Abstract
The p53 gene product is an attractive target for tumor immunotherapy. The present study aims to understand the potential of MVAp53 vaccine to induce expansion of p53-specific cytotoxic T lymphocyte ex vivo in cancer patients. The result indicated that 14 of 23 cancer patients demonstrated p53-specific IFN-γ production, degranulation, cell proliferation, and lysis of p53 overexpressed human tumor cell lines. These experiments show that MVAp53 stimulation has the potential to induce the expansion of p53-specific cytotoxic T lymphocyte from the memory T cell repertoire. The data suggest that MVAp53 vaccine is an ideal candidate for cancer immunotherapy.
Keywords: : MVA, p53, Peptide library, PBMC, CTL expansion, Cancer patients
INTRODUCTION
A novel approach to cancer treatment involves the use of vaccines, which target tumor-associated antigens (TAA) to promote T-cell-mediated antitumor immune responses (1–3). The human p53 gene product is an ideal target for the enhancement of the cellular immune response to malignancy. Over 50% of all malignancies have p53 mutations (4). Mutations of p53, which abrogate its function as a suppresser of cell division, are associated with a high nuclear and cytoplasmic concentration of the p53 protein (5). Nonmutated p53 is expressed at low levels normally, which would be most likely to escape an enhanced immune response to overexpressed mutant p53 (6, 7). Extensive preclinical murine tumor model studies using p53-based vaccine demonstrate that this approach can induce tumor rejection (8–14). Several groups have generated human cytotoxic T lymphocyte (CTL) against HLA Class I binding motif peptides from wild-type p53 using techniques of in vitro stimulation (IVS), and the resulting cells are capable of lysing human tumor cells which overexpress p53 (15–18). Prior preclinical and clinical studies have targeted p53 using a number of peptide- and viral-vaccine-based approaches (19–21). Although modest p53-specific cellular immune responses were identified, the vaccine vectors were not robust enough to generate strong p53-specific immunity. Because p53 is an autoantigen widely expressed throughout development, tolerance to p53 may limit the effectiveness of p53-directed immunotherapy.
Preclinical results from our laboratory demonstrate that recombinant modified vaccinia virus Ankara (MVAp53) immunization can overcome tolerance to p53, induce p53-specific cellular immune responses, and reject established p53-overexpressing tumor (11–14). The MVAp53 vaccine approach has advantages over specific epitope vaccine approaches because epitope-specific immunization strategies might not stimulate responses to cryptic epitopes or stimulate a p53-specific T-helper (Th) response.
MVA is an ideal vector for the generation of a therapeutic response to overexpressed p53. The development of MVA as a recombinant vaccine delivery vehicle stemmed from its benign safety profile as a smallpox vaccine in Europe in the late 1970s. It was administered to over 120,000 individuals including the aged and very young as a smallpox vaccine without serious side effects. MVA was administered to immuno-compromised nonhuman primates without adverse outcome (22). Its development into a vaccine vehicle was only initiated in the early 1990's (23), when it became clear that nonattenuated poxviruses such as the Western Reserve (WR) strain could not be safely administered to immuno-compromised persons. Although MVA is able to efficiently replicate DNA in mammalian cells, it is noninfectious, because of the loss of two important host range genes among at least 25 additional mutations and deletions that occurred during its 570 serial passages through chicken embryo fibroblasts (CEF) (24). Despite its restricted host range and inability to produce infectious progeny in human cells, and in contrast to NYVAC (attenuated Copenhagen strain) and ALVAC (host range restricted avipox), both early and late transcription are unimpaired, making MVA a suitable vaccine candidate. In fact, in our preclinical mouse studies, we have found it to be more immunogenic than the WR strain, and most critical is its ability to be used in conditions of pre-existing poxvirus immunity.
In preclinical models, p53-based immunotherapy approaches have demonstrated tumor rejection without stimulating autoimmunity (11–12, 14). Like other tumor-antigen-directed T-cell-based immunotherapies, effective p53-based immunotherapy will be dependent on patients’ ability to mount a responsive to wild-type p53 and the ability of the tumor to present p53 epitopes for T cell recognition. In this report, we described the capacity of MVAp53 to induce the expansion of specific CTL from the memory T cell repertoire of cancer patients. These experiments demonstrate that MVAp53 stimulation has the potential to induce p53-specific CTL expansion from the memory T cell repertoire. The data suggest that MVAp53 vaccine is an ideal candidate for cancer immunotherapy.
MATERIALS AND METHODS
Human subjects
Blood samples (50cc) were collected from 23 HLA-A2+ cancer patients (Table 1). Peripheral blood mononuclear cells (PBMC) were used either fresh or after cryopreservation. These studies were conducted under a City of Hope Institutional Review Board approved protocol.
Table 1 .
Patient Characteristics and IFN-γ responses to MVAp53 stimulation
| All Patients Number (%) | IFN-γ+ Induction Number (%)* | |
|---|---|---|
| Sex | ||
| Male | 13 (56.5) | 7 (53.8) |
| Female | 10 (43.5) | 7 (70.0) |
| Total | 23 (100) | 14 (60.9) |
| Age | ||
| Average | 60.8 | |
| Median | 64 | |
| Range | 47–64 | 8 (66.7) |
| Range | 65–85 | 6 (54.5) |
| Tumor type | ||
| HNSCC | 1 (4.3) | 1 (100) |
| Breast | 5 (21.7) | 3 (60.0) |
| Gastric | 1 (4.3) | 1 (100) |
| Colon | 6 (26.1) | 4 (66.7) |
| Prostate | 7 (30.4) | 3 (42.9) |
| Duodenal | 1 (4.3) | 1 (100) |
| Bile duct | 1 (4.3) | 1 (100) |
| Lung | 1 (4.3) | 0 (0.0) |
| Tumor stage | ||
| I | 5 (21.7) | 3 (60.0) |
| II | 5 (21.7) | 3 (60.0) |
| III | 9 (39.1) | 7 (77.8) |
| IV | 4 (17.4) | 1 (25.0) |
*Percentage of IFN-γ production is calculated in each category.
Generation of recombinant MVA
The generation of recombinant MVA has been described previously (14). Briefly, full-length wild-type human p53 and CEA proteins were inserted into the MVA shuttle plasmid pLW22. Recombinant MVA was generated by transfecting the insertion shuttle plasmid into wild-type MVA (wtMVA)-infected BHK-21 cells. MVAp53 was purified after 8–10 rounds of screening in the presence of Bluo-gal (5-Bromo-3-indolyl-β-d-galactopyranoside; Sigma-Aldrich, St Louis, MO). DNA was extracted from the infected cell lysate, and the absence of wtMVA was confirmed by PCR. Expression of recombinant protein was assessed by western blot analysis.
The p53 peptides and overlapping peptide libraries
The p53 peptides corresponding to positions 149–157 and 264–272 and 15-mer peptides derived from wild-type human p53 were synthesized in our laboratory by using a 12-position parallel Symphony organic synthesizer (PTI Technologies, Oxnard, CA). The 15-mer libraries spanned the respective proteins with an 11 amino acid overlap between peptides. The p53 peptide library was composed of 96 peptides, which covered all 393 amino acids of p53 protein. Purity was ascertained by reverse phase HPLC analysis. The peptide library was created into two groups, superpool-1 and superpool-2. Superpool-1 covered the first 48 peptides, and superpool-2 covered the remaining 48 peptides. The 15-mer Pepmix peptide libraries spanning BKV-VP1 were purchased from JPT Peptide Technologies GmbH (Berlin, Germany).
Antibodies
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated mAbs to human CD107a/CD107b, CD8, and IFN-γ were purchased from BD Pharmingen (San Diego, CA).
Cell lines
The cell lines MDA-MB231, SK-BR3, and SAOS-2 were obtained from the ATCC and maintained in RPMI (supplemented with 10% fetal Bovine Serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and fresh glutamine). SAOS/p53 cell was HLA A2.1+, p53 deficient human osteosarcoma cell line SAOS-2 transfected with mutated p53 (r to h at position 175), and maintained in 400 μg/mL Geneticin (G418, GIBCO BRL, Grand Island, NY).
In vitro stimulation (IVS)
CD8+ T cells, enriched by positive selection with antibody coated microbeads using a magnetic purification system (Miltenyi Biotec MACS, Auburn, CA), were used as effector cells. Autologous-antigen-presenting cells (APC) were generated by CD8- PBMC incubated with 5 μg/mL of CpG-A ODN 2216 and 5 μg/mL CpG-B ODN 2006 from TriLink (San Diego, CA) for 3 days. The resulting APCs were infected with at an MOI = 1 with recombinant MVA for 6 hr and irradiated at 3000 rad. The APCs were co-incubated with CD8+ effector cells in 20% human AB serum, RPMI with 10 IU/mL IL-2 for 7 days. In some experiments, cells were subjected to a second and/or third round of IVS with p53 peptide library pulsed APC and cultured for another week before ICC assay.
Intracellular cytokine (ICC) assays
Stimulated cells were tested for intracellular IFN - γ production following stimulation with 10 μg/mL of peptide library. FITC-labeled antibody to CD107a/b and pure antibodies to CD28 and CD49d (BD Pharmingen) were added for degranulation assay. GolgiStop was added to cultures prior to overnight incubation. The cells were then washed with 3 mL PBS/0.5% BSA before labeling for 20 min at 4°C with a PE-conjugated antibody to CD8. The cells were then washed again with PBS/0.5% BSA before permeabilization (Cytofix/Cytoperm, BD Pharmingen, San Diego, CA) and labeling with APC-conjugated antibody to IFN-γ for 30 min at 4°C. The cells were washed and analyzed on a FACSCanto flow cytometer (BD Biosciences).
Tetramer and tetramer-binding assay
The HLA-A*02 BKV VP1p108, HLA-A*02 p53149–257, and HLA-A*02 p53264–272 tetramers were refolded, purified, and conjugated to APC in our laboratory by using previously described methods (25). The stimulated cells were labeled and analyzed on the FACScanto flow cytometer (BD Biosciences).
CFSE-based ICC
The effector cells were labeled with 10 μM CFSE for 10 min at 37°C. The reaction was stopped by the addition of two volumes of culture medium, followed by 10-min incubation on ice. After two washes, the CFSE-labeled effector cells were cultured with p53 peptide library pulsed APC for a week. The stimulated cells were used to perform the ICC assays.
Chromium release assay (CRA)
To assess specific CTL killing, MDA-MB231, SK-BR3, SAOS-2, and SAOS-2/p53 tumor cell lines were pretreated with 20 ng/mL IFN-γ and 3 ng/mL TNF-α for 24 hr and then labeled with Na51CrO4 for 1 hr. The labeled target cells and diluted effector cells were coincubated for 4 hr at 37°C. Supernatants were harvested and counted using a gamma counter. Percent specific lysis was calculated using the following formula: percent specific release = (experimental release − spontaneous release) / (total release − spontaneous release) × 100.
Statistical methods
Data were analyzed by GraphPad Prizm 5 software. Values of the results were expressed as means and SEs. Differences were considered to be statistically significant when p value < .05. The percentage of specific lysis was evaluated using two-tailed unpaired t test.
RESULTS
Patient characteristics
Twenty-three HLA-A2+ patients (13 males, 10 females) with solid tumors were enrolled in the study. The characteristics of patients are shown in Table 1. The majority of patients had adenocarcinoma of prostate, colon, and breast origin and had stage III or IV cancer. After stimulation of PBMC with MVAp53 and the human-p53-derived peptide library, over 60% of the patients demonstrated p53-specific IFN-γ production. IFN-γ responses were present in PBMC from patients with all types of solid tumor malignancy with the exception of the one patient with lung cancer. Over 53% of male patients and 70% of female patients demonstrated p53-specific immune responses. In 8 (66.7%) of the younger patients and 6 of the older patients (p = .68), p53-specific responses were identified. Patients with stage IV tumor were less likely to mount p53-specific responses than the group of patients with stage I–III malignancy (p = .26), but this difference was not significant. The status of human p53 expression in tumor did not correlate with p53-specific IFN-γ production (data not shown).
MVAp53 stimulation results in p53-specific IFN-γ+-secreting CD8+ cell expansion
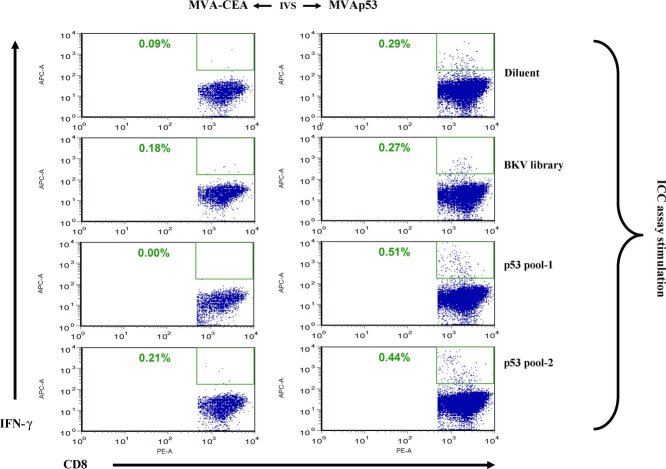
Activation of tumor-specific CD8+ T cells and subsequent amplification of sustained effector CTL responses is of particular importance in tumor immunity. To assess specific IFN-γ-secreting CD8+ cell expansion followed by MVAp53 stimulation, CD8+-enriched cells from a patient with head and neck squamous-cell carcinoma (HNSCC) were stimulated by MVAp53 or MVA-CEA infected CpG-activated autologous PBMC blast APC (Figure 1). The production of p53-specific IFN-γ was identified following stimulation with MVAp53 but not with an MVA expressing the irrelevant control protein CEA (1).
To further evaluate the potential of MVAp53 to stimulate p53-specific IFN-γ+-secreting CD8+ cells, CD8+ cells from an HLA-A2+ gastric cancer patient were stimulated with MVAp53 following by an IVS with the human-p53-derived overlapping peptide library. The resulting cell population demonstrated tetramer-specific binding for the previously described HLA-A2 restricted epitopes of p53149–157 and p53264–272 [see Figure 2(a)]. IFN-γ ICC analysis indicated that 0.5% of the cells were positive by ICC for p53149–157, while 0.25% were positive by ICC for p53264–272. By comparison, 1.00% + 2.85% or 3.85% of the cell population responded to the 2 subfractions of the p53-derived overlapping peptide library [see Figure 2(b)]. This suggests that the p53-specific responses seen following MVAp53 stimulation are not only restricted to the two well-established HLA-A2 epitopes but also to other less well-defined p53-derived epitopes.
Figure 2 .
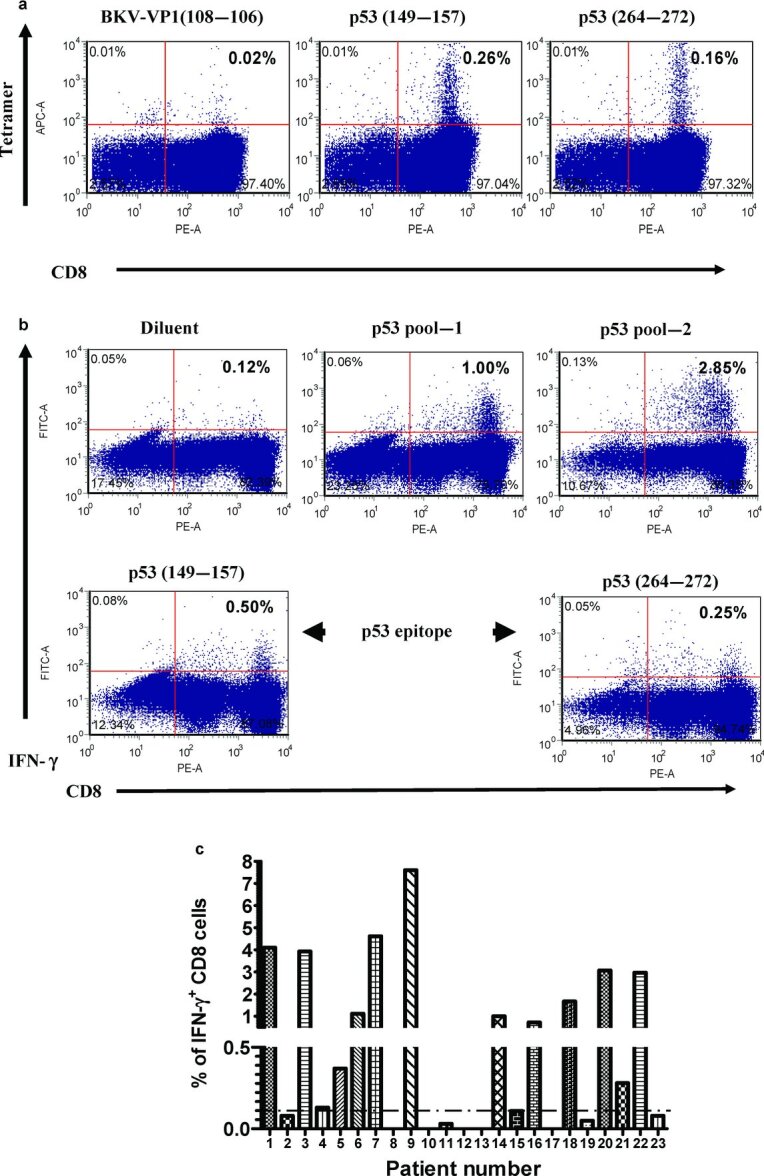
Stimulated cells were analyzed by tetramer-binding assay and ICC assay. CD8+ cells from the PBMC of cancer patient were subjected to MVAp53 stimulation and IVS with the human-p53-derived peptide library. (a) A tetramer-binding assay was performed on the stimulated effector cells. (b) IFN-γ production analysis was performed on stimulated effector cells following an overnight incubation with HLA-A2-restricted p53-specific epitopes or the p53-derived peptide library. (c) The stimulated effector cells from PBMC of all 23 cancer patients were analyzed for IFN-γ production by ICC assay after overnight incubation with p53 peptide library. Cutoff value was set up at 0.10% IFN-γ+ in CD8+ T cells after background subtraction. Results are gated CD8+ T cells.
Figure 1 .
IFN-γ production after MVA stimulation. CD8+-enriched cells from an HNSCC patient were stimulated with MVA-infected autologous APC for 7 days. An ICC assay was used to evaluate IFN-γ production following overnight incubation with the reagents shown on the right of the figure (top to bottom) including the peptide diluent, a control peptide library derived from BK virus (BKV library), or pools of overlapping p53-derived peptides (pool-1 and pool-2). Results are gated on CD8+ T cells.
Figure 2(c) shows IFN-γ production from PBMC of all 23 cancer patients after stimulation with MVAp53 and p53 peptide library. Among them, 14 patients demonstrated p53-specific immune responses. Table 1 depicts IFN-γ responses to MVAp53 stimulation of the different tumor types and stages (Table 1).
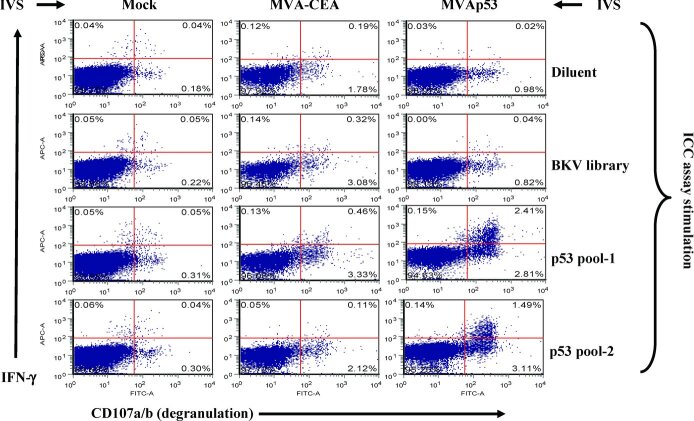
p53-specific IFN-γ+-producing cells demonstrated degranulation and proliferation
Because there is an established correlation between CTL degranulation and specific lysis of target cells, the CD107 mobilization assay can be used as an adjunct to a standard killing assay. To evaluate if the expanded IFN-γ+ CD8 cells have the capacity for p53-specific T-cell degranulation, CD8+-enriched cells were stimulated with MVA-CEA, MVAp53, or mock stimulation. All cells were restimulated with p53 peptide library pulsed autologous CpG-activated APC. The p53-specific production of IFN-γ and degranulation were demonstrated following IVS with MVAp53, but not following IVS with MVA-CEA or mock stimulation (see Figure 3).
Figure 3 .
ICC assay on human PBMC following IVS with MVA and human p53 peptide library. CD8+ T cells isolated from the PBMC of a HNSCC patient were subjected to IVS under the conditions shown at the top of the figure (left to right), including mock stimulation, MVA-CEA, or MVAp53. All cells were restimulated with p53 peptide library pulsed autologous CpG-stimulated blasts. ICC assay was performed on 7-day-cultured CD8+ cells following overnight incubation with the peptide or control reagents shown on the right of the figure (top to bottom) including the peptide diluent, a BKV peptide library, or p53-derived peptide library pool-1 and pool-2. Results are gated on CD8+ T cells.
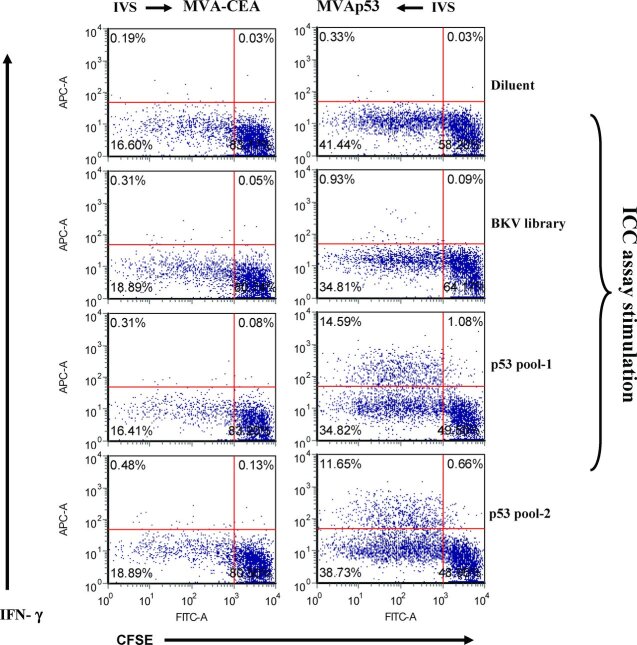
The cells derived as described above were labeled with CFSE and further amplified following an additional IVS with the p53 peptide library. After 7 days, the resulting cells were analyzed by ICC. The p53-specific production of IFN-γ was demonstrated in the MVAp53-stimulated cells but not in the MVA-CEA-stimulated cells. More than 90% of IFN-γ+ CD8 cells demonstrated active proliferation (see Figure 4). This suggests that MVAp53 stimulated a specific p53 IFN-γ+ CD8+ cells expansion from the T cell repertoire.
Figure 4 .
IFN-γ-producing cells underwent cell division in response to the human-p53-derived peptide library. Stimulated cells derived as in Figure 3 were labeled with CFSE and cultured with p53 peptide library pulsed autologous APC for 7 days. ICC assay was performed to analyze cell proliferation and IFN-γ production.
IFN-γ+ CD8 cells lysed specifically tumor cell lines
The potential of MVAp53-stimulated cells to mediate direct cytotoxicity was measured using a51Cr release assay. CD8+ cells from cancer patients were stimulated with MVAp53 and the p53 peptide library and were used as an effector cells in a standard 4 hr CRA. The resulting cells did not recognize and lyse the target of p53-null SAOS-2 osteosarcoma cell line unless they were first transfected with p53 [see Figure 5(a)]. We also evaluated the ability of the CTL to recognize endogenous p53 overexpression in the context of HLA-A2 expression. The stimulated HLA-A2+ CD8 cells only lysed p53 overexpressing HLA-A2+ restricted mammary carcinoma cell line MDA-MB231 but did not lyse the SK-BR3, which overexpresses p53 but does not express HLA-A2 [see Figure 5(b)].
Figure 5 .
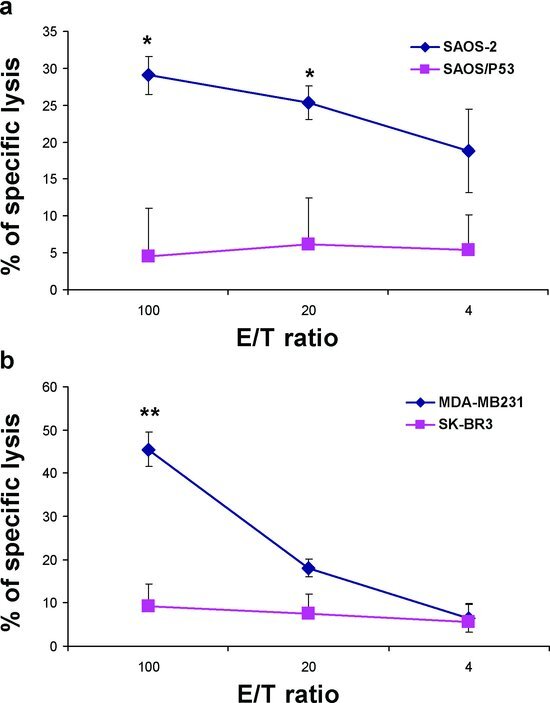
Cytotoxicity of the expanded CTL against various tumor cell lines. CD8+ T cells stimulated with MVAp53 and restimulated for 2 sequential IVS with the human p53 peptide library were used as an effector cells in a standard 4-hr CRA. (a) Cytotoxic activity was assessed against SAOS-2 (osteosarcoma cell line, p53 null, and HLA-A2+) and SAOS–p53 (p53 transfected SAOS-2 cell line). (b) Cytotoxic activity was assessed against mammary carcinoma cell lines, MDA-MB231 (p53+ and HLA-A2+), and SK-BR3 (p53+ and HLA-A2-). *p < .05, **p < .01 by two-tailed unpaired t test.
DISCUSSION
There has been extensive clinical experience with unmodified MVA as a smallpox vaccine since the 1970s (26). Significant past experience with MVA supports its use as a vector for the generation of a therapeutic response to tumor antigens (11, 13, 14, 27–29). Several phase I trials have evaluated MVA-based vaccines showing no toxicity and some clinical response (30–32). A phase I vaccination study using recombinant MVA expressing human tyrosinase (MVA-hTyr) was conducted in patients with stage II melanoma. Twenty patients were vaccinated three times at 4-week intervals with 5 × 108 IU of MVA-hTyr each time. The vaccine was demonstrated to be safe and did not have side effects above grade 2 (31). However, it did not elicit a measurable immune response to its transgene product in patients with stage II melanoma after repeated combined intradermal and subcutaneous vaccination.
A phase I clinical trial using MVA expressing MUC1 and IL-2 (TG4010) for MUC1+ cancers demonstrated clinical responses and cellular immune responses to MUC1 in some patients (30). MUC1-specific T-cell activity was observed in 5 of 13 patients and 1 patient experienced stabilization of disease. At present, there are a number of ongoing clinical trials using MVA-based vaccines that target a variety of cancers. A phase I safety and immunogenicity trial of MVA expressing HER2 (MVA-BN-HER2) vaccine is being conducted in HER-2-positive breast cancer patients following adjuvant therapy (NCT01152398) (33). A phase II clinical trial assessing the efficacy of MVA-MUC1-IL2 (TG4010) as a therapeutic vaccine combined with chemotherapy in comparison with chemotherapy alone is being conducted in patients with advanced nonsmall cell lung cancer (NCT00415818) (34). A phase II study to access the activity of MVA-5T4 (TroVax) plus docetaxel versus docetaxel alone is being conducted in subjects with progressive hormone refractory prostate cancer (NCT01194960) (35). A phase I study of MVA-FCU1 (TG4023) combined with systemic administration of 5-fluorocytosine is conducted in patients with primary or secondary hepatic tumors (NCT00978107) (36).
Tolerance to p53 is a crucial issue to overcome in the development of a therapeutic p53-specific immune response (9, 14). During ontogeny, most T cells directed against epitopes from self-proteins such as p53 are deleted in the thymus. Because p53 is an autoantigen widely expressed throughout development, tolerance to p53 might limit the effectiveness of p53-directed immunotherapies. Functional and tetramer studies in mice have clearly demonstrated tolerance to p53 at the CTL level (37). To achieve successful p53-directed immunotherapy, it will be necessary to break immunological tolerance to p53. Small numbers of self-reacting T cells escape during the processes involved in the immune tolerance. In mice, MVAp53 immunization alone can generate modest p53-specific CTL and rejection of early established tumors that overexpress p53 (13, 14). Several studies have demonstrated the ability to induce p53-specific response in cancer patients and therefore indicate that p53-specific CTLs have not been eliminated completely (14, 20, 38). Wild-type p53-specific CTL and Th cells have been detected in PBMC cultures in vitro (39, 40). Studies by Zwaveling et al. have demonstrated that CD4+ p53-specific Th cells are able to help tumor-specific CTL in controlling p53 overexpressing tumors (40). Other groups have shown that MHC Class I restricted wild-type p53 epitope pulsed dendritic cells (DCs) are able to induce CTL expansion from normal donors and cancer patients (10, 18, 41). Although CTLs from human PBMC have been derived against a number of HLA-A2-binding peptides of p53, these CTLs are less effective against p53 overexpressing tumor cells. Immunization with whole p53 protein delivered by viral vectors has the advantage of potential stimulation of responses targeting multiple MHC Classes I and II restricted epitopes (13, 42, 43). In the current study, the diverse nature of an immune response to p53 was confirmed by the demonstration of a higher percentage of IFN-γ ICC positive cells in response to the p53 peptide library compared with both of the well-known HLA-A2 epitopes combined [see Figure 2(b)]. This suggests that an epitope-specific strategy may not take full advantage of the potential p53-specific memory T-cell repertoire.
Mutations in p53 might represent true TAA and would be an ideal target for a tumor vaccine (8, 44). Unfortunately, mutations in p53 occur at many sites, and most p53 mutation does not correspond to immunologic T-cell epitopes. To be widely applicable, p53-directed immunotherapy would need to target wild-type epitopes of p53. Several groups have generated human CTL against HLA Class I binding motif peptides from wild-type p53 by using IVS techniques, and some wild-type p53-specific CTL derived from human PBMC are capable of lysing human tumor cells which overexpress p53 (16, 18, 45, 46). In addition, there appears to be a lack of p53-specific tolerance at the Th-cell level. Nikitina et al. used Ad-p53-infected DC to restimulate p53-specific responses from the PBMC of patients with aerodigestive tract cancers (9). They were able to generate p53-specific CTL which would recognize and lyse p53 overexpressing cancer cells, from 8 of 9 patients were evaluated. Using IVS techniques with variant peptide epitopes, Hoffmann et al. were able to generate wild-type p53-specific CTL from 5 of 7 healthy donors (47). These CTLs recognized p53 overexpressing tumor cells. The relevance of the p53-specific immune response is supported by tetramer analyses demonstrating an increased frequency of wild-type p53-specific CD8+ cells localized to tumor sites. Several vaccine approaches have targeted p53 as a tumor antigen with no evidence of autoimmunity, immunogenicity, and clinical response (42, 48). A phase I/II dose-escalation study evaluated the effect of a recombinant canarypox virus (ALVAC) vaccine encoding wild-type human p53 in patients with advanced colorectal cancer (48). Potent T-cell and IgG antibody responses against the vector component of the ALVAC vaccine were induced in the majority of the patients. Analysis of vaccine-induced immunity revealed the presence of weak IFN-γ-secreting T-cell responses against p53. The immunologic and clinical effect of another p53-based cancer vaccine, which consisted of DCs transduced with the full-length wild-type p53 gene delivered via an adenoviral vector, was studied in patients with extensive stage small cell lung cancer (NCT00049218) (42). Vaccination resulted in the development of weak p53-specific T-cell responses in over half of the treated patients (57.1%).
The objective of the current study was to determine the capacity of MVAp53 to stimulate p53-specific IFN-γ+-secreting CD8+ T cells capable of expansion and direct tumor cell lysis. We investigated this potential by modeling MVAp53 immunization with an in vitro assay system using PBMC from cancer patients. The p53-specific responses with therapeutic potential could be generated from the majority of patients with a variety of solid tumor malignancies. The ability to generate p53-specific immunity was not age or stage dependent, as responses could be identified in PBMC from elderly and stage III and IV patients.
We utilized an overlapping peptide library to interrogate p53-specific immune responses, allowing the p53-specific response to be elucidated in the absence of exogenous protein. Recently, Quintarelli et al. (49) reported that high avidity CTLs were generated ex vivo by stimulation with peptide library spanning the entire PRAME protein from PBMC of cancer patients and healthy donors. These high avidity polyclonal PRAME-specific CTL lines recognized new HLA-A2 epitope and were capable of killing primary leukemic blasts and tumor cell lines. In our study, we utilized MVAp53 and p53 peptide library IVSs to induce p53-specific CTL expansion from PBMC of cancer patients. Since 15-mer overlapping peptide libraries contain all possible epitopes for both CD4+ and CD8+ T cells, the induction and examination of immune responses bypasses the concern for epitope mapping. IFN-γ produced by CD8+ cells from MVAp53 and p53 peptide library stimulations distribute into different peptide pools and further divide into variety of peptide subpools (data not shown). The relevance of the expanded T cells is demonstrated by the capacity for p53-specific T-cell degranulation and cytotoxic potential as measured by expression of CD107 (see Figure 3) and specific cell proliferation measured by CFSE labeling (see Figure 4). The resulting human CTLs are able to recognize and lyse HLA-A2+, p53 overexpressing tumor cell lines but not HLA-A2 negative or p53 negative cell lines (see Figure 5).
In conclusion, we performed a comprehensive analysis of p53-specific CD8+ CTL expansion from 23 HLA-A2+ cancer patients after MVAp53 stimulation in vitro. The MVAp53 vaccine was capable of inducing p53-specific CD8+ IFN-γ producing cells in 60.9% of the solid tumor patients. The demonstrated ability of the MVAp53-stimulated cells to mediate lysis of p53 overexpressing cell lines supports the potential of the vaccine. These studies support the evaluation of MVAp53 in a clinical trial setting involving patients with solid tumor malignancy.
ACKNOWLEDGMENTS
The authors thank nurses and doctors in the operating room at the City of Hope Medical Center for blood draw before surgery. These studies have been partially supported by AI062496 NIH, CA077544 NIH, CA030206 NIH, and CA1148890 NIH to Don J. Diamond and by CA1148890, Riley Foundation, and FAMRI (042275) to Joshua D.I. Ellenhorn.
DECLARATION OF INTERESTS
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- 1.Rosenblatt J, Vasir B, Uhl L, Blotta S, Macnamara C, Somaiya P, Wu Z, Joyce R, Levine JD, Dombagoda D, Yuan YE, Francoeur K, Fitzgerald D, Richardson P, Weller E, Anderson K, Kufe D, Munshi N, Avigan D. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood 2011;117:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciesielski MJ, Ahluwalia MS, Munich SA, Orton M, Barone T, Chanan-Khan A, Fenstermaker RA. Antitumor cytotoxic T-cell response induced by a survivin peptide mimic. Cancer Immunol Immunother 2010;59:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest 2010;120:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991;253:49–53. [DOI] [PubMed] [Google Scholar]
- 5.Zambetti GP, Levine AJ. A comparison of the biological activities of wild-type and mutant p53. FASEB J 1993;7:855–865. [DOI] [PubMed] [Google Scholar]
- 6.Theobald M, Offringa R. Anti-p53-directed immunotherapy of malignant disease. Expert Rev Mol Med 2003;5:1–13. [DOI] [PubMed] [Google Scholar]
- 7.Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol 2010;2:a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, Storkus WJ, Appella E, DeLeo AB. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med 1996;183:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikitina EY, Chada S, Muro-Cacho C, Fang B, Zhang R, Roth JA, Gabrilovich DI. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther 2002;9:345–352. [DOI] [PubMed] [Google Scholar]
- 10.DeLeo AB, Whiteside TL. Development of multi-epitope vaccines targeting wild-type sequence p53 peptides. Expert Rev Vaccines 2008;7:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daftarian P, Song GY, Ali S, Faynsod M, Longmate J, Diamond DJ, Ellenhorn JD. Two distinct pathways of immuno-modulation improve potency of p53 immunization in rejecting established tumors. Cancer Res 2004;64:5407–5414. [DOI] [PubMed] [Google Scholar]
- 12.Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, Ellenhorn JD. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol 2003;170:3401–3407. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaki H, Song GY, Srivastava T, Carroll KD, Shahabi V, Manuel ER, Diamond DJ, Ellenhorn JD. Heterologous prime/boost immunization with p53-based vaccines combined with toll-like receptor stimulation enhances tumor regression. J Immunother 2010;33:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song GY, Gibson G, Haq W, Huang EC, Srivasta T, Hollstein M, Daftarian P, Wang Z, Diamond D, Ellenhorn JD. An MVA vaccine overcomes tolerance to human p53 in mice and humans. Cancer Immunol Immunother 2007;56:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakakura K, Chikamatsu K, Furuya N, Appella E, Whiteside TL, Deleo AB. Toward the development of multi-epitope p53 cancer vaccines: an in vitro assessment of CD8(+) T cell responses to HLA class I-restricted wild-type sequence p53 peptides. Clin Immunol 2007;125:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chikamatsu K, Nakano K, Storkus WJ, Appella E, Lotze MT, Whiteside TL, DeLeo AB. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res 1999;5:1281–1288. [PubMed] [Google Scholar]
- 17.Tokunaga N, Murakami T, Endo Y, Nishizaki M, Kagawa S, Tanaka N, Fujiwara T. 2005. Human monocyte-derived dendritic cells pulsed with wild-type p53 protein efficiently induce CTLs against p53 overexpressing human cancer cells. Clin Cancer Res 2005;11:1312–1318. [PubMed] [Google Scholar]
- 18.Wurtzen PA, Claesson MH. A HLA-A2 restricted human CTL line recognizes a novel tumor cell expressed p53 epitope. Int J Cancer 2002;99:568–572. [DOI] [PubMed] [Google Scholar]
- 19.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A 1995;92:11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speetjens FM, Kuppen PJ, Welters MJ, Essahsah F, Voet van den Brink AM, Lantrua MG, Valentijn AR, Oostendorp J, Fathers LM, Nijman HW, Drijfhout JW, van de Velde CJ, Melief CJ, van der Burg SH. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009;15:1086–1095. [DOI] [PubMed] [Google Scholar]
- 21.van der Burg SH, Menon AG, Redeker A, Bonnet MC, Drijfhout JW, Tollenaar RA, van de Velde CJ, Moingeon P, Kuppen PJ, Offringa R, Melief CJ. Induction of p53-specific immune responses in colorectal cancer patients receiving a recombinant ALVAC-p53 candidate vaccine. Clin Cancer Res 2002;8:1019–1027. [PubMed] [Google Scholar]
- 22.Stittelaar KJ, Kuiken T, de Swart RL, van Amerongen G, Vos HW, Niesters HG, van Schalkwijk P, van der Kwast T, Wyatt LS, Moss B, Osterhaus AD. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine 2001;19:3700–3709. [DOI] [PubMed] [Google Scholar]
- 23.Tartaglia J, Pincus S, Paoletti E. Poxvirus-based vectors as vaccine candidates. Crit Rev Immunol 1990;10:13–30. [PubMed] [Google Scholar]
- 24.Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 1998;244:365–396. [DOI] [PubMed] [Google Scholar]
- 25.La Rosa C, Wang Z, Brewer JC, Lacey SF, Villacres MC, Sharan R, Krishnan R, Crooks M, Markel S, Maas R, Diamond DJ. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood 2002;100:3681–3689. [DOI] [PubMed] [Google Scholar]
- 26.Moss B. Smallpox vaccines: targets of protective immunity. Immunol Rev 2011;239:8–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tykodi SS, Thompson JA. Development of modified vaccinia Ankara-5T4 as specific immunotherapy for advanced human cancer. Expert Opin Biol Ther 2008;8:1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkord E, Dangoor A, Drury NL, Harrop R, Burt DJ, Drijfhout JW, Hamer C, Andrews D, Naylor S, Sherlock D, Hawkins RE, Stern PL. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother 2008;31:820–829. [DOI] [PubMed] [Google Scholar]
- 29.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, Wassan H, Habib N, Anthoney A. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res 2007;13:4487–4494. [DOI] [PubMed] [Google Scholar]
- 30.Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, Tartour E, Zhao Y, Bizouarne N, Baudin M, Acres B. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med 2003;5:690–699. [DOI] [PubMed] [Google Scholar]
- 31.Meyer RG, Britten CM, Siepmann U, Petzold B, Sagban TA, Lehr HA, Weigle B, Schmitz M, Mateo L, Schmidt B, Bernhard H, Jakob T, Hein R, Schuler G, Schuler-Thurner B, Wagner SN, Drexler I, Sutter G, Arndtz N, Chaplin P, Metz J, Enk A, Huber C, Wolfel T. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr). Cancer Immunol Immunother 2005;54:453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrop R, Shingler W, Kelleher M, de Belin J, Treasure P. Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother 2010;33:999–1005. [DOI] [PubMed] [Google Scholar]
- 33.Mandl SJ, Delcayre A, Curry D, Dal Pazzo K, Fernandez L, Njoku T, Riggs R, Treiger B, Laus R. MVA-BN-HER2: a novel vaccine for the treatment of breast cancers which overexpress HER-2. J Immunother 2006;29:652. [Google Scholar]
- 34.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, Krzakowski M, Hess D, Tartour E, Chenard MP, Limacher JM, Bizouarne N, Acres B, Halluard C, Velu T. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol 2008;3: 735–744. [DOI] [PubMed] [Google Scholar]
- 35.Amato RJ. 5T4-modified vaccinia Ankara: progress in tumor-associated antigen-based immunotherapy. Expert Opin Biol Ther 2010;10:281–287. [DOI] [PubMed] [Google Scholar]
- 36.Erbs P, Findeli A, Kintz J, Cordier P, Hoffmann C, Geist M, Balloul JM. Modified vaccinia virus Ankara as a vector for suicide gene therapy. Cancer Gene Ther 2008;15:18–28. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez J, Lee PP, Davis MM, Sherman LA. The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol 2000;164: 596–602. [DOI] [PubMed] [Google Scholar]
- 38.Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, Hepkema BG, Willemse PH, Molmans BH, Hollema H, Drijfhout JW, Sluiter WJ, Valentijn AR, Fathers LM, Oostendorp J, van der Zee AG, Melief CJ, van der Burg SH, Daemen T, Nijman HW. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer 2009;125:2104–2113. [DOI] [PubMed] [Google Scholar]
- 39.Lambeck A, Leffers N, Hoogeboom BN, Sluiter W, Hamming I, Klip H, ten Hoor K, Esajas M, van Oven M, Drijfhout JW, Platteel I, Offringa R, Hollema H, Melief K, van der Burg S, van der Zee A, Daemen T, Nijman H. P53-specific T cell responses in patients with malignant and benign ovarian tumors: implications for p53 based immunotherapy. Int J Cancer 2007;121:606–614. [DOI] [PubMed] [Google Scholar]
- 40.Zwaveling S, Vierboom MP, Ferreira Mota SC, Hendriks JA, Ooms ME, Sutmuller RP, Franken KL, Nijman HW, Ossendorp F, Van Der Burg SH, Offringa R, Melief CJ. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res 2002;62:6187–6193. [PubMed] [Google Scholar]
- 41.Andrade Filho PA, Ito D, Deleo AB, Ferris RL. CD8+ T cell recognition of polymorphic wild-type sequence p53(65–73) peptides in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2010;59:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, Menander K, Chada S, Gabrilovich DI. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006;12:878–887. [DOI] [PubMed] [Google Scholar]
- 43.Ren SP, Wu CT, Huang WR, Lu ZZ, Jia XX, Wang L, Lao MF, Wang LS. Adenoviral-mediated transfer of human wild-type p53, GM-CSF and B7-1 genes results in growth suppression and autologous anti-tumor cytotoxicity of multiple myeloma cells in vitro. Cancer Immunol Immunother 2006;55:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanuck M, Carbone DP, Pendleton CD, Tsukui T, Winter SF, Minna JD, Berzofsky JA. A mutant p53 tumor suppressor protein is a target for peptide-induced CD8 +cytotoxic T-cells. Cancer Res 1993;53:3257–3261. [PubMed] [Google Scholar]
- 45.Wurtzen PA, Pedersen LO, Poulsen HS, Claesson MH. Specific killing of P53 mutated tumor cell lines by a cross-reactive human HLA-A2-restricted P53-specific CTL line. Int J Cancer 2001;93:855–861. [DOI] [PubMed] [Google Scholar]
- 46.Umano Y, Tsunoda T, Tanaka H, Matsuda K, Yamaue H, Tanimura H. Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br J Cancer 2001;84:1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann TK, Loftus DJ, Nakano K, Maeurer MJ, Chikamatsu K, Appella E, Whiteside TL, DeLeo AB. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53 (264–272) epitope. J Immunol 2002;168:1338–1347. [DOI] [PubMed] [Google Scholar]
- 48.Menon AG, Kuppen PJ, van der Burg SH, Offringa R, Bonnet MC, Harinck BI, Tollenaar RA, Redeker A, Putter H, Moingeon P, Morreau H, Melief CJ, van de Velde CJ. Safety of intravenous administration of a canarypox virus encoding the human wild-type p53 gene in colorectal cancer patients. Cancer Gene Ther 2003;10:509–517. [DOI] [PubMed] [Google Scholar]
- 49.Quintarelli C, Dotti G, Hasan ST, De Angelis B, Hoyos V, Errichiello S, Mims M, Luciano L, Shafer J, Leen AM, Heslop HE, Rooney CM, Pane F, Brenner MK, Savoldo B. High-avidity cytotoxic-T-lymphocytes specific for a new preferentially expressed antigen of melanoma (PRAME)-derived peptide can target leukemic- and leukemic-precursor cells. Blood 2011;117:3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]