIV/SIV vaccine design.
Keywords: Interleukin-21, T cells, B cells, Natural Killer cells, Rhesus macaques, SIV
Introduction
Interleukin (IL)-21 is a member of a family of cytokines which includes IL-2, IL-4, IL-7, IL-9 and IL-15 all of which utilize a common γ chain in their individual receptor complexes for delivering intracellular signals in their target cells. IL-21 is produced by CD4+ T cells, in particular T follicular helper cells, Th17 cells and NK cells. It is involved in the regulation of both innate and adaptive immunity [1, 2], due to the wide range of cellular targets that express the IL-21 receptor (IL-21R), namely B cells, T cells, Natural killer (NK) cells, NKT cells, dendritic cells, macrophages, keratinocytes and intestinal fibroblasts. There is compelling evidence for a critical role of IL-21 in protection against lymphocytic choriomeningitis virus (LCMV) infection of mice [3–5]. Higher levels of IL-21 produced by virus–specific CD4 T cells were demonstrated to be critical for sustaining antiviral effector functions of CD8+ T cells without the cells becoming exhausted.
Recent ex vivo studies have demonstrated that IL-21 is also an important contributor for the control of HIV in patients identified as natural viral controllers [6, 7]. While correlates of immune protection in HIV infection are not well understood, cytotoxic T cells [8–10] and more recently, NK cells have been shown to play important roles in virologic control [11, 12]. The pore-forming protein perforin, together with granzyme, constitute key cytotoxic effector molecules that transmit cell death signals [13, 14] to virus infected target cells. [8, 9, 15]. In HIV infection, expression of perforin is abundant in antigen specific CD8 T cells of long term non progressors but deficient in chronic progressors and the killing of HIV infected CD4 T cells is directly linked to expression of cytotoxic molecules [10, 16, 17]. We have previously shown that perforin expression is augmented in CD8 T cells [18] and NK cells [19] of HIV infected persons following ex-vivo treatment of the cells with IL-21.
Based on the above findings we conducted a pilot study of recombinant MamuIL-21 (rMamuIL-21) in Rhesus macaques (RM) to determine IL-21 safety and activity. IL-21 was safe, and demonstrated biologic activity with increase in cytotoxic molecules in CD8 T cells and NK cells. It was also associated with an amplified frequency of circulating memory B cells and a modest increase in serum SIV antibody titers.
Materials and Methods
Animals
Seven colony-bred Indian RM infected intrarectally with SIVmac251 for periods greater than 6 months were housed at Advanced BioScience Laboratories, Inc. These animals were highly viremic with mean plasma viral loads (VL) of 5.5 ± 1.4 log10 (range, 2.7–6.9) SIV RNA copies/ml [20, 21]. Two healthy RM housed at the Yerkes National Primate Research Center were used as uninfected controls to measure IL-21 bioactivity in the absence of SIV infection. The care and use of all animals were in compliance with the National Institutes of Health- and relevant institutional guidelines. SIV infected animals were euthanized upon completion of this study.
Production and testing of rMamu IL-21
rMamuIL-21 was produced in the E coli BL21 codon+ (Novagen, Madison, WI) using the pET32a vector system similar to the production of IL-15 described previously [22, 23] by the Resource for Nonhuman Primate Immune Reagents. All batches of IL-21 were tested for the presence of residual endotoxin and purified. Content and bioactivity of the cytokine batches were verified by EIA (B–D antibody pairs J148–1134 and I76–539), its capacity to upregulate perforin expression in vitro and by administration to healthy RM.
IL-21 administration
SIV infected RM were randomized based on gender and viral load into the IL-21 arm, (group 1, n = 4, M897; 5yrs, P004; 5yrs, P005; 5 yrs, and P046; 7yrs) and the control arm, (group 2, n = 3, M893; 5yrs, M905; 5yrs, and P003; 5yrs). Animals in group 1 were given rMamuIL-21 in a manner aimed at maximizing its biological effect based on administration of other recombinant cytokines [23–25]. Two intravenous (i.v.) doses of rMamu IL 21, 50μg/kg given 7 days apart were followed by a third s.c. dose of 100μg/kg 23 days after the 2nd dose (Fig 1A). Animals were sacrificed five days after the third IL-21dose. PB, serum and plasma were collected at baseline and at three days after 1st and 2nd doses of IL-21 and five days after 3rd dose of IL-21. Peripheral LN biopsies were collected at baseline and 3 days after the 2nd dose of IL-21, while mesenteric lymph node (mesLN) and another peripheral LN were collected when animals at sacrifice, 5 days after the third dose of IL-21. The two healthy monkeys were given subcutaneous (s.c.) injections of IL-21 at escalating doses of 1, 10, 50 and 100 μg/kg body weight at 2 week intervals. After each IL-21 administration, complete blood count, chemistry profile and plasma anti-IL-21 antibodies were monitored. Peripheral blood was collected at baseline before administration of each IL-21 dose, and 48 hrs after IL-21 administration.
Figure 1. IL-21 administration in SIV infected RM did not alter T cells, B cells, NK cells numbers.
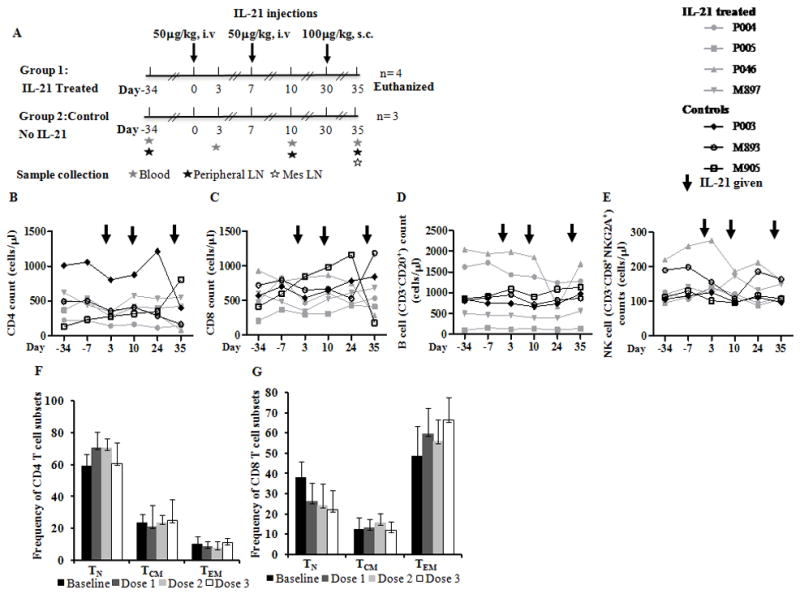
(A), Schematic representation of dose and route of rMamuIL-21 administration in SIV infected viremic RM. Animals were given two i.v. injections of IL-21, 50μg/kg (depicted as day 0 and day 7) and one s.c. dose, 100μg/kg, 23 days after the second dose (depicted as day 30 Times at which samples of blood and LN biopsies were collected are indicated. Line graph showing absolute numbers of (B), CD4 T cells; (C), CD8 T cells; (D), B cells and (E), NK cells pre and post IL-21 administration in control (black) and in IL-21 treated (grey) animals. Bar graph showing mean frequencies of TN, TCM and TEM subsets in (F), CD4 T cell and (G), CD8 T cell subsets in IL-21 treated animals.
Virus load determination
Plasma SIVmac251 RNA levels were measured by nucleic acid sequence based amplification (NASBA) technique [26]. The copy number was expressed per 100 μl of plasma or per microgram of RNA, and the detection limit of the assay was 2 × 103 RNA copies.
Cell preparations
Fresh PBMC were isolated using standard density gradient centrifugation method using Ficoll Paque (GE health care biosciences, SE). In some instances PBMC were cryopreserved in 10% DMSO and 90% fetal calf serum in liquid nitrogen for immunologic studies. Immunologic analyses were conducted in freshly isolated PBMC or in cryopreserved cells as indicated. Thawed cryopreserved cells were rested for 12 hrs at 37°C and 5% CO2 and 100% humidity prior to using them for assays. Cell recovery was >80% and viability was >95% in all instances. The cells were stained with fluorochrome tagged monoclonal antibody (mAb) reagents for surface antigens and with the amine reactive violet dead cell discriminator dye (ViViD) for 20 min at room temperature in the dark. For intracellular staining, surface stained cells were washed, fixed and permeabilized for 20 min in BD cytofix/cytoperm solution stained for 30 min at 4°C for intracellular perforin and granzyme B (GrB) [27]. Intracellular staining for proliferation marker Ki67 was performed at room temperature for 30 min. Isotype Ab staining was performed along with test Abs to minimize the errors due to nonspecific binding.
Flow cytometry analysis
Samples processed as above were acquired on a LSRII flow cytometer (BD Biosciences, San Jose, CA). Flow cytometry analysis was performed after proper instrument setting, calibration and compensation to minimize and correct the spillover induced background [28, 29]. Forward scatter (FSC)-width versus FSC-height parameters were used to exclude cell doublets followed by exclusion of dead cells stained by ViViD dye; 200,000–500,000 events were collected and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Staining cells for T cell phenotype analysis, immune activation and proliferation by flow cytometry
One million PBMC were washed and surface stained with antibodies against CD3, CD4, CD8, CD28, CD95 and HLA-DR followed by intracellular staining for Ki67. T cell maturation subsets were identified in gated CD3+CD4 T cells and CD3+CD8 T cells based on the surface expression of CD28 and CD95 as naïve (TN: CD28+CD95−), central memory (TCM: CD28+CD95+) and effector memory (TEM: CD28−CD95+). Expression of proliferation marker Ki67 and activation marker HLA-DR were analyzed in CD4 and CD8 T cells and on maturation subsets based on isotype gating.
Perforin and GrB analysis
Perforin and GrB staining was performed using macaque specific antibodies [30, 31] in PBMC or lymph node (LN) cells with antibodies to CD3, CD4, CD8, CD28 and CD95, NKG2A followed by intracellular staining for perforin and GrB. Flow cytometry analysis was carried out for the expression of perforin and GrB in CD4 and CD8 T cells and their maturation subsets and NK cells (CD3−CD8+NKG2A+) based on isotype gating.
Functional T cell analysis
Cells were examined for intracellular expression of degranulation marker CD107a, and cytokines IFN-γ, IL-2 and TNF-α for polyfunctional T cells [27]. Briefly, one million PBMC were washed and incubated with 1 μg/ml each anti-CD28 and anti-CD49d mAb and pooled overlapping SIV Gag peptides (2 μg/ml of each) in a final volume of one ml. Ab against granular membrane protein CD107a was added to the cells before stimulation. A negative control with the addition of only anti-CD28 and anti-CD49d mAb, and a positive control of staphylococcal enterotoxin B (SEB) at 2 μg/ml were included. Cultures were incubated for 6 h at 37°C in 5% CO2 in the presence of the secretion inhibitor monensin, 0.7 μl/ml, and brefeldin A, 10 μg/ml. Thereafter, PBMC were washed, surface stained with mAb for CD3, CD4, CD8 and ViViD, permeabilized using BD cytofix/cyotperm for 20 min at 4°C, stained with mAbs for intracellular cytokines for 30 min at 4°C and resuspended in 1% paraformaldehyde in PBS for flow cytometry. Boolean gate analysis was performed using the FlowJo platform to identify cells with one or more functions (CD107a, IFN-γ, IL-2 and TNF-α) in CD4 and CD8 T cells. SPICE (Simplified Presentation of Incredibly Complex Evaluations, Version 5.05013, courtesy of Mario Roederer, Vaccine Research Center, NIAID, NIH) was used to depict the polyfunctional T cell data obtained after Boolean gating.
Phenotypic analysis of B cells
PBMC (1×106 cells) were stained with antibodies against CD3, CD20, CD27, IL-21R or isotype antibodies and ViViD dye for flow cytometry. B cells were identified as CD3−CD20+ cells and were further characterized based on the expression of CD27 as memory (CD20+CD27+) and naïve (CD20+CD27−) subsets [32]. Total, naïve and memory B cells were further analyzed for the expression of IL-21R based on gating performed on isotype controls.
Anti-SIV antibody detection by ConA ELISA
Serum samples from IL-21 treated and control macaques were analyzed for their reactivity to viral antigens in a concanavalin A (ConA) ELISA [33]. Briefly, 96-well plates were coated with 5 μg of ConA for 1 hr, washed and incubated with the viral supernatant which is first incubated with triton detergent. After blocking, sera at different dilution were added to the wells and incubated for 1 hr at room temperature. The plates were washed and anti-SIV IgG Ab was detected using after incubating 20 min with anti-human IgG biotin/Streptavidin HRP.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 4.01. Differences between pre- and post-treatment values were assessed with a paired 2-tailed Student’s t test. Comparisons between different groups of animals (IL-21 treated versus control animals) were made using an unpaired, 2-tailed Student’s t test. P values less than 0.05 were considered significant.
Results
IL-21 was safe and biologically active in healthy RM
Doses of rMamu IL-21 up to 100μg/kg body weight were well tolerated in the two healthy SIV uninfected RM without evidence of dose limiting toxicities other than modest increases in serum liver enzymes that did not appear to correlate with IL-21 doses (Table S1). No major effects on the absolute numbers and/or frequencies of CD4, CD8, NK and B cells were noted. Biological activity was documented at all four doses - 1, 10, 50 and 100 μg/kg, as shown in Fig S1, with transient induction of Ki67, Perforin and GrB in CD8 T cells and increases in CD27+ memory B cells and IL21R+ B cells. Among CD8 T cell maturation subsets, maximum induction of perforin was noted predominantly in TEM and GrB in TN, TCM and TEM (data not shown). Of note, there were only minor differences in the induction of cytotoxic molecules between the 10 and 100 μg/kg doses suggesting maximal effect for these parameters in healthy monkeys. Expression of immune activation marker HLA-DR on T cells was unchanged (not shown). Based on these data, a maximum tolerated dose was not established and doses of 50 and 100μg/kg IL-21 were considered safe and inducing maximal upregulation of cytotoxic molecules in healthy macaques.
T cells, B cells, NK cells numbers, plasma virus load and T cell immune activation profile were unchanged after IL-21 administration in SIV infected RM
IL-21 administration to SIV infected macaques did not result in significant changes in the absolute numbers of PB CD4T cells (Fig 1B), CD8 T cells (Fig 1C), CD20+ B cells (Fig 1D) and CD8+NKG2A+ NK cells (Fig 1E). Similarly, frequencies of CD4 and CD8 T cell maturation subsets (TN, TCM and TEM) did not show appreciable changes after IL-21 administration when compared to baseline levels (Fig 1F & 1G) or control animals (not shown). IL-21 administration did not alter the plasma SIV RNA (Fig S2) and did not result in increases in immune activation markers HLA-DR or Ki67 in CD4 and CD8 T cells and their maturation subsets in PB, peripheral LN and mesLN in comparison to baseline levels or control animals (not shown).
IL-21 administration in SIV infected viremic RM results in induction of GrB and perforin in T cells
As shown in Figs 2A and 2B, IL-21 treated animals manifested significant increases in the absolute numbers of GrB and perforin expressing CD8 T cells respectively in PB with maximal increases after the third IL-21 dose. Increases in GrB occurred in all CD8 T cell maturation subsets (TN, TCM and TEM), predominantly after the 3rd dose of IL-21. Perforin expressing CD8 T cells increased after each IL-21 dose in TCM and TEM, but as expected not in TN. Interestingly, PB CD4 T cells also exhibited increases in absolute numbers of GrB (Fig 2C) and perforin (Fig 2D) positive cells after each IL-21 dose. Increase in GrB+ cells occurred in all CD4 T cell maturation subsets (Fig 2C) and a significant increase was noted in in perforin expressing CD4 TCM subset (Fig 2D). Representative dot plot analyses for CD4 and CD8 T cells and maturation subsets for perforin and GrB are depicted in Fig S3. The MFI of GrB was higher in total CD8 and CD4 T cells and in their maturation subsets with maximum MFI after the third dose of IL-21 (data not shown). MFI of perforin was higher in PB CD8 T cells of IL-21 treated animals compared to controls (data not shown).
Figure 2. Granzyme B and perforin induction in PB CD8 T cells and CD4 T cells after IL-21 administration in SIV infected RM.
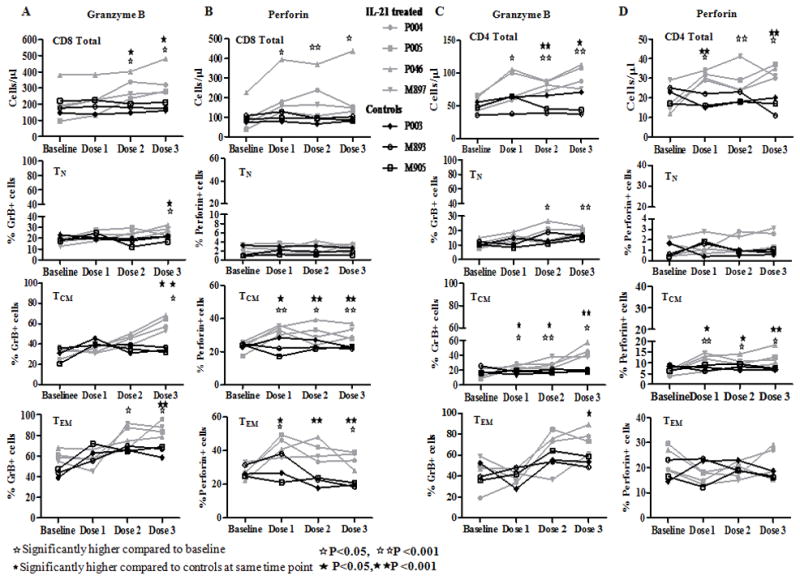
One million cells were stained with antibodies against CD3, CD4, CD8, CD95, CD28 and ViViD, fixed, permeabilized and stained for intracellular GrB and perforin. CD3+ T cells were gated from singlets followed by gating for CD4 and CD8 T cells. T cell maturation subsets were identified based on the surface expression of CD28 and CD95 as naïve (TN: CD28+CD95−), central memory (TCM: CD28+CD95+) and effector memory (TEM: CD28−CD95+) subsets. Intracellular expression of perforin and GrB was analyzed in CD4, CD8 T cells and maturation subsets based on isotype controls. Line graphs shown for control (black) and IL-21 treated (grey) animals at baseline (pre-IL-21) and after dose 1, dose 2 and dose 3 of IL-21for (A), GrB; (B), perforin in gated live CD3+CD8+ T cells and maturation subsets and (C), GrB; (D), perforin in gated live CD3+CD4+ T cells and maturation subsets.
The increase in frequencies of GrB and perforin positive cells was also evident in LN T cells (Fig 3A). In peripheral LN, IL-21 treated animals manifested significant increases from baseline in frequency of GrB expressing cells (2.8 fold in CD8 T cells and 5 fold in CD4 T cells). These changes were most evident after the 3rd 100 μg/kg dose of IL-21, suggesting either compartmentalization of such responders relative to blood and/or the need for a higher dose to generate noticeable LN enhancement by IL-21 in SIV infected and viremic macaques. Among CD8 T cell maturation subsets, significant increases from baseline were noted in GrB+ TCM and TEM cells (Fig 3B), and in GrB+CD4 TCM (Fig 3C). Compared to GrB, the increase in frequency of perforin positive CD8 and CD4 T cells in peripheral LN was less prominent (CD8, 1.5 fold and CD4, 2.1 fold) and did not reach significance (data not shown). In mesLN collected after the 3rd dose of IL-21, increase in frequency of GrB positive cells was 1.8 fold in total CD8 T cells, 5.5 fold in TCM and 1.4 fold in TEM subsets compared to untreated SIV infected control animals (Fig S4). In IL-21 treated versus control animals increase in frequencies of perforin positive mesLN CD8 T cells was 1.5 fold (17.8 ± 2.1% versus 13.3 ± 1.2%, p=0.03) with a 1.6 fold increase in TEM, data not shown.
Figure 3. Granzyme B induction in ingLN T cells of SIV infected RM.
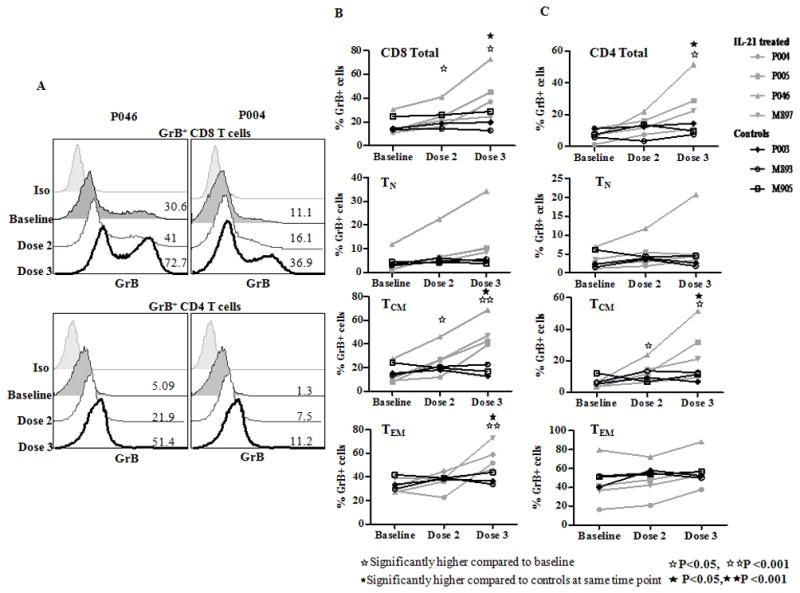
Cryoporeserved cells were thawed and stained with antibodies against CD3, CD4, CD8, CD95, CD28 and ViViD, fixed, permeabilized and stained for GrB and perforin. Data is shown for intracellular GrB in gated live CD3+CD4+ and CD3+CD8+ T cells and maturation subsets. (A), Representative histogram of GrB expression in total CD8 and CD4 T cells of two IL-21 treated animals at baseline, after dose 2 and dose 3.of IL-21. Line graph showing frequency of GrB expressing (B), CD8 and (C), CD4 T cells and their maturation subsets in control (black) and in IL-21 treated (grey) animals at baseline (pre-IL-21), does 2 and dose 3 of IL-21.
Dual function positive SIV-specific IFN-γ plus CD107a expressing cells are moderately augmented by IL-21 administration
One or more of the following functions: CD107a (degranulation marker), or cytokines IL-2, IFN-γ and TNF-α were examined following ex-vivo stimulation of PBMC/LN cells with SIV gag pool for 6 hrs (Fig 4A). At baseline, only single SIV specific functional cells were detected in the following order of frequency: CD107a> IFN-γ > TNF-α >IL-2. Data for cells positive for more than one function is depicted in fig 4B. Following IL-21 treatment, significant increases were noted in SIV-specific polyfunctional SIV-specific CD8 T cells with dual function (CD107a+ IFN-γ+) in PB and LN. A slightly higher frequency of SIV specific CD107a+IFN-γ+TNF-α+ triple function cells were observed but the change was not significant. There was no induction of T cells with 4 functions (Fig 4B). In PB, after the 3rd dose of IL-21, a 3.8 fold increase in mean frequencies of SIV-specific CD107a+IFN-γ+ CD8 T cells was noted compared to baseline levels (Fig 4C). CD8 T cells from peripheral LN also showed an increase in SIV-specific CD107a+IFN-γ+ cells after the 3rd dose of IL-21 compared to baseline levels (1.8 fold; Fig 4D) or as compared to control animals (2.3 fold; p<0.01). SIV-specific CD4 T cells in PB (Fig 4E) and in peripheral LN (Fig 4F) also showed a significant increase in the frequencies of CD107a+IFN-γ+ cells after the third dose of IL-21 compared to baseline levels or control animals.
Figure 4. Effect of IL-21 administration on SIV gag specific CD8 T cells.
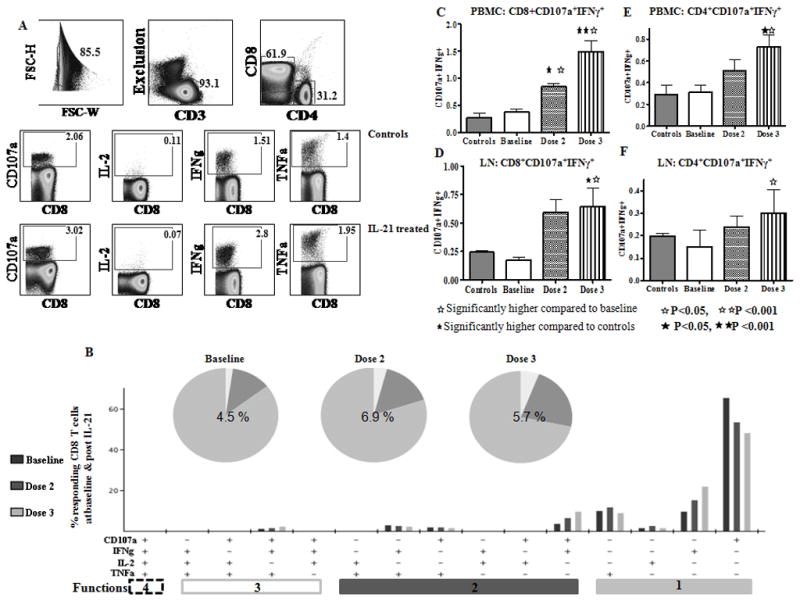
PBMC or LN cells were thawed and stimulated with SIV gag pool for 6 hrs in the presence of monensin and brefeldin A and CD107a antibody. SEB was used as positive control and medium alone as negative control. Cells were stained for CD3, CD4, CD8 along with ViViD, fixed, permeabilized and stained for intracellular IL-2, IFNγ and TNFα. CD4 and CD8 T cells were gated from live CD3+ cells and analyzed for the expression of different cytokines along with degranulation marker CD107a. (A), Representative figure showing SIV gag specific cytokine and CD107a in PB CD3+CD8 T cells in a control animal and in an IL-21 treated animal after the third dose. (B), Functional combinations in PB CD8 T cells were identified after Boolean gating. Pie chart represents 1, 2 and 3 functions and bar chart shows 15 possible functional combinations at baseline and after doses 2 and 3 of IL-21. Number in the center of each pie represents the mean percentage of cells responding to gag stimulation. (C–E), Bar charts showing mean CD107a+IFNγ+ (2 function+ cells) in (C), PBMC CD8 T cells; (D), IngLN CD8 T cells; (E), PBMC CD4 T cells and (F), IngLN CD4 T cells in control animals and in IL-21 treated animals at baseline and after dose 2 and dose 3 of IL-21.
IL-21 administration enhances cytotoxic molecules in NK cells
In addition to T cells, NK cells in PB also showed an increased numbers of GrB (Fig 5A) and perforin (Fig 5B) expressing cells as compared to baseline levels or control animals, with maximum increase after the third dose of IL-21. The observed increase in perforin and GrB in NK cells after IL-21 administration occurred in the absence of changes in numbers or activation state of NK cells (data not shown).
Figure 5. Effect of IL-21 administration on NK cells.
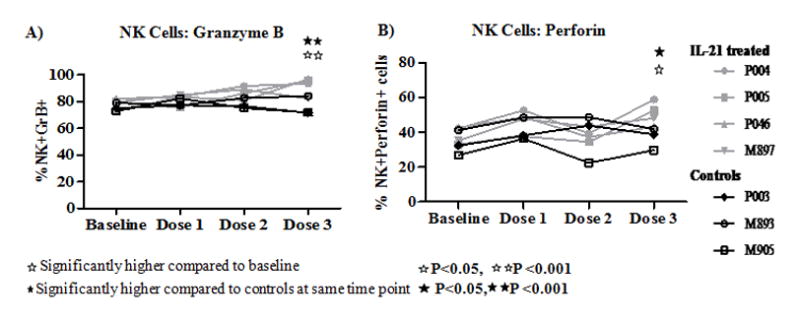
NK cells were identified in PBMC as CD3−CD8+NKG2A+ cells and analyzed for intracellular expression of (A), GrB and (B), perforin in NK cells of control (black) and in IL-21 treated (grey) animals.
IL-21 administration increases the frequencies of CD27+ memory B cells in SIV infected RM
Compared to baseline levels and control animals, a significant increase in numbers of memory B cells (CD20+CD27+) in PB and LN were noted after the 2nd and 3rd IL-21 doses. Moreover, both CD27+ memory (Fig 6B) and CD27 negative naïve B cells (Fig 6C) upregulated IL-21R expression after IL-21 administration. In addition to PB, peripheral LN also showed an increase in CD27+ memory B cells with increased expression of IL-21R (not shown).
Figure 6. Effect of IL-21 administration on B cells and levels of SIV antibodies in SIV infected RM.
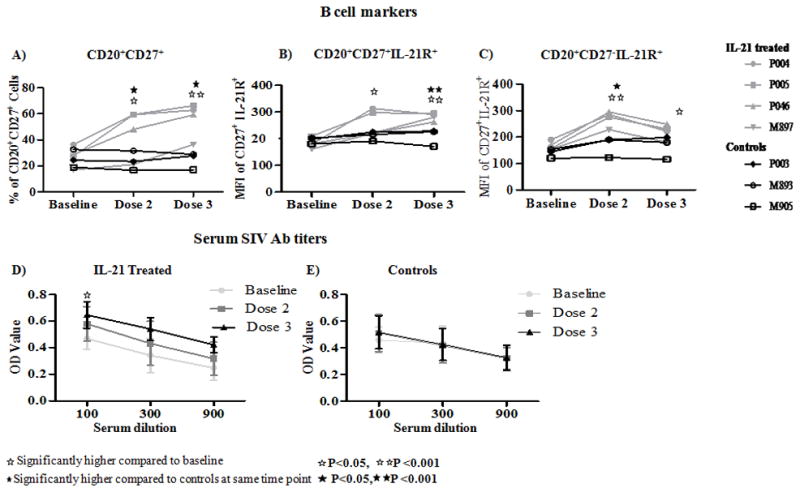
B cells were identified as CD3-CD20+ cells and analyzed for Line graph showing (A), frequency of CD20+ CD27+ as memory B cells and for MFI of IL-21R expression in (B), memory B cells and (C), naïve (CD20+CD27−) B cells in control (black) and in IL-21 treated (grey) animals at baseline and after dose 2 and dose 3. Mean absorbance value (OD) for SIV antibodies in (D), IL-21 treated animals and (E), control animals at different dilutions of plasma.
IL-21 administration led to increases in anti-SIV antibodies
Since IL-21 is known for enhancing B cell function [34], and since we had observed an increase in CD27+ memory B cells along with increase in frequency of IL-21R expression, we measured anti SIV antibodies in serum. Compared to baseline levels, significantly higher mean OD values for anti-SIV antibodies were observed after the 3rd dose of IL-21 (p=0.02, Fig 6D). Control animals did not show changes in the anti-SIV antibody levels (Fig 6E).
Discussion
Several cytokines have been investigated in HIV/SIV infection for potential use as immunotherapeutic modalities or as vaccine adjuvants [22, 23, 25, 35–40]. We are reporting the first administration of IL-21 in vivo in chronically SIV infected RM demonstrating safety and biologic activity of IL-21. Even at late stages of SIV infection, this cytokine was well tolerated up to the highest dose tested of 100 μg/kg body weight, and rapidly enhanced the expression of cytotoxic molecules perforin and GrB in T cells and NK cells within 72 hours of each dose. Additionally, increases in SIV antigen stimulated CD107+IFN-γ+ dual function T cells and of resting memory B cell pool were noted, with modest increase in anti-SIV antibody levels. Importantly, unlike IL-2, IL-7 and IL-15, members of the cytokine family that signal via the common γ-chain, IL-21 did not lead to measurable immune activation or enhanced plasma viremia, in the absence of ART. This activity of IL-21 in RM chronically infected with SIV confirms and extends prior observations of ex-vivo IL-21 activity [6, 7, 18, 19, 41] and underscores the need for further studies to evaluate the full range of IL-21 effects.
CD8 T cells and NK cells are believed to play an important role in control of viremia in HIV/SIV infection through the upregulation of cytotoxic molecules perforin and GrB [8–12]. We observed an increase in the GrB and perforin expression in CD8 T cells in PB and peripheral LN and mesLN after IL-21 administration in healthy and SIV infected animals. In addition, an increase in GrB and perforin was observed in NK cells of IL-21 treated animals. This property of IL-21 is appealing as qualitative defects in CTL have been attributed to reduced perforin in total and HIV specific CD8 T cells in patients who are on highly active antiretroviral therapy (HAART) [42–44]. The ability of ex-vivo treatment with rIL-21 to induce cytotoxic molecules GrB and perforin in CD8 T cells [18] and NK cells [19] of HIV infected persons was first documented by us and we recently extended these studies to show potential mechanisms of IL-21 activity and induction of antiviral activity in purified sorted T cells of healthy donors [45]. Several reports now ascribe an important role to this cytokine and to HIV-specific IL-21 producing CD4 T cells in virus control in HIV infected patients [6, 7, 41, 46, 47]. These findings parallel the role of IL-21 in controlling chronic LCMV infection by sustaining the antiviral effector functions of CD8 T cells and protecting them from undergoing cellular exhaustion [3–5].
In the studies reported here, the maximum induction of Perforin and GrB occurred in CD8 TEM and TCM subsets. Interestingly in our studies the effect of IL-21 on T cells was restricted to CD8 T cells in healthy RM but was more widespread in SIV infected animals, involving CD4 TEM and TCM T cells in PB and LN. Moreover, even a fraction of TN cells exhibited increased GrB expression, which could be a consequence of generalized immune activation, even though the same subsets generally failed to express perforin. These data are consistent with our prior studies showing that a state of inherent immune activation enhances the activity of IL-21 which by itself is unable to induce cellular activation [18, 45]. Of note though, perforin and GrB upregulation in T and NK cells required markedly more IL-21 in SIV infected monkeys that in uninfected controls, suggesting decreased capacity to respond or higher threshold needed following infection, similar to the observations with IL-15 therapy [25], suggesting a new form of immune impairment during chronic lentivirus infection. This lowered response potential may however not be uniform as B cell responses were observed after the initial IL-21 dose in SIV infected monkeys, although a clear dose response remains to be performed in the context of SIV infection. Polyfunctional T cells, considered to mediate antiviral activity were not detectable in these SIV infected animals before IL-21 injections and did not get significantly induced with IL-21. Instead, post IL-21, frequencies of dual positive SIV gag specific CD107a+IFN-γ+ T cells were increased; these properties, together with perforin and GrB induction could potentially lead to heightened T cell cytotoxic activity. Importantly, anti-IL-21 antibodies which could potentially mask the actual effect of IL-21were not detected in SIV infected RM (data not shown).
Despite the apparent augmentation in the frequency of T cells with cytotoxic molecules, plasma VL did not decrease; this was not unexpected as the animals had been infected for more than 6 months, had minimal polyfunctional T cells, and it is not known if they also had preexisting virus escape mutations making them non-responsive to CTL [48, 49]. A reduction in virus load in chronically infected animals with single cytokine treatment alone would be an unrealistic expectation, and no reduction in virus load has been previously noted following administration of common γ-chain cytokines, notably IL-7 [35, 37], IL-2 [23] and IL-15 [22, 23, 25]. Even IL-12 could not suppress VL in SIV infected RM [50, 51] despite clear increases in frequency of long-lived effector memory CD8 T cells [25]. This outcome may be the effect of translation of opposing forces, driving viral replication while improving control, or simply that the magnitude of the increase in antiviral response is insufficient for an effect to be detected. It has been reported that in HIV infection, disease progression does not solely depend on VL, and we did not follow the animals long enough to address this issue [52, 53].
IL-21 administation was associated with an increase in the frequencies of CD27+ memory B cells with upregulation of IL-21R on both CD27+ memory and CD27 negative naïve B cells. The role of IL-21 in promoting B cell differentiation and development of memory B cells is well established [34, 54–57] and it is known to be a critical regulator of B cell responses [34, 58]. It enhances T-dependent B cell proliferation, and promotes isotype switching and differentiation of B cells into plasma cells [34, 59, 60]. HIV and SIV infection lead to a state of intense B cell activation which alters frequency of the maturational B cell subsets due to expansion of transitional and activated B cells with reduction in memory B cells, which are important contributors to antibody responses [61, 62]. Improvement in the survival and function of immune cells including B cells by immunotherapeutic approaches are desirable objectives in control of HIV. The increase in anti-SIV antibodies in serum of IL-21 treated animals are suggestive of biologic activity on the B cell populations and are worthy of attention in future studies.
Despite the modest number of animals tested, the overall results of the present study in RM suggest that IL-21 can not only increase the cytotoxic property of T and NK cells but can also improve B cell survival and augment antibody responses. In a study by Bolesta et al [63], treatment of mice with an expression vector for either IL-21 alone or in combination with an IL-15 expression vector enhanced both cellular and humoral immune response to a HIV-1 Env DNA vaccine. Similar observations have been made when IL-21 has been co-expressed with DNA vaccines encoding glycoprotein B of herpes simplex virus (HSV)-1[64] or tuberculosis antigens [65]. In humans, the safety of IL-21 has been established in Phase II trials of patients with renal cell carcinoma and metastatic melanoma, with evidence for improved anti-tumor CD8 T cell responses in IL-21 treated patients [66–68]. In these studies IL-21 administration has resulted in increases in mRNA for IFN-γ, perforin, and GrB in CD8 T cells and NK cells [66, 67]. Together, the data support the need to further explore the properties of IL-21 in well controlled experimental models of HIV/SIV infection in early and chronic stages in conjunction with ART and also in SIV/HIV vaccine or immunotherapeutic strategies.
Supplementary Material
Highlights.
IL-21 administration to chronically SIV infected RM was safe and biologically active
IL-21 was well tolerated up to the highest tested dose of 100 μg/kg body weight
Rapid induction of perforin and GrB in T cells and NK cells post IL-21administration
Increases in memory B cell pool and modest increase in anti-SIV Ab in treated animals
Increases in SIV antigen stimulated CD107+IFN-γ+ T cells in treated animals
IL-21 administration did not lead to immune activation or enhanced plasma viremia
Acknowledgments
We thank the Laboratory Sciences Core of the Developmental Center for AIDS Research, University of Miami Miller School of Medicine for flow cytometry. We acknowledge the animal facilities at Advanced BioScience Laboratories, Inc and Yerkes Regional Primate Research Center for the study. This work was supported by NIH grant A1077501 (SP), R24RR016988 (FV) and RR00165 (Yerkes NPRC).
Abbreviations used in this paper
- ART
antiretroviral therapy
- rMamuIL-21
recombinant rhesus interleukin 21
- rIL-21
Recombinant IL-21 (rIL-21)
- LN
Lymph node
- LCMV
lymphocytic choriomeningitis virus
- TCM
central memory
- TEM
effector memory
- NASBA
nucleic acid sequence-based amplification
- mesLN
mesenteric lymph node
- IngLN
inguinal lymph node
- GrB
granzyme B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000 Nov 2;408(6808):57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 2.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 3.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009 Jun 19;324(5934):1569–72. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009 Jun 19;324(5934):1576–80. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 5.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009 Jun 19;324(5934):1572–6. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011 Jan;85(2):733–41. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010 Jul 1;185(1):498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 8.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6(5):e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008 Dec 19;29(6):1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 11.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009 Jan;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiemessen CT, Shalekoff S, Meddows-Taylor S, Schramm DB, Papathanasopoulos MA, Gray GE, et al. Natural killer cells that respond to human immunodeficiency virus type 1 (HIV-1) peptides are associated with control of HIV-1 infection. J Infect Dis. 2010 Nov 1;202(9):1444–53. doi: 10.1086/656535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenne DE, Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 14.Young JD, Hengartner H, Podack ER, Cohn ZA. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–59. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- 15.Harari A, Enders FB, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol. 2009 Apr;83(7):2862–71. doi: 10.1128/JVI.02528-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001 Mar 1;410(6824):106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 17.Mueller YM, Petrovas C, Do DH, Altork SR, Fischer-Smith T, Rappaport J, et al. Early establishment and antigen dependence of simian immunodeficiency virus-specific CD8+ T-cell defects. J Virol. 2007 Oct;81(20):10861–8. doi: 10.1128/JVI.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007 May 1;109(9):3873–80. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strbo N, de Armas L, Liu H, Kolber MA, Lichtenheld M, Pahwa S. IL-21 augments natural killer effector functions in chronically HIV-infected individuals. AIDS. 2008 Aug 20;22(13):1551–60. doi: 10.1097/QAD.0b013e3283089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000 Oct;6(10):1140–6. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 21.Nacsa J, Edghill-Smith Y, Tsai WP, Venzon D, Tryniszewska E, Hryniewicz A, et al. Contrasting effects of low-dose IL-2 on vaccine-boosted simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cells in macaques chronically infected with SIVmac251. J Immunol. 2005 Feb 15;174(4):1913–21. doi: 10.4049/jimmunol.174.4.1913. [DOI] [PubMed] [Google Scholar]
- 22.Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005 Apr;79(8):4877–85. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004 Sep 3;22(25–26):3510–21. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Leone A, Rohankhedkar M, Okoye A, Legasse A, Axthelm MK, Villinger F, et al. Increased CD4+ T cell levels during IL-7 administration of antiretroviral therapy-treated simian immunodeficiency virus-positive macaques are not dependent on strong proliferative responses. J Immunol. 2010 Aug 1;185(3):1650–9. doi: 10.4049/jimmunol.0902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006 Jun;116(6):1514–24. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano JW, Williams KG, Shurtliff RN, Ginocchio C, Kaplan M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol Invest. 1997 Jan-Feb;26(1–2):15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- 27.Hryniewicz A, Price DA, Moniuszko M, Boasso A, Edghill-Spano Y, West SM, et al. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J Immunol. 2007 Mar 15;178(6):3492–504. doi: 10.4049/jimmunol.178.6.3492. [DOI] [PubMed] [Google Scholar]
- 28.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006 Sep 1;69(9):1037–42. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 29.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1(3):1522–30. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- 30.Zahn RC, Rett MD, Korioth-Schmitz B, Sun Y, Buzby AP, Goldstein S, et al. Simian immunodeficiency virus (SIV)-specific CD8+ T-cell responses in vervet African green monkeys chronically infected with SIVagm. J Virol. 2008 Dec;82(23):11577–88. doi: 10.1128/JVI.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuber B, Quigley MF, Critchfield JW, Shacklett BL, Abel K, Miller CJ, et al. Detection of macaque perforin expression and release by flow cytometry, immunohistochemistry, ELISA, and ELISpot. J Immunol Methods. 2006 May 30;312(1–2):45–53. doi: 10.1016/j.jim.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Kuhrt D, Faith SA, Leone A, Rohankedkar M, Sodora DL, Picker LJ, et al. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: rapid depletion of naive and memory B-cell subsets with delayed reconstitution of the naive B-cell population. J Virol. 2010 Mar;84(5):2466–76. doi: 10.1128/JVI.01966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson JE, Holton D, Liu J, McMurdo H, Murciano A, Gohd R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Methods. 1990 Aug 28;132(1):63–71. doi: 10.1016/0022-1759(90)90399-g. [DOI] [PubMed] [Google Scholar]
- 34.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005 Dec 15;175(12):7867–79. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 35.Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006 Jan 15;176(2):914–22. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 36.Craiu A, Barouch DH, Zheng XX, Kuroda MJ, Schmitz JE, Lifton MA, et al. An IL-2/Ig fusion protein influences CD4+ T lymphocytes in naive and simian immunodeficiency virus-infected Rhesus monkeys. AIDS Res Hum Retroviruses. 2001 Jul 1;17(10):873–86. doi: 10.1089/088922201750290005. [DOI] [PubMed] [Google Scholar]
- 37.Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003 Mar 15;101(6):2294–9. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 38.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009 Apr;119(4):997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moniuszko M, Edghill-Smith Y, Venzon D, Stevceva L, Nacsa J, Tryniszewska E, et al. Decreased number of CD4+ and CD8+ T cells that express the interleukin-7 receptor in blood and tissues of SIV-infected macaques. Virology. 2006 Dec 5–20;356(1–2):188–97. doi: 10.1016/j.virol.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009 Jun 18;113(25):6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, et al. Interleukin-21-Producing HIV-1-Specific CD8 T Cells Are Preferentially Seen in Elite Controllers. J Virol. 2011 Mar;85(5):2316–24. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson J, Kinloch S, Sonnerborg A, Nilsson J, Fehniger TE, Spetz AL, et al. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J Infect Dis. 2002 May 1;185(9):1355–8. doi: 10.1086/340124. [DOI] [PubMed] [Google Scholar]
- 43.Jansen CA, Piriou E, Bronke C, Vingerhoed J, Kostense S, van Baarle D, et al. Characterization of virus-specific CD8(+) effector T cells in the course of HIV-1 infection: longitudinal analyses in slow and rapid progressors. Clin Immunol. 2004 Dec;113(3):299–309. doi: 10.1016/j.clim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Shacklett BL, Cox CA, Quigley MF, Kreis C, Stollman NH, Jacobson MA, et al. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J Immunol. 2004 Jul 1;173(1):641–8. doi: 10.4049/jimmunol.173.1.641. [DOI] [PubMed] [Google Scholar]
- 45.Parmigiani A, Pallin MF, Schmidtmayerova H, Lichtenheld MG, Pahwa S. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Hum Immunol. 2011 Oct 25; doi: 10.1016/j.humimm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010 Jan 1;184(1):114–26. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 47.Iannello A, Boulassel MR, Samarani S, Tremblay C, Toma E, Routy JP, et al. IL-21 enhances NK cell functions and survival in healthy and HIV-infected patients with minimal stimulation of viral replication. J Leukoc Biol. 2010 May;87(5):857–67. doi: 10.1189/jlb.1009701. [DOI] [PubMed] [Google Scholar]
- 48.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004 Aug;4(8):630–40. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 49.Mandl JN, Regoes RR, Garber DA, Feinberg MB. Estimating the effectiveness of simian immunodeficiency virus-specific CD8+ T cells from the dynamics of viral immune escape. J Virol. 2007 Nov;81(21):11982–91. doi: 10.1128/JVI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villinger F, Bucur S, Chikkala NF, Brar SS, Bostik P, Mayne AE, et al. In vitro and in vivo responses to interleukin 12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res Hum Retroviruses. 2000 May 20;16(8):751–63. doi: 10.1089/088922200308756. [DOI] [PubMed] [Google Scholar]
- 51.Ansari AA, Mayne AE, Sundstrom JB, Bostik P, Grimm B, Altman JD, et al. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002 Feb;76(4):1731–43. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008 Jan 1;197(1):126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madec Y, Boufassa F, Porter K, Meyer L. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005 Nov 18;19(17):2001–7. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 54.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008 Aug 1;181(3):1767–79. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 55.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004 Nov 1;173(9):5361–71. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 56.Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004 May 1;172(9):5154–7. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 57.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010 Jan 18;207(1):155–71. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007 Mar 1;178(5):2872–82. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 59.Borte S, Pan-Hammarstrom Q, Liu C, Sack U, Borte M, Wagner U, et al. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood. 2009 Nov 5;114(19):4089–98. doi: 10.1182/blood-2009-02-207423. [DOI] [PubMed] [Google Scholar]
- 60.Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009 Feb 15;182(4):1781–7. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- 61.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009 Apr;9(4):235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 Aug 4;205(8):1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, et al. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. J Immunol. 2006 Jul 1;177(1):177–91. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui FD, Asada H, Jin ML, Kishida T, Shin-Ya M, Nakaya T, et al. Cytokine genetic adjuvant facilitates prophylactic intravascular DNA vaccine against acute and latent herpes simplex virus infection in mice. Gene Ther. 2005 Jan;12(2):160–8. doi: 10.1038/sj.gt.3302393. [DOI] [PubMed] [Google Scholar]
- 65.Dou J, Tang Q, Zhao F, Chu L, Chen J, Cao M, et al. Comparison of immune responses induced in mice by vaccination with DNA vaccine constructs expressing mycobacterial antigen 85A and interleukin-21 and Bacillus Galmette-Guerin. Immunol Invest. 2008;37(2):113–27. doi: 10.1080/08820130701690741. [DOI] [PubMed] [Google Scholar]
- 66.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009 Mar 15;15(6):2123–9. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 67.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008 Oct;57(10):1439–49. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008 Apr 20;26(12):2034–9. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


