Abstract
Interface tissue engineering is a promising new strategy aimed at the regeneration of tissue interfaces and ultimately enabling the biological fixation of soft tissue grafts utilized in orthopaedic repair and sports medicine. Many ligaments and tendons with direct insertions into subchondral bone exhibit a complex enthesis consisting of several distinct yet continuous regions of soft tissue, noncalcified fibrocartilage, calcified fibrocartilage and bone. Regeneration of this multi-tissue interface will be critical for functional graft integration and improving long term clinical outcome. This review will highlight current knowledge of the structure-function relationship at the interface, the mechanism of interface regeneration, and the strategic biomimicry implemented in stratified scaffold design for interface tissue engineering and multi-tissue regeneration. Potential challenges and future directions in this emerging field will also be discussed. It is anticipated that interface tissue engineering will lead to the design of a new generation of integrative fixation devices for soft tissue repair, and it will be instrumental for the development of integrated musculoskeletal tissue systems with biomimetic complexity and functionality.
Keywords: Interface tissue engineering, Enthesis, Anterior cruciate ligament, Rotator cuff, Scaffold, Co-culture, Tri-culture
I. INTRODUCTION
A significant challenge in orthopedic reconstruction surgery resides in achieving extended functional integration of soft tissue grafts with subchondral bone. The biological fixation of these grafts is particularly critical in the repair of injuries to ligaments and tendons, as integration between soft and hard tissues is essential for musculoskeletal motion. Many soft tissues, such as the anterior cruciate ligament (ACL) or the supraspinatus tendon, exhibit direct insertions into subchondral bone through a complex enthesis consisting of three distinct yet continuous regions of soft tissue, fibrocartilage, and bone1–3. The fibrocartilage region is further divided into calcified and uncalcified zones. This multi-tissue organization serves several purposes, from mediating load transfer between two distinct types of tissue2,4 to minimizing the formation of stress concentrations2,5,6, and to supporting the heterotypic cellular communication necessary for interface function and homeostasis7. The insertion site is, however, prone to injury, and mechanical fixation of current ligament or tendon reconstruction grafts often fail to preserve or re-establish an anatomic soft tissue-to-bone enthesis post surgery. Absence of this critical interface has been reported to compromise graft stability and long term clinical outcome8–11. Consequently, there exists a significant need for integrative graft fixation systems which can promote interface regeneration and facilitate functional graft-to-bone integration.
In the past decade, tissue engineering12,13 has emerged as a promising approach to musculoskeletal tissue repair and regeneration. Utilizing a combination of cells, growth factors and/or biomaterials, tremendous advances have been made whereby bone-14–18, cartilage-19–23, tendon-,24–28 and ligament-like29–34 tissues have been engineered in vitro and in vivo. Design methodologies developed from these efforts can be readily applied to regenerate the enthesis between soft tissue and bone through interface tissue engineering. Focusing on the anterior cruciate ligament (ACL)-to-bone insertion site, this review highlights recent work in interface tissue engineering, aimed at promoting the biological fixation of grafts utilized for ACL reconstruction surgery. Current knowledge of the mechanism of interface regeneration, elucidation of the structure and function relationship inherent at the ligament-to-bone insertion, as well as implementation of strategic biomimicry in stratified scaffold design for interface regeneration will be discussed. Extension of these interface tissue engineering strategies to rotator cuff repair will also be highlighted. Finally, potential challenges and future directions in this emerging field will be considered. It is emphasized that biological fixation through interface tissue engineering will be instrumental in the development of a new generation of integrative fixation devices, as well as the design of complex musculoskeletal tissue systems which integrate seamlessly with the body.
II. DESIGN CONSIDERATIONS IN ACL-BONE INTERFACE TISSUE ENGINEERING
Ligaments or tendons insert into bone through either direct or indirect entheses, with the latter characterized by soft tissue attachment to the periosteum and Sharpey’s fibers traversing directly from the soft tissue to bone4. In contrast, direct insertions, exhibited by the ACL or supraspinatus tendon, are much more complex, transiting from soft tissue to bone through a characteristic fibrocartilage interface, which is further divided into non-mineralized and mineralized regions1–3,35–41. The ACL-to-bone junction exhibits controlled spatial variations in cell type and matrix composition (Fig. 1), with the ligament proper comprised of fibroblasts embedded in a type I and type III collagen matrix. The non-mineralized fibrocartilage matrix consists of ovoid chondrocytes, and types I and II collagen are present within a proteoglycan-rich matrix. In the mineralized fibrocartilage zone, hypertrophic chondrocytes are surrounded by a calcified matrix containing type X collagen40,42. The last region is the subchondral bone, within which osteoblasts, osteocytes and osteoclasts reside in a mineralized type I collagen matrix. This controlled matrix heterogeneity observed at the interface reduces the accumulation of stress concentrations and facilitates the transfer of complex loads between soft and hard tissues2,6,43.
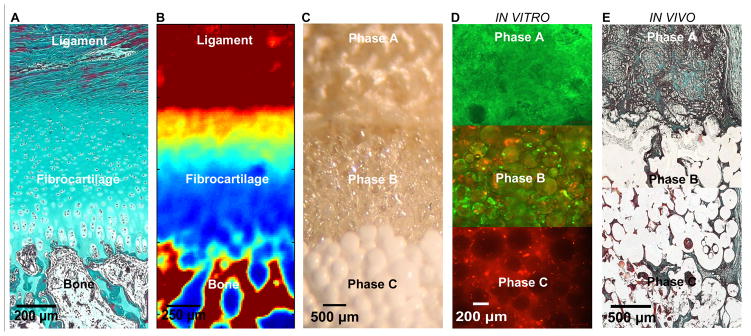
Figure 1. Biomimetic Scaffold Design and Evaluation for Orthopaedic Interface Tissue Engineering.
A) The native ACL-bone interface exhibits distinct yet continuous tissue regions, including ligament, fibrocartilage, and bone. (Neonatal Bovine, Modified Goldner Masson Trichrome Stain, bar = 200 μm).
B) Fourier Transform Infrared Spectroscopic Imaging or (FTIR-I) revealed that relative collagen content is the highest in the ligament and bone regions, with a decrease in collagen across the fibrocartilage interface from ligament to bone (neonatal bovine, bar=250 μm, with blue to red representing low to high collagen content, respectively).
C) A tri-phasic stratified scaffold has been designed to mimic the three distinct yet continuous interface regions (bar=500 μm).
D) In vitro co-culture of fibroblasts and osteoblasts on the tri-phasic scaffold resulted in phase-specific cell distribution and the formation of controlled matrix heterogeneity. Fibroblasts (Calcein AM, green) were localized in Phase A and osteoblasts (CM-DiI, red) in Phase C over time. Both osteoblasts and fibroblasts migrated into Phase B by day 28 (bar = 200 μm).
E) In vivo evaluation of the tri-phasic scaffold tri-cultured with Fibroblasts (Phase A), Chondrocytes (Phase B), and Osteoblasts (Phase C) revealed abundant host tissue infiltration and matrix production (week 4, Modified Goldner Masson Trichrome Stain, bar = 500 μm).
The ACL is also the most frequently injured knee ligament44, with 200,000 injuries and approximately 100,000 reconstruction procedures reported annually in the United States alone45,46. The long-term performance of ACL grafts depends the structural and material properties of the graft, initial graft tension47–51, the intra-articular position of the graft52,53, and graft fixation9,10. Increased emphasis has been placed on graft fixation since post-surgical rehabilitation regimens require the immediate ability to regain the full range of motion, reestablish neuromuscular function, and bear weight11,54. Autologous hamstring or allografts are increasingly utilized for ACL reconstruction due to donor site morbidity associated with bone-patellar tendon-bone grafts (BPTB) 55,56. The BPTB graft has been the gold standard, in part because of its ability to integrate with subchondral bone via its bony ends. Moreover, it possesses intact insertion sites or entheses that can serve as functional transitions between soft tissue and bone. In contrast, the tendinous grafts must be fixed mechanically within the bone tunnel. Although the physiological range of motion may be possible via mechanical fixation, graft-to-bone integration is not achieved as the native insertion site is lost during surgery, with nonmineralized soft tissue found instead within the bone tunnels9,11,57. Thus graft fixation at the tibial and femoral tunnels, instead of the isolated strength of the graft, represents the weakest point during the early postoperative healing period9,10,58. Despite improvement in fixation with interference screws, the clinical outcomes of ACL reconstructions with hamstring tendon grafts have continued to be afflicted with greater laxity and failure rates compared to BPTB reconstructions59–66. In the absence of an anatomical interface, the graft-bone junction exhibits poor mechanical stability9,10,58 which remains one of the primary causes of graft failure8–10,67,68.
Based on the intricate multi-tissue organization observed at the soft tissue-to-bone junction, it is likely that interface formation will require multiple types of cells, a multi-phased scaffold system which supports interactions between these different cell populations, and the development of distinct yet continuous multi-tissue regions mimicking that of the native insertion through physical and biochemical stimuli. Moreover, the success of any interface tissue engineering effort will first require an in-depth understanding of the structure-function relationship at the native insertion in order to identify interface-relevant design parameters. In addition, the mechanism governing interface regeneration must be determined, especially regarding the role of heterotypic cellular interactions in interface repair and homeostasis. Multi-scale co-culture or tri-culture models may be used to decipher the relative contribution of homotypic and heterotypic cellular communication in multi-tissue regeneration. This knowledge will enable the design of stratified scaffolds optimized for supporting heterotypic cellular interactions, as well as promote the development of controlled matrix heterogeneity which is essential for interface tissue engineering. Recent advances in each of the above three critical areas in interface tissue engineering will be highlighted in the following sections.
III. STRUCTURE-FUNCTION RELATIONSHIP AT THE LIGAMENT-TO-BONE INTERFACE
From a structure-function perspective, the complex multi-tissue organization and heterogeneity in matrix composition at the interface are likely related to the nature and distribution of the mechanical stress experienced at the ligament-bone junction. It has been reported that matrix organization at soft tissue-to-bone transition is optimized to sustain both tensile and compressive stresses4,69,70. Recently, using ultrasound elastography71, Spalazzi et al. mapped the strain distribution at the ACL-to-bone interface72. As shown in Figure 2A, elastography analyses revealed that when the joint is loaded in tension, the deformation across the insertion site is region-dependent, with the highest displacement observed at the ACL, followed by a decrease from the fibrocartilage interface to bone. These regional differences suggest an increase in tissue stiffness from ligament to bone. In addition, both tensile and compressive strain components were detected at the insertion while the knee was loaded in tension.
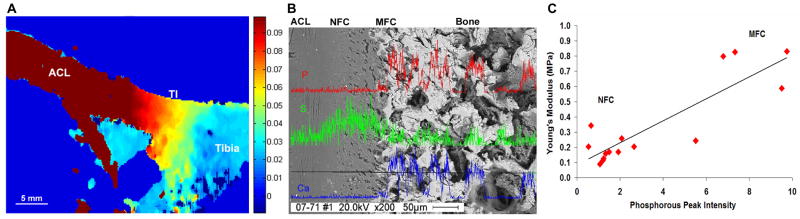
Figure 2. Structure-Function Relationship at the Ligament-to-Bone Insertion Site.
A) Elastographic analysis of the tibial ACL-to-Bone insertion (TI) under applied uniaxial tension. Displacement map calculated from ultrasound radiofrequency data (increase in magnitude: blue to red, bar = 5 mm). A region-dependent decrease in displacement is related to increase in tissue stiffness from the ligament to fibrocartilage interface and then to bone.
B) Energy Dispersive X-ray Analysis (EDAX) across the ACL-to-Bone insertion revealed region-dependent changes in mineral content from the non-mineralized (NFC) to the mineralized fibrocartilage region (MFC), and to bone. Calcium (Ca, blue) and phosphorous (P, red) peaks are detected only within the MFC and bone regions; whereas the sulfur (S, green) peak intensity diminished from the NFC to the MFC region (200x, scale = 50 μm).
C) Correlation of Young’s modulus and phosphorous peak intensity for the NFC and MFC regions of the ACL-to-Bone insertion site. An increase in Young’s modulus strongly correlates (R = 0.868) with higher phosphourous peak intensity, suggesting a structure-function relationship between insertion site mechanical properties and mineral distribution.
Direct measurement of interface mechanical properties has been difficult due to the complexity and the relative small scale of the interface, in general ranging from 100 μm to 1 mm in length1,3,43,73. Thus existing knowledge of insertion material properties has been largely derived from theoretical models41,69. Recently, Moffat et al. performed the first experimental determination of the compressive mechanical properties of the ACL-to-bone interface6. Specifically, the incremental displacement field of the fibrocartilage tissue under the applied uniaxial strain was evaluated by coupling microcompression with optimized digital image correlation (DIC) analysis of the pre- and post-loading images74. Similar to the elastography findings72, deformation decreased gradually from the fibrocartilage interface to bone. Moreover, these region-dependent changes were accompanied by a gradual increase in compressive modulus. The interface also exhibited a region-dependent decrease in strain, with a significantly higher elastic modulus found in the mineralized fibrocartilage when compared to the nonmineralized region6. In the neonatal bovine model, the compressive modulus of the non-mineralized fibrocartilage region is 0.32 ± 0.14 MPa6, representing less than 50% of the mineralized fibrocartilage modulus (0.68 ± 0.39 MPa)6. Both of these values are lower than that of trabecular bone which is reported to be 173 ± 97 MPa in the same model75. These interface region-specific mechanical properties enable a gradual transition rather than an abrupt increase in tissue strain across the insertion, and provide valuable cues for interface scaffold design.
Given the structure-function dependence inherent in the biological system, the regional changes in mechanical properties reported by Moffat et al.6 are likely correlated to differences in matrix organization and composition across the interface. Partition of the fibrocartilage interface into non-mineralized and mineralized regions is anticipated to have a functional significance, as increases in matrix mineral content have been associated with higher mechanical properties in connective tissues76–78. Evaluation of the insertion site using Fourier Transform Infrared Imaging (FTIR-I, Fig. 1B)79 and X-ray analysis6 both revealed an increase in calcium and phosphorous content progressing from ligament, interface, and then to bone (Fig 2B). An abrupt transition, instead of a gradient of mineral distribution, was detected progressing from the non-mineralized to the mineralized interface regions. Similar to other connective tissues35, the increase in elastic modulus progressing from the non-mineralized to the mineralized fibrocartilage interface region was shown to be positively correlated6 with the presence of calcium phosphate (Fig. 2C).
The aforementioned elucidation of the structure-function relationship inherent at the ligament-to-bone insertion has yielded invaluable clues for the design of biomimetic scaffolds for regenerating this complex multi-tissue interface. The intricate multi-tissue organization and controlled matrix heterogeneity observed at the ACL-to-bone junction suggest that interface scaffold design must consider the need to regenerate more than one type of tissue, as well as exercising spatial control over the respective cell populations indigenous to the ACL-to-bone interface regions. Additionally, a gradual increase in mechanical properties across the scaffold phases is needed in order to prevent the formation of stress concentrations. This may be achieved by regulating the distribution and concentration of calcium phosphate on the scaffold phases.
IV. ROLE OF CELLULAR INTERACTIONS IN THE MECHANISM OF INTERFACE REGENERATION
As described above, the native ACL-to-bone insertion consists of a linear progression of three distinct matrix regions: ligament, fibrocartilage, and bone, with each region exhibiting a characteristic cellular phenotype and matrix composition. It is likely that communication amongst the three resident cell populations, namely fibroblasts, fibrochondrocytes and osteoblasts, are important for interface homeostasis and regeneration. The insertion fibrochondrocyte phenotype is not well defined as fibrocartilaginous tissues differ in composition and structure depending on the anatomic site23,80. Sun et al.81 compared the response of fibrochondrocytes isolated directly from the ACL-to-bone insertion to those of inner- and outerring meniscal fibrochondrocytes, as well as ligament fibroblasts and articular chondrocytes. It was found that the greatest increase in proteoglycan synthesis was detected in insertion fibrochondrocytes and articular chondrocytes. In addition, the fibrochondrocytes produced a matrix containing both type I and type II collagen. Cell alkaline phosphatase activity peaked at one week for the insertion fibrochondrocytes and was significantly higher compared to that of articular chondrocytes or meniscal fibrochondrocytes. Aside from its mineralization potential, these findings suggest that the ACL insertion fibrochondrocytes appear to be similar to articular chondrocytes, while differing significantly from the meniscal fibrochondrocytes and ligament fibroblasts.
Currently, the mechanism of interface regeneration is not known. A fundamental question in interface tissue engineering is how distinct boundaries between different types of connective tissues are re-established post-injury. When Fujioka et al. sutured the Achilles tendon to its original attachment site, cellular organization resembling that of the native insertion and the deposition of collagen type X were observed in vivo82. It is also well established that while tendon-to-bone healing following ACL reconstruction does not lead to the re-establishment of the native insertion, a layer of fibrocartilage-like tissue is formed within the bone tunnel11,57,83. These observations collectively suggest that when trauma or injury to the interface results in non-physiologic exposure of normally segregated tissue types (e.g., bone, ligament or tendon), interactions between the resident cell populations in these tissues (osteoblast-fibroblast) are likely critical for initiating and directing the repair response that lead to the re-establishment of a fibrocartilage interface between soft tissue and bone. In vivo cell-tracking studies have also revealed that the tendon graft is usually invaded by host cells within one week of implantation84, indicating that cell types other than the osteoblasts and fibroblasts populating the graft-bone junction may be involved in fibrocartilage regeneration. Based on these observations, Lu and Jiang7 proposed a working hypothesis for interface regeneration, suggesting that osteoblast-fibroblast interactions mediate interface regeneration through heterotypic cellular interactions that can lead to phenotypic changes or trans-differentiation of osteoblasts and/or fibroblasts. In addition, these interactions can promote the differentiation of stem cells or progenitor cells into fibrochondrocytes and promote the regeneration of the fibrocartilage interface.
Several in vitro studies evaluating the role of heterotypic cellular interactions on interface regeneration have been reported85,86. Co-culture and tri-culture models of interface-relevant cell populations were used to determine the effects of cellular communication on the development of fibrocartilage-specific markers in vitro. Wang et al. examined the interaction between osteoblasts and ligament fibroblasts85, whereby a 2-D co-culture model permitting both cell physical contact and soluble factor interactions was designed to emulate the in vivo condition in which the tendon graft is in direct contact with bone tissue following ACL reconstruction (Fig. 3A, inset). Osteoblasts and fibroblasts were first separated by a hydrogel divider, and upon reaching confluence, the divider was removed, allowing the osteoblasts and fibroblasts to migrate and interact directly within the interface region (Fig. 3A). It was reported that these controlled interactions decreased cell proliferation (Fig. 3B), altered the alkaline phosphatase (ALP) activity profile, and promoted the expression of matrix proteins characteristic of the fibrocartilage interface, such as types I and II collagen, and cartilage oligomeric matrix protein (COMP). Subsequent conditioned media studies have revealed that both autocrine and paracrine factors were responsible for the changes in phenotype observed during osteoblast-fibroblast co-culture87. Although it is unknown which or if any of the two cell populations are directly responsible for interface regeneration, these observations suggest that osteoblast-fibroblast interactions are key modulators of cell phenotype at the graft-to-bone junction. These cellular interactions will certainly have a down-stream effect, either in terms of inducing trans-differentiation into fibrochondrocytes or in the recruitment and differentiation of progenitor or stem cells for fibrocartilage formation.
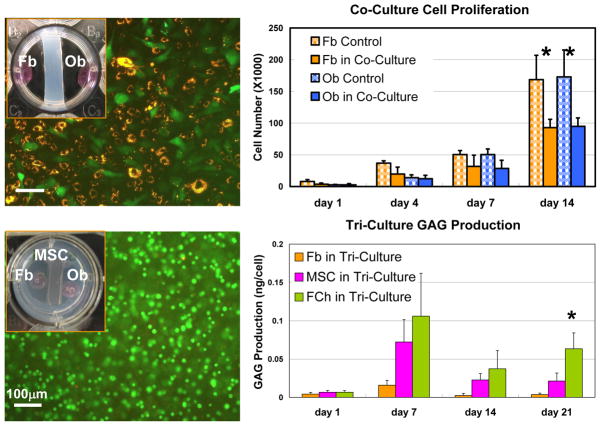
Figure 3. Co-Culture and Tri-Culture Models for Evaluating the Interaction between Interface-Relevant Cell Populations.
A) In vitro co-culture model of fibroblasts (Fb) and osteoblasts (Ob) (inset) and the cellular interactions between Fb (CM-DiI) and Ob (CFDA-SE) in co-culture (bar = 100 μm).
B) Co-culture modulated the proliferation of fibroblasts and osteoblasts (*p<0.05).
C) In vitro tri-culture model (inset) to evaluate the effects of osteoblast-fibroblast interactions on the fibrochondrogenic differentiation of bone marrow-derived mesenchymal stem cells (MSC) as well as ligament fibroblasts (Fb). Insertion fibrochondrocytes (FCh) served as the positive control. (Live-dead stain, MSC in hydrogel, day 40, bar = 100 μm).
D) Co-culture modulated glycosaminoglycan (GAG) production by interface relevant cells (Fb, MSC, FCh) (*p<0.05).
While osteoblast-fibroblast interactions resulted in phenotypic changes and the expression of interface-relevant markers in co-culture, a fibrocartilage-like interface was not formed in vitro. Moreover, when Lim et al.88 coated tendon grafts with mesenchymal stem cells embedded in a fibrin gel, the formation of a zone of cartilaginous tissue between graft and bone was observed, suggesting a potential role for stem cells in fibrocartilage formation. Thus other cell types such as fibrochondrocyte precursors or stem cells may be involved in interface regeneration, and it is likely that osteoblast-fibroblast interactions may direct the fibrochondrogenic differentiation of these cells. In addition, the insertion site is derived from the ligament during development38,39,89,90, and dermal fibroblasts as well as cells residing in tendon or ligament have been shown to exhibit fibrochondrocyte- or chondrocyte-like phenotype under controlled conditions91–94. Building on the 2-D co-culture model, Wang et al. designed a tri-culture system (Fig. 3C, inset) of fibroblasts, osteoblasts and interface-relevant cell populations such as fibroblasts and bone marrow-derived mesenchymal stem cells (MSC)95. The response of MSC or fibroblasts in tri-culture was compared to those of ACL-to-bone insertion fibrochondrocytes or articular chondrocytes cultured under similar conditions. In tri-culture, fibroblasts and osteoblasts were each seeded on cover-slips on the opposite sides of the well, with either fibroblasts or MSC pre-loaded into the hydrogel insert. In addition to being able to assess the response of individual cell types in tri-culture, another advantage of this model system is that physiologically relevant 3-D instead of monolayer culture can be maintained at the interface region (Fig. 3C).
Under the influence of osteoblast-fibroblast interactions, it was found that cell number for the MSC, fibrochondrocyte and articular chondrocyte groups remained relatively constant, while ligament fibroblasts proliferated readily in tri-culture. Unlike fibroblasts, MSC in tri-culture exhibit a level of alkaline phosphatase activity similar to that of insertion fibrochondrocytes, with both groups peaking by day 7 and decreasing thereafter. In addition, while minimal proteoglycan deposition was seen in the fibroblast group, MSC measured significantly higher proteoglycan synthesis in tri-culture, although the level of response was below that of insertion fibrochondrocytes (Fig. 3D). Moreover, under stimulation by osteoblast-fibroblast interactions, both insertion fibrochondrocytes and MSC produced a type II collagen-containing matrix, while no such matrix was observed for fibroblasts following tri-culture.
The multi-scale co-culture and tri-culture models described above are simple and elegant systems that can be used to systematically investigate the mechanisms governing interface regeneration. Findings from the reported in vitro studies of heterotypic cellular interactions provide preliminary validation of the hypothesis that osteoblast-fibroblast interactions play a regulatory role in the induction of interface-specific markers in progenitor or stem cells, and demonstrate the effects of heterotypic cellular interactions in regulating the maintenance of soft tissue-to-bone junctions. While the mechanisms of interaction and the nature of the regulatory cytokines secreted remain elusive, cell communication is likely to be significant for interface regeneration as well as homeostasis. Therefore the optimal interface scaffold must promote interactions between the relevant cell populations residing in each interface region.
V. STRATIFIED SCAFFOLD FOR LIGAMENT-BONE INTERFACE TISSUE ENGINEERING
Investigations of the interface structure-function relationships as well as the role of cellular interactions in interface regeneration have provided invaluable insight into biomimetic scaffold design for orthopaedic interface tissue engineering. The multi-tissue transition (ligament, fibrocartilage, bone) represents a significant challenge as several distinct yet contiguous tissue regions constitute the complex insertion site. A stratified scaffold design will therefore be essential for recapturing the aforementioned complexity of the native ligament-to-bone interface. The ideal scaffold for interface tissue engineering, in addition to supporting the growth and differentiation of relevant cell populations, must also direct heterotypic and homotypic cellular interactions while promoting the formation and maintenance of controlled matrix heterogeneity. Consequently, the scaffold should exhibit a gradient of structural and mechanical properties mimicking those of the native insertion site. Compared to a homogenous structure, a scaffold with pre-designed, tissue-specific matrix inhomogeneity can better sustain and transmit the distribution of complex loads inherent at the ACL-to-bone interface. It is emphasized that while the scaffold is stratified or consisted of different phases, a key criteria is that these phases must be interconnected and pre-integrated with each other, thereby supporting the formation of distinct yet continuous multi-tissue regions. The interface scaffold must also possess mechanical properties comparable to those of the ligament-to-bone interface. In addition, the scaffold phases should be biodegradable so it is gradually replaced by living tissue, although its degradation must be controlled in order to sustain physiological loading and promote neo-interface function. Finally, for in vivo graft integration, the interface scaffold must be easily adaptable with current ACL reconstruction grafts, or pre-incorporated into the design of ligament replacement grafts.
Traditional efforts in synthetic or tissue engineered alternatives for ACL reconstruction have focused on regenerating the ligament proper30,32,96. Recently, a more complex design of a synthetic ACL graft with a ligament proper as well as two bony regions33,34 was fabricated from 3-D braiding of polylactide-co-glycolide (PLGA) fibers, with the extended goal of promoting ACL graft integration within the bone tunnels. In vitro34 and in vivo97 evaluations demonstrated biocompatibility, healing and extended mechanical strength in a rabbit model, While the strength of the ligament region is necessary for the success of the ACL graft, establishment of a stable graft-to-bone interface will also be critical for the long term functionality of the tissue engineered graft. Recently, Spalazzi et al. reported on the design and evaluation of a tri-phasic scaffold (Fig. 1C) for the regeneration of the ACL-to-bone interface98,99. Modeled after the multi-tissue native insertion site, the scaffold consists of three distinct yet continuous phases, each pre-engineered for a particular interface cell population and tissue region: Phase A is designed with PLGA (10:90) mesh for fibroblast culture and soft tissue formation, Phase B consists of PLGA (85:15) microspheres and is the interface region intended for fibrochondrocyte culture, and Phase C is comprised of sintered PLGA (85:15) and 45S5 bioactive glass composite microspheres18 for osteoblast culture and bone formation. It is noted that the innovative stratified scaffold design and fabrication method resulted in essence in a “single” scaffold system with three distinct yet continuous phases, intended to support to the formation of the multi-tissue regions observed across the ACL-bone junction.
Interactions between interface relevant cell populations (e.g. fibroblasts, chondrocytes, osteoblasts) on the tri-phasic scaffold have been evaluated both in vitro and in vivo98–100. For co-culture, human ligament fibroblasts and osteoblasts were seeded onto Phase A and Phase C, respectively98, while Phase B was left unseeded. The migration of both cell types into Phase B was monitored over time. It was observed that fibroblasts and osteoblasts were localized primarily at opposite ends of the scaffolds post-seeding, with very few cells found in Phase B. After four weeks, each cell type proliferated within their respective phases as well as migrated into Phase B. The stratified scaffold design promoted phase-specific cell distribution with osteoblasts and fibroblasts localized in their respective regions, while their interaction was restricted to Phase B, the interface region (Fig. 1D). Spatial control over cell distribution also resulted in the elaboration of cell type-specific matrix on each phase of the scaffold, with a mineralized matrix detected only on Phase C, and an extensive type I collagen matrix found on both Phases A and B. When the tri-phasic scaffold co-cultured with osteoblasts and fibroblasts was evaluated in a subcutaneous athymic rat model99,100, abundant tissue formation was observed on Phase A and Phase C. Cells migrated into Phase B and increased matrix production was found in this interface region. Moreover, tissue continuity was maintained across all three scaffold phases. Interestingly, extracellular matrix production compensated for the decrease in mechanical properties accompanying scaffold degradation, while the phase-specific controlled matrix heterogeneity was maintained in vivo.
Similar to the findings of the 2-D co-culture model, while both anatomic ligament- and bone-like matrices were formed on the tri-phasic scaffold in vitro and in vivo, no fibrocartilage-like tissue was observed in the interface phase through osteoblast-fibroblast co-culture. Spalazzi et al. extended the in vivo evaluation to tri-culture of fibroblasts, chondrocytes, and osteoblasts on the stratified scaffold99. Articular chondrocytes encapsulated in a hydrogel matrix were injected into Phase B of the scaffold, while fibroblasts and osteoblasts were pre-seeded onto Phase A and Phase C, respectively. At two months post-implantation, an extensive collagen-rich matrix was prevalent in all three phases of the tri-cultured scaffolds (Fig. 1E), and the mineralized matrix was again confined to Phase C. The fibrocartilage region formed in tri-culture exhibited characteristic markers such as types I and II collagen as well as proteoglycan production. Interestingly, both cell shape and matrix morphology of the neo-fibrocartilage resembled that of the neonatal fibrocartilage tissue observed at the ACL-bone insertion3. Moreover, the neo-fibrocartilage formed was continuous with the ligament-like tissue observed in Phase A as well as the bone-like tissue found in Phase C100.
These promising results demonstrate that biomimetic stratified scaffold design coupled with spatial control over the distribution of interface relevant cell populations result in the formation of cell type- and phase-specific matrix heterogeneity in vitro and in vivo, with a fibrocartilage-like interface formed in tri-culture. These observations not only demonstrate the feasibility of the stratified scaffold for promoting biological fixation, but also highlight the potential for continuous multi-tissue regeneration on a single scaffold system. It is envisioned that the tri-phasic scaffold can be used to guide the re-establishment of an anatomic fibrocartilage interfacial region directly on soft tissue grafts. Specifically, the scaffold can be used as a graft collar or a circumferential interference screw during ACL reconstruction surgery. As a graft collar, it can be fabricated as a hollow cylinder through which the ACL graft will be inserted, seeded with interface relevant cells on each phase, and secured to the ends of the graft. It is anticipated that the phase-specific matrix heterogeneity and optimized cellular interactions, combined with application of both mechanical and chemical stimuli, will be able to induce the formation of a fibrocartilage interface directly onto the soft tissue graft. For use as an interference screw, the tri-phasic scaffold can be fabricated as matching halves of the hollow cylinder, with each half containing the three scaffold phases. The two matching halves will encase the soft tissue graft on all sides. The relative position of each phase of the tri-phasic scaffold would be in the anatomical position, i.e., with Phase A (soft tissue) exposed to the joint cavity, Phase B (fibrocartilage interface) flushed with articular cartilage, and Phase C (bone) encased within the bone tunnel. The feasibility of such a system for interface regeneration was recently demonstrated in a study by Spalazzi et al.101, where a mechanoactive scaffold system was formed based on a composite of poly-α-hydroxyester nanofibers and sintered microspheres. It was observed that scaffold-induced compression of tendon grafts resulted in significant matrix remodeling and the expression of fibrocartilage interface-related markers such as type II collagen, aggrecan, and transforming growth factor-β3 (TGF-β3). These results suggest that the stratified scaffold can be used to induce the formation of an anatomic fibrocartilage enthesis directly on ACL reconstruction grafts.
It is emphasized here that fixation of the aforementioned graft collar or interference screw is achieved by inserting the collar-graft complex into the bone tunnel, with Phases A and B remaining within the joint cavity. The ACL graft can also be augmented with mechanical fixation until an anatomic interface has been regenerated on the graft. Controlled cellular interactions coupled with mechanical loading will promote the formation of a fibrocartilage region directly on the ACL reconstruction graft. In parallel, graft osteointegration within the bone tunnel may be promoted by Phase C and the delivery of growth factors (e.g., bone morphogenetic proteins)58,102–104 to stimulate tendon mineralization within the bone tunnel. The optimal scenario is to have a completely mineralized tendon within the bone tunnel, accompanied by the formation of an anatomic fibrocartilage insertion directly on the ACL reconstruction graft. In addition, for functional ligament tissue engineering, the tri-phasic scaffold may be coupled with synthetic ACL grafts either as a graft collar or pre-incorporated into degradable polymer-based ACL prostheses34. It is anticipated that by focusing on engineering soft tissue-to-bone integration ex vivo, the complexity of intra-articular graft reconstruction would be reduced to bone-to-bone integration in vivo, which is relatively less challenging when compared to soft tissue-to-bone integration.
VI. STRATIFIED SCAFFOLD FOR TENDON-BONE INTERFACE TISSUE ENGINEERING
As soft tissue-to-bone interfaces are ubiquitous in the musculoskeletal system, the biomimetic scaffold design and multi-lineage cell culture methods described above are applicable to the regeneration of other soft tissue-to-bone insertions such as that of the rotator cuff tendons and bone. Similar to the ACL insertion site, a zonal distribution of extracellular matrix components and cell types are found at the supraspinatus tendon-to-bone interface41,43,105–107. Additionally, the repair of the supraspinatus tendon is characterized by disorganized scar tissue and the lack of fibrocartilage regeneration at the insertion site108,109. The debilitating effect of rotator cuff tears coupled with the high incidence of failure associated with existing repair techniques110–113 underscore the clinical need for functional solutions for supraspinatus tendon-to-bone repair.
Several groups have evaluated the feasibility of integrating tendon grafts with bone or biomaterials through the formation of anatomic insertion sites82,114. Fujioka et al. reported that cellular reorganization occurred at the site of surgical reattachment of the Achilles tendon, along with the formation of non-mineralized and mineralized fibrocartilage-like regions82. Additionally, Inoue et al. used a bone marrow-infused bone graft to promote supraspinatus tendon integration with a metallic implant82,114. Promising results from these early studies demonstrate that the tendon-bone interface may be regenerated, and emphasize the need for functional grafting solutions that can promote biological fixation. The ideal scaffold for supraspinatus tendon repair must be able to meet the physiological demand of the native tendon by matching its mechanical properties, as well as promoting host cell-mediated healing by mimicking the ultrastructural organization of the native tendon. In addition, the scaffold should be biodegradable in order to be gradually replaced by new tissue while maintaining physiologically relevant mechanical properties. Finally, the scaffold must be able to integrate with the host tendon and surrounding bone tissue by promoting the regeneration of the native tendon-to-bone enthesis.
Guided by these design criteria, the potential of a degradable polylactide-co-glycolide (PLGA) nanofiber-based scaffold system (Fig. 4) for rotator cuff repair was recently evaluated in vitro28. Nanofibers are advantageous for orthopaedic tissue engineering due to their superior biomimetic potential and physiological relevance. To date, nanofibers have been investigated for bone115,116, meniscus117, intervertebral disk118, cartilage119, and ligament120,121 tissue engineering. A distinct advantage of nanofiber scaffolds is that they can be tailored to resemble the native tendon extracellular matrix, exhibiting high aspect ratio, surface area, permeability and porosity122–126. Moreover, nanofiber organization and alignment can be modulated during fabrication126,127, which allows the scaffold structural and material properties to be readily tailored to meet the functional demands of the rotator cuff tendons.
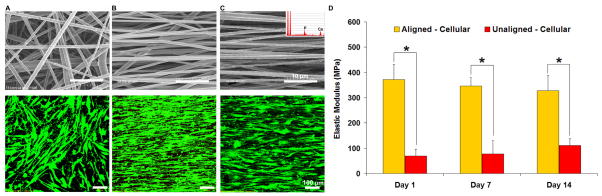
Figure 4. Nanofiber-Based Scaffold for Tendon-to-Bone Integration.
A) Unaligned nanofibers based on polylactide-co-glycolide (PLGA) supported the attachment and growth of human rotator cuff tendon fibroblasts (Top, as-fabricated scaffold bar = 10 μm; Bottom, Live-dead stain, day 14, 20x, bar = 100 μm).
B) Aligned PLGA nanofibers guided the alignment of human rotator cuff tendon fibroblasts (Top, as-fabricated scaffold bar=10 μm; Bottom, Live-dead stain, day 14, 20x, bar = 100 μm).
C) Nanofiber composite of PLGA and hydroxyapatite particles also supported tendon fibroblast growth and alignment (Top, as-fabricated scaffold with HA particles (inset), bar = 10 μm; Bottom, Live-dead stain, day 14, 20x, bar = 100 μm).
D) Mechanical properties of the aligned and unaligned nanofiber scaffolds seeded with supraspinatus tendon fibroblasts as a function of in vitro culture time (*p<0.05).
Recently, the effects of nanofiber organization on cellular attachment and alignment as well as gene expression and matrix deposition were evaluated28. It was reported that nanofiber organization (aligned vs. unaligned) is the primary factor guiding tendon fibroblast morphology (Fig. 4), alignment and integrin expression. Moreover, both types I and III collagen, the primary collagen types found in the native supraspinatus tendon, were synthesized on the nanofiber scaffolds and interestingly, their deposition was also controlled by the underlying fiber organization. Scaffold mechanical properties are directly related to fiber alignment and while they decreased as the polymer degraded, both the elastic modulus (Fig. 4) and ultimate tensile strength remain within range of those reported for the native supraspinatus tendon128.
Building upon the aligned nanofiber system, Moffat et al. later designed a composite nanofiber system of PLGA and hydroxyapatite (HA) nanoparticles129, with the extended goal of regenerating both the non-mineralized and mineralized fibrocartilage regions of the supraspinatus tendon-to-bone insertion site. The response of interface-relevant cell populations, including rotator cuff fibroblasts and osteoblasts, have been examined on the polymer-ceramic composite nanofibers with promising results (Fig. 4C). These observations demonstrate the potential of the biodegradable nanofiber-based scaffold system for tendon tissue engineering, and underscore the need for the development of stratified scaffolds for integrative rotator cuff repair and augmentation.
VII. SUMMARY AND CHALLENGES IN INTERFACE TISSUE ENGINEERING
Interface tissue engineering focuses on the regeneration of the anatomic interface between distinct tissue types, and has the potential to provide integrative graft solutions that will expedite the translation of tissue engineered technologies to the clinical setting. Building upon the solid foundation of tissue engineering methods already validated in past studies, interface tissue engineering aims to develop innovative technologies for the formation of complex tissue systems, with the extended goad of achieving the biological fixation of tissue engineered grafts with each other and with the host environment. Current efforts in this emerging area have centered on the formation of a functional interface between distinct tissue types, guided by the working hypothesis that tissue interfaces may be regenerated from the controlled interaction of relevant cell types on a biomimetic stratified scaffold with pre-designed gradient of structural and functional properties.
The broader question to be addressed in orthopaedic interface tissue engineering is how distinct boundaries between different types of connective tissues are formed, re-established post-injury, and maintained in the body. The success of any interface tissue engineering effort will require a thorough understanding of the structure-function relationship existing at the native insertion site, as well as the elucidation of the mechanisms governing interface regeneration and homeostasis. While the majority of research has focused on interface formation, the engineering of multiple tissue types must also address the problem of maintaining the stability of pre-formed tissue regions. It is likely that heterotypic cellular interactions will also play a critical role in interface homeostasis130. Moreover, the effects of biological, physical and chemical stimulation on interface regeneration are not known and remain to be explored.
In summary, re-establishment of an anatomic, functional and stable interface on biologic or synthetic soft tissue grafts through interface tissue engineering represents a promising strategy for achieving biological graft fixation for ligament or tendon reconstruction, as well as augmenting the clinical translation potential of tissue engineered orthopaedic grafts. The multiphasic scaffold design principles and co-culturing methodologies optimized through these efforts will lead to the development of a new generation of integrative fixation devices for orthopedic repairs. Moreover, by bridging distinct types of tissue, interface tissue engineering will be instrumental for the ex vivo development and in vivo translation of integrated musculoskeletal tissue systems with biomimetic complexity and functionality.
Acknowledgments
The authors gratefully acknowledge the contribution of all students, fellows and collaborators who have worked on the orthopaedic interface tissue engineering research described in this review. We also thank the National Institutes of Health (NIH/NIAMS AR052402, HHL; AR056459, HHL; and AR055280-A2, HHL/SAR), the Wallace H. Coulter Foundation (HHL/SAR) and the National Science Foundation GK-12 Graduate Fellowship (GK-12 0338329, KLM) for funding support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERECE LIST
- 1.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970;52:1–20. [PubMed] [Google Scholar]
- 2.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang IE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–1755. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 4.Woo SL, et al. In: Injury and Repair of the Musculosketal Soft Tissues. Woo SL, Bulkwater JA, editors. American Academy of Orthopaedic Surgeons; Savannah, Georgia: 1988. pp. 133–166. [Google Scholar]
- 5.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1:22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 6.Moffat KL, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci USA. 2008;105:7947–7952. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 8.Friedman MJ, et al. Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop. 1985:9–14. [PubMed] [Google Scholar]
- 9.Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225–229. doi: 10.1177/036354658701500306. [DOI] [PubMed] [Google Scholar]
- 10.Robertson DB, Daniel DM, Biden E. Soft tissue fixation to bone. Am J Sports Med. 1986;14:398–403. doi: 10.1177/036354658601400512. [DOI] [PubMed] [Google Scholar]
- 11.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Skalak R. Tissue engineering: proceedings of a workshop, held at Granlibakken; Lake Tahoe, California. February 26–29, 1988; New York, NY: Liss; 1988. [Google Scholar]
- 13.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 14.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 15.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 16.Laurencin CT, Ambrosio AA, Borden M, Cooper JA. In: Annual Review of Biomedical Engineering. Yarmush ML, Diller KR, Toner M, editors. 1999. pp. 19–46. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. Journal of Biomedical Materials Research. 2001;55:141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Lu HH, El Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J Biomed Mater Res. 2003;64A:465–474. doi: 10.1002/jbm.a.10399. [DOI] [PubMed] [Google Scholar]
- 19.Freed LE, et al. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 20.Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. Effects of mixing on the composition and morphology of tissue- engineered cartilage. Aiche Journal. 1996;42:850–860. [Google Scholar]
- 21.Mauck RL, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Zhu X, Valenzuela RG, Currier BL, Yaszemski MJ. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop. 2001:S251–S270. doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 23.Almarza AJ, Athanasiou KA. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32:2–17. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 24.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 25.Zhang AY, Chang J. Tissue engineering of flexor tendons. Clin Plast Surg. 2003;30:565–572. doi: 10.1016/s0094-1298(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 26.Goh JC, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31–S44. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 27.Juncosa N, West JR, Galloway MT, Boivin GP, Butler DL. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech. 2003;36:483–488. doi: 10.1016/s0021-9290(02)00459-1. [DOI] [PubMed] [Google Scholar]
- 28.Moffat KL, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Engineering. 2008 doi: 10.1089/ten.tea.2008.0014. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson DW, Heinrich JT, Simon TM. Biologic and synthetic implants to replace the anterior cruciate ligament. Arthroscopy. 1994;10:442–452. doi: 10.1016/s0749-8063(05)80197-5. [DOI] [PubMed] [Google Scholar]
- 30.Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363–1371. doi: 10.1002/jbm.820291107. [DOI] [PubMed] [Google Scholar]
- 31.Woo SL, et al. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999:S312–S323. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 32.Altman GH, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 33.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–1532. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Lu HH, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–4816. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin M, Evans EJ, Rao RD, Findlay JA, Pemberton DJ. Quantitative differences in the histology of the attachment zones of the meniscal horns in the knee joint of man. J Anat. 1991;177:127–134. [PMC free article] [PubMed] [Google Scholar]
- 36.Niyibizi C, Visconti CS, Kavalkovich K, Woo SL. Collagens in an adult bovine medial collateral ligament: immunofluorescence localization by confocal microscopy reveals that type XIV collagen predominates at the ligament-bone junction. Matrix Biol. 1995;14:743–751. doi: 10.1016/s0945-053x(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 37.Sagarriga VC, Kavalkovich K, Wu J, Niyibizi C. Biochemical analysis of collagens at the ligament-bone interface reveals presence of cartilage-specific collagens. Arch Biochem Biophys. 1996;328:135–142. doi: 10.1006/abbi.1996.0153. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Messner K. The postnatal development of the insertions of the medial collateral ligament in the rat knee. Anat Embryol (Berl) 1996;193:53–59. doi: 10.1007/BF00186833. [DOI] [PubMed] [Google Scholar]
- 39.Messner K. Postnatal development of the cruciate ligament insertions in the rat knee. morphological evaluation and immunohistochemical study of collagens types I and II. Acta Anatomica. 1997;160:261–268. doi: 10.1159/000148020. [DOI] [PubMed] [Google Scholar]
- 40.Petersen W, Tillmann B. Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol (Berl) 1999;200:325–334. doi: 10.1007/s004290050283. [DOI] [PubMed] [Google Scholar]
- 41.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variations of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 42.Niyibizi C, Sagarrigo VC, Gibson G, Kavalkovich K. Identification and immunolocalization of type X collagen at the ligament-bone interface. Biochem Biophys Res Commun. 1996;222:584–589. doi: 10.1006/bbrc.1996.0787. [DOI] [PubMed] [Google Scholar]
- 43.Woo SL, Buckwalter JA AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. J Orthop Res. 1988;6:907–931. doi: 10.1002/jor.1100060615. [DOI] [PubMed] [Google Scholar]
- 44.Johnson RJ. The anterior cruciate: a dilemma in sports medicine. Int J Sports Med. 1982;3:71–79. doi: 10.1055/s-2008-1026066. [DOI] [PubMed] [Google Scholar]
- 45.American Academy of Orthopaedic Surgeons. How old is too old to repair the ACL? United States: 2008. Press Release. [Google Scholar]
- 46.Gotlin RS, Huie G. Anterior cruciate ligament injuries. Operative and rehabilitative options. Phys Med Rehabil Clin N Am. 2000;11:895–928. [PubMed] [Google Scholar]
- 47.Fleming BC, Abate JA, Peura GD, Beynnon BD. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 48.Fleming B, Beynnon B, Howe J, McLeod W, Pope M. Effect of tension and placement of a prosthetic anterior cruciate ligament on the anteroposterior laxity of the knee. J Orthop Res. 1992;10:177–186. doi: 10.1002/jor.1100100204. [DOI] [PubMed] [Google Scholar]
- 49.Beynnon B, et al. A sagittal plane model of the knee and cruciate ligaments with application of a sensitivity analysis. J Biomech Eng. 1996;118:227–239. doi: 10.1115/1.2795965. [DOI] [PubMed] [Google Scholar]
- 50.Beynnon BD, et al. The effect of functional knee bracing on the anterior cruciate ligament in the weightbearing and nonweightbearing knee. Am J Sports Med. 1997;25:353–359. doi: 10.1177/036354659702500314. [DOI] [PubMed] [Google Scholar]
- 51.Gregor RJ, Abelew TA. Tendon force measurements and movement control: a review. Med Sci Sports Exerc. 1994;26:1359–1372. [PubMed] [Google Scholar]
- 52.Loh JC, et al. Knee stability and graft function following anterior cruciate ligament reconstruction: Comparison between 11 o'clock and 10 o'clock femoral tunnel placement. Arthroscopy. 2003;19:297–304. doi: 10.1053/jars.2003.50084. [DOI] [PubMed] [Google Scholar]
- 53.Markolf KL, et al. Effects of femoral tunnel placement on knee laxity and forces in an anterior cruciate ligament graft. J Orthop Res. 2002;20:1016–1024. doi: 10.1016/S0736-0266(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 54.Brand J, Jr, Weiler A, Caborn DN, Brown CH, Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28:761–774. doi: 10.1177/03635465000280052501. [DOI] [PubMed] [Google Scholar]
- 55.Beynnon BD, et al. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am. 2002;84-A:1503–1513. doi: 10.2106/00004623-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Barrett GR, Noojin FK, Hartzog CW, Nash CR. Reconstruction of the anterior cruciate ligament in females: A comparison of hamstring versus patellar tendon autograft. Arthroscopy. 2002;18:46–54. doi: 10.1053/jars.2002.25974. [DOI] [PubMed] [Google Scholar]
- 57.Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25:554–559. doi: 10.1177/036354659702500420. [DOI] [PubMed] [Google Scholar]
- 58.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 59.Berg EE. Autograft bone-patella tendon-bone plug comminution with loss of ligament fixation and stability. Arthroscopy. 1996;12:232–235. doi: 10.1016/s0749-8063(96)90018-3. [DOI] [PubMed] [Google Scholar]
- 60.Matthews LS, Soffer SR. Pitfalls in the use of interference screws for anterior cruciate ligament reconstruction: brief report. Arthroscopy. 1989;5:225–226. doi: 10.1016/0749-8063(89)90177-1. [DOI] [PubMed] [Google Scholar]
- 61.Kurzweil PR, Frogameni AD, Jackson DW. Tibial interference screw removal following anterior cruciate ligament reconstruction. Arthroscopy. 1995;11:289–291. doi: 10.1016/0749-8063(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 62.Burkart A, Imhoff AB, Roscher E. Foreign-body reaction to the bioabsorbable suretac device. Arthroscopy. 2000;16:91–95. doi: 10.1016/s0749-8063(00)90134-8. [DOI] [PubMed] [Google Scholar]
- 63.Allum RL. BASK Instructional Lecture 1: graft selection in anterior cruciate ligament reconstruction. Knee. 2001;8:69–72. doi: 10.1016/s0968-0160(01)00070-9. [DOI] [PubMed] [Google Scholar]
- 64.Shellock FG, Mink JH, Curtin S, Friedman MJ. MR imaging and metallic implants for anterior cruciate ligament reconstruction: assessment of ferromagnetism and artifact. J Magn Reson Imaging. 1992;2:225–228. doi: 10.1002/jmri.1880020217. [DOI] [PubMed] [Google Scholar]
- 65.Weiler A, et al. Tendon healing in a bone tunnel. Part I: Biomechanical results after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:113–123. doi: 10.1053/jars.2002.30656. [DOI] [PubMed] [Google Scholar]
- 66.Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26:536–539. doi: 10.1177/03635465980260041101. [DOI] [PubMed] [Google Scholar]
- 67.Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Cruciate reconstruction using freeze dried anterior cruciate ligament allograft and a ligament augmentation device (LAD). An experimental study in a goat model. Am J Sports Med. 1987;15:528–538. doi: 10.1177/036354658701500602. [DOI] [PubMed] [Google Scholar]
- 68.Yahia L. Ligaments and Ligamentoplasties. Springer Verlag; Berlin Heidelberg: 1997. [Google Scholar]
- 69.Matyas JR, Anton MG, Shrive NG, Frank CB. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. J Biomech. 1995;28:147–157. doi: 10.1016/0021-9290(94)00058-c. [DOI] [PubMed] [Google Scholar]
- 70.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998;193(Pt 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konofagou EE, Ophir J. Precision estimation and imaging of normal and shear components of the 3D strain tensor in elastography. Phys Med Biol. 2000;45:1553–1563. doi: 10.1088/0031-9155/45/6/311. [DOI] [PubMed] [Google Scholar]
- 72.Spalazzi JP, Gallina J, Fung-Kee-Fung SD, Konofagou EE, Lu HH. Elastographic imaging of strain distribution in the anterior cruciate ligament and at the ligament-bone insertions. J Orthop Res. 2006;24:2001–2010. doi: 10.1002/jor.20260. [DOI] [PubMed] [Google Scholar]
- 73.Gao J, Messner K. Quantitative comparison of soft tissue-bone interface at chondral ligament insertions in the rabbit knee joint. J Anat. 1996;188:367–373. [PMC free article] [PubMed] [Google Scholar]
- 74.Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J Biomech. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 75.Swartz DE, Wittenberg RH, Shea M, White AA, III, Hayes WC. Physical and mechanical properties of calf lumbosacral trabecular bone. J Biomech. 1991;24:1059–1068. doi: 10.1016/0021-9290(91)90022-f. [DOI] [PubMed] [Google Scholar]
- 76.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 77.Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203:191–202. doi: 10.1046/j.1469-7580.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radhakrishnan P, Lewis NT, Mao JJ. Zone-specific micromechanical properties of the extracellular matrices of growth plate cartilage. Ann Biomed Eng. 2004;32:284–291. doi: 10.1023/b:abme.0000012748.41851.b4. [DOI] [PubMed] [Google Scholar]
- 79.Spalazzi JP, Boskey AL, Lu HH. Region-dependent variations in matrix collagen and mineral distribution across the femoral and tibial anterior cruciate ligament-to-bone insertion sites. Trans Orthop Res Soc. 2007 [Google Scholar]
- 80.Landesberg R, Takeuchi E, Puzas JE. Differential activation by cytokines of mitogen-activated protein kinases in bovine temporomandibular-joint disc cells. Arch Oral Biol. 1999;44:41–48. doi: 10.1016/s0003-9969(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 81.Sun WS, Moffat KL, Lu HH. Characterization of Fibrochondrocytes Derived from the Ligament-Bone Insertion. Trans Orthop Res Soc. 2007 [Google Scholar]
- 82.Fujioka H, et al. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37:205–218. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 83.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–351. doi: 10.1177/036354659402200309. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi M, et al. The fate of host and graft cells in early healing of bone tunnel after tendon graft. Am J Sports Med. 2005;33:1892–1897. doi: 10.1177/0363546505277140. [DOI] [PubMed] [Google Scholar]
- 85.Wang IE, et al. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J Orthop Res. 2007;25:1609–1620. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 86.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–770. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 87.Shan JM, Wang IE, Lu HH. Osteoblast-Fibroblast Interactions Modulate Cell Phenotypes Through Paracrine and Autocrine Regulations. Trans Orthop Res Soc. 2007 [Google Scholar]
- 88.Lim JK, et al. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 89.Nawata K, Minamizaki T, Yamashita Y, Teshima R. Development of the attachment zones in the rat anterior cruciate ligament: changes in the distributions of proliferating cells and fibrillar collagens during postnatal growth. J Orthop Res. 2002;20:1339–1344. doi: 10.1016/S0736-0266(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 90.Gao J, Messner K, Ralphs JR, Benjamin M. An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol (Berl) 1996;194:399–406. doi: 10.1007/BF00198542. [DOI] [PubMed] [Google Scholar]
- 91.French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32:50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 92.Nicoll SB, Wedrychowska A, Smith NR, Bhatnagar RS. Modulation of proteoglycan and collagen profiles in human dermal fibroblasts by high density micromass culture and treatment with lactic acid suggests change to a chondrogenic phenotype. Connect Tissue Res. 2001;42:59–69. doi: 10.3109/03008200109014249. [DOI] [PubMed] [Google Scholar]
- 93.Vogel KG. The effect of compressive loading on proteoglycan turnover in cultured fetal tendon. Connect Tissue Res. 1996;34:227–237. doi: 10.3109/03008209609000701. [DOI] [PubMed] [Google Scholar]
- 94.Vogel KG, Ordog A, Pogany G, Olah J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993;11:68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 95.Wang IE, Lu HH. Role of cell-cell interactions in the regeneration of soft tissue-to-bone interface. Proceedings of the IEEE Engineering Biology and Medicine Society. 2006;1:783–786. doi: 10.1109/IEMBS.2006.259456. [DOI] [PubMed] [Google Scholar]
- 96.Dunn MG, et al. Anterior cruciate ligament reconstruction using a composite collagenous prosthesis. A biomechanical and histologic study in rabbits. Am J Sports Med. 1992;20:507–515. doi: 10.1177/036354659202000504. [DOI] [PubMed] [Google Scholar]
- 97.Cooper JA, Jr, et al. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci U S A. 2007;104:3049–3054. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of Controlled Matrix Heterogeneity on a Triphasic Scaffold for Orthopedic Interface Tissue Engineering. Tissue Engineering. 2006;12:3497–3508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 99.Spalazzi JP, et al. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res. 2008;86A:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 100.Spalazzi JP, Moffat KL, Rodeo SA, Lu HH. Design of a novel stratified scaffold for ACL-to-Bone interface tissue engineering. 8th International Symposium on Ligaments and Tendons; 2008. [Google Scholar]
- 101.Spalazzi JP, et al. Scaffold-induced compressive loading of tendon grafts promotes matrix remodeling and the expression of fibrocartilage-related markers. Clinical Orthopaedics and Related Research. 2008;466(8):1938–1948. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan SN, Bostrom MP, Lane JM. Bone growth factors. Orthop Clin North Am. 2000;31:375–388. doi: 10.1016/s0030-5898(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 103.Ramp WK, et al. A serum substitute promotes osteoblast-like phenotypic expression in cultured cells from chick calvariae. Bone Miner. 1991;15:1–17. doi: 10.1016/0169-6009(91)90107-b. [DOI] [PubMed] [Google Scholar]
- 104.Lu HH, Kofron MD, El Amin SF, Attawia MA, Laurencin CT. In vitro bone formation using muscle-derived cells: a new paradigm for bone tissue engineering using polymer-bone morphogenetic protein matrices. Biochem Biophys Res Commun. 2003;305:882–889. doi: 10.1016/s0006-291x(03)00858-1. [DOI] [PubMed] [Google Scholar]
- 105.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 106.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J Anat. 1994;185(Pt 2):279–284. [PMC free article] [PubMed] [Google Scholar]
- 107.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28:1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 108.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81:1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 109.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:S191–S197. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 110.Iannotti JP, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 111.Coons DA, Alan BF. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14:185–190. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 112.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004;13:538–541. doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 114.Inoue N, et al. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002;20:957–966. doi: 10.1016/S0736-0266(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 115.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 116.Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomol Eng. 2007;24:75–80. doi: 10.1016/j.bioeng.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 117.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 119.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 120.Lee CH, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 121.Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27:5681–5688. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 122.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 123.Christenson EM, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25:11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 124.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 125.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murugan R, Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007;13:1845–1866. doi: 10.1089/ten.2006.0078. [DOI] [PubMed] [Google Scholar]
- 127.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 128.Itoi E, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578–584. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 129.Moffat KL, Levine WN, Lu HH. In Vitro Evaluation of Rotator Cuff Tendon Fibroblasts on Aligned Composite Scaffold of Polymer Nanofibers and Hydroxyapatite Nanoparticles. Trans Orthop Res Soc. 2008 [Google Scholar]
- 130.Jiang J, Leong NL, Mung J, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis and Cartilage. 2008;16:70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]