Abstract
The incidence and prevalence of heart failure have increased significantly over the past few decades. Available data suggest that patients with heart failure independent of the aetiology have viable but dysfunctional myocardium that is potentially salvageable. Although a great deal of research effort has focused on characterizing the molecular basis of heart failure, cardiac metabolism in this disorder remains an understudied discipline. It is known that many aspects of cardiomyocyte energetics are altered in heart failure. These include a shift from fatty acid to glucose as a preferred substrate and a decline in the levels of ATP. Despite these demonstrated changes, there are currently no approved drugs that target metabolic enzymes or proteins in heart failure. This is partly due to our limited knowledge of the mechanisms and pathways that regulate cardiac metabolism. Better characterization of these pathways may potentially lead to new therapies for heart failure. Targeting myocardial energetics in the viable and potentially salvageable tissue may be particularly effective in the treatment of heart failure. Here, we will review metabolic changes that occur in fatty acid and glucose metabolism and AMP-activated kinase in heart failure. We propose that cardiac energetics should be considered as a potential target for therapy in heart failure and more research should be done in this area.
Keywords: Energetics, Cardiac metabolism, AMP-activated kinase, Heart failure
Heart failure (HF) is a complex syndrome with several features, including abnormal myocardial function and excessive, continuous neurohormonal activation. HF is costly and deadly; disease prevalence is 6–10%, with an annual incidence of 1% in adults over the age of 65 and costs in excess of US$35 billion. One-year mortality is 20% and 5-year mortality is 50%, a figure worse than that for many cancers.1 Data from an Italian registry of >6200 unselected outpatients with HF indicate that ischaemic heart disease is the most common risk factor for HF (40%), followed by dilated cardiomyopathy, primary valvular disease, and hypertension (32, 12, and 11%, respectively).2 Less commonly, HF may be caused by viral myocarditis, infiltrative disorders, human immunodeficiency virus (HIV) cardiomyopathy, alcohol, cocaine, and connective tissue disease.
From a mechanistic standpoint, reduced myocardial performance may be related to anatomic loss of myocardial tissue, reduced function of viable myocytes, or a combination of both. However, contractile failure is more often the expression of adaptive responses of viable myocytes to various forms of non-lethal damage, and as such is potentially reversible with appropriate treatment.3 Indeed, viable but dysfunctional myocardium, which can be identified by imaging techniques and appears to be present in a significant number of patients independent of their aetiology, predicts the response to therapy in patients with HF.4 Traditional treatments for HF have mostly focused on haemodynamics and neurohormonal pathways. Thus, targeting specific proteins and processes in the myocardial cells that are defective may provide additional benefits in patients with this disorder.
Myocardial energetics refers to the energy that is produced and utilized by cardiomyocytes to generate the contraction and active relaxation of the beating heart. The healthy human heart is a highly efficient pump that propels ∼5 L/min of blood, totalling >7000 L/day and >2.6 million L/year.5 To perform this work, the myocardium hydrolyses >6 kg of adenosine triphosphate (ATP) daily,6 which is produced by transferring chemical energy from nutrients taken up into the myocytes from the bloodstream. From a metabolic standpoint, cardiac muscle can be considered an ‘omnivorous’ tissue, since it can oxidize free fatty acids (FFAs) and glucose simultaneously and in variable ratios according to substrate availability and disease state.7 The workload of the heart requires a high level of architectural efficiency, which is maintained by constant turnover and rebuilding. In a healthy person, extracellular and intracellular structural renewal occurs rapidly from a steady supply of amino acids, lipids, and carbohydrates.8 As the myocardium fails, there are significant changes in the heart's ability to supply adequate energy for its needs. The early observations that a decrease in oxidative phosphorylation capacity accompanies HF have been validated with more modern tools such as 31P-nuclear magnetic resonance (NMR) spectroscopy, which allows for non-invasive measurements of cardiac energy reserves. Decreased cardiac energy reserve in patients suffering from dilated cardiomyopathy is associated with significantly increased mortality.9 While these clinical observations do not prove causation, the contribution of deranged cardiac energetics to the development of HF has been studied in numerous animal models. Specific metabolic gene mutations or knockouts result in cardiac hypertrophy and a decrease in contractile reserve, resulting in overt HF or decreased ability to respond to cardiac stressors.10 These in vivo models highlight the consequences of altering cardiac metabolism and their impact on preserving normal cardiac functional reserve.
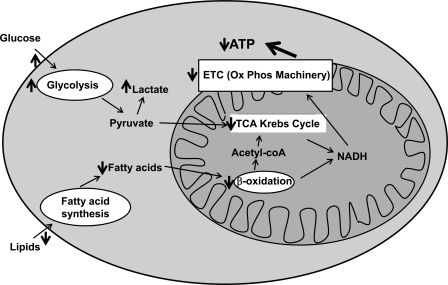
Multiple myocardial metabolic abnormalities occur in HF. The failing myocardium is characterized by altered substrate utilization (increased dependence on glucose), decreased oxidative phosphorylation, decreased high-energy phosphate content, generation of reactive oxygen species, and mitochondrial dysfunction (Figure 1).11 These changes are not due to a lack of substrate availability, since the coronary circulation is able to provide an excess of substrates, but rather to a change in substrate flux and modification of the enzymatic repertoire.12 Thus, myocyte structure remains relatively intact but myocyte function is impaired, resulting from the metabolic imbalance, which is expressed clinically by the presence of viable, but dysfunctional, myocardium. Importantly, this can be further exacerbated by the increasing metabolic demands triggered by the excessive and continuous activation of the sympathetic nervous system typical of HF.13
Figure 1.
Cardiac metabolism and defects in heart failure. Under normal conditions, cardiomyocytes mostly utilize free fatty acids as their primary substrate. However, with pressure overload and heart failure, cardiomyocytes switch substrate preference to glucose. There is also decreased activity of fatty acid β-oxidation, tricarboxylic acid (TCA) cycle enzymes, and complexes involved in the electron transport chain (ETC) in heart failure. Red arrows show defects in cellular metabolism in heart failure. For more details, please refer to the text.
Despite recent progress, our knowledge of myocardial energetic abnormalities in HF is still limited. Whether and how they differ according to aetiology, duration, and severity of the disease remains poorly understood. There is, however, a unique opportunity for therapeutic intervention at the metabolic level in patients with HF since only a portion of myocytes may be irrevocably injured at a given time. In the last few years, a number of promising therapies modulating the metabolic function of the heart have been tested, and some of them appear to improve the performance of viable myocardium. In this article, we will review the main aspects of cardiac metabolism in relation to the pathophysiology of HF, we will highlight the potential targets for pharmacometabolic therapy, and we will report the effects of new metabolic drugs. We propose that by targeting myocardial energetic pathways, we may be able to prevent the eventual death of viable myocardial cells in heart failure. This review starts with a description of animal models of HF, followed by an overview of glucose and FFA metabolism and the role of AMP-activated kinase (AMPK) in HF. It should be noted that other metabolic aspects of the heart, such as mitochondrial function, oxygen consumption, production of reactive oxygen species, hibernation and stunning, and micronutrients will not be discussed here, and the focus will be on the changes that occur in glucose and FFA metabolism and AMPK activity in HF.
Animal models of heart failure
Multiple animal models of HF exist. The common pathophysiological basis of all of these models is either pressure overload or volume overload leading to cardiomyopathy and HF. Pressure- or volume-overloaded hearts were classically believed to undergo ventricular remodelling to normalize systolic wall stress.14,15 Pressure overload leads to concentric hypertrophy through an increase in myocyte volume but not length.16 The classic clinical example is that of aortic stenosis. This concentric hypertrophy initially normalizes wall stress, typically called ‘compensatory hypertrophy’, according to the law of Laplace. However, it is known that mechanical function in the heart with concentric hypertrophy is not normal; in particular, diastolic dysfunction develops as an early consequence.17 Further, the capillary network may be inadequate for supplying these thickened myocytes.16 Volume overload causes eccentric hypertrophy through an increase in myocyte length and maintenance of mitochondrial and capillary density. Eccentric hypertrophy develops to allow the ventricle to accommodate the increased volume; this normalizes systolic wall stress at the price of increased end-diastolic wall stress.14 Clinically, pure volume overload is produced by atrioventricular valve regurgitation or intracardiac shunts such as an atrial septal defect.
The classic pressure overload model is thoracic aortic constriction (TAC) whereby the aorta is constricted, resulting in chronic elevation of left ventricular (LV) end-systolic pressure.18 Animals subjected to TAC classically display hypertrophy with progression to overt heart failure. Volume overload is typically produced experimentally by introduction of an aorto-venous (AV) shunt. Animals subjected to AV shunt formation develop increased ventricular volume overload and concomitant ventricular dilatation. The animal model of myocardial infarction represents a mix between pressure and volume overload.19 The infarcted heart is initially subjected to a volume overload. This sudden volume overload results in a pressure overload.
These animal models have been useful in delineating underlying pathways and signalling cascades that regulate HF, as discussed below. However, there are inherent limitations of animal models. HF in humans is, in most cases, a process that develops slowly over many years. Animal models, particularly those with experimentally induced HF such as the TAC or AV shunt models, rely on rapid development of HF. This rapid induction of HF may not result in the same underlying molecular pathways that occur in the much more slowly progressive disease process in humans. Further, humans with HF frequently have many co-morbid conditions that contribute to their underlying heart disease, which probably results in significant molecular heterogeneity not present in animal models. These confounding issues need to be considered when applying the results of animal models to humans.
While the final common pathway of these models is often the development of end-stage dilated cardiomyopathy with severe systolic dysfunction and overt HF, it is important to recognize that the molecular pathways leading to this phenotype from pressure overload and volume overload are distinct. Pressure overload cardiomyopathy is characterized by re-expression of the fetal gene programme. Numerous molecular pathways stimulate concentric hypertrophy associated with pressure overload. Some pathways promote adaptive hypertrophy with maintenance of normal myocardial mechanics, such as the ERK (extracellular signal-regulated kinase) pathway, while other pathways promote maladaptive remodelling, such as that induced by myocardial production of angiotensin II.19 Recently, comparison between TAC and AV shunt models demonstrated distinctly different molecular signatures with >160 differentially expressed transcripts.20 Further distinct molecular perturbations occur in the progression to end-stage HF.
Glucose and glycogen metabolism in heart failure
Fatty acid oxidation is the primary means of energy production under resting conditions in the normal adult human heart; glycolysis makes only a small contribution towards myocardial ATP production.21 Regulation of glycolysis is complex and determined by intracellular glucose concentrations, transcriptional rates and activity of glycolytic enzymes, and metabolic demands of the myocardium. However, as the heart remodels in response to hypertrophy and ischaemia, marked changes in cardiomyocyte glucose and ATP metabolism occur (Figure 2). Enzymes involved in glycolytic pathways are up-regulated even during early stages of cardiac dysfunction; however, it is unclear if the shift is mediated by increased adrenergic signalling, up-regulation of fetal gene programmes, or hypoxia.22–24 The shift towards glucose metabolism improves myocardial contractile efficiency by increasing the stochiometric ratio of ATP production to oxygen consumption in addition to minimizing oxidative losses through mitochondrial respiratory chain uncoupling associated with FFA metabolism.25 Abnormally high myocardial dependence on FFA metabolism, as seen during ischaemia or high adrenergic states, increases cardiac oxygen consumption by 30–50% adjusted for equivalent stroke work indices.26,27 Strategies to increase glucose oxidation and decrease fatty acid metabolism can improve myocardial energy efficiency by up to 30%.28
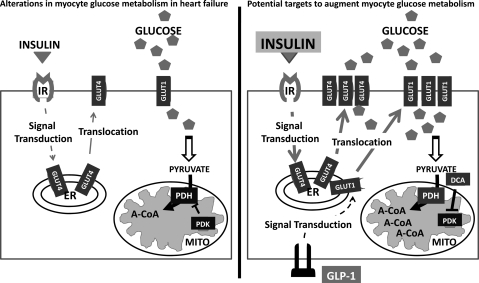
Figure 2.
Insulin resistance in heart failure leads to decreased glucose uptake by cardiomyocytes via decreased translocation of GLUT4 to the sarcolemma. Fewer GLUT4 transporters result in decreased glucose flux into the myocyte. Strategies to augment glucose metabolism in heart failure include increasing glucose uptake and oxidation by the cardiomyocyte. Administration of exogenous insulin may increase GLUT4 transporter translocation. In addition, GLP-1 has been shown to increase GLUT1 translocation from intracellular vesicles to the plasma membrane. Glucose oxidation can be increased by blocking the inhibitory effects of PDK on PDH. Increased PDH activity allows for increased oxidation of pyruvate into acetyl-CoA which can enter the citric acid cycle to generate ATP. A-CoA, acetyl-CoA; DCA, dichloroacetate; ER, endoplasmic reticulum; GLP-1, glucagon-like peptide-1; GLUT4, glucose transporter type 4; IR, insulin receptor; MITO, mitochondria; PDH, pyruvate dehyrdogenase; PDK, pyruvate dehydrogenase kinase.
At a basic level, glucose metabolism is dependent on intracellular glucose concentrations, which in turn are regulated by glucose flux across the sarcolemma. Glucose enters the cell down a concentration gradient primarily via two isoforms of the glucose transporter (GLUT) family. GLUT1 transporters are constitutively expressed, whereas GLUT4 exists in intracellular vesicles and translocates to the plasma membrane in response to hormonal stimuli such as insulin and catecholamines or in response to muscle contraction. GLUT4 transporters are the most abundant and are responsible for the bulk of basal glucose uptake in the beating heart, but may increase further in response to hormonal stimulation or increased workload.29
During myocardial ischaemia and hypertrophy, enhanced glucose uptake occurs and appears to be both cardioprotective and adaptive.30 This observation has been validated in animal models. Deficiencies in glucose transport are detrimental to myocardial function. Mice with either global and cardiac-specific deletions of GLUT4 transporters develop myocardial hypertrophy, have decreased cardiac contractility, and show compensatory increases in gene expression of enzymes involved in fatty acid oxidation.31 Decreased GLUT4 expression and decreased insulin-dependent myocardial glucose uptake have also been seen in non-diabetic patients with cardiac hypertrophy from aortic stenosis.32
However, GLUT4 levels are not the sole determinant of myocardial glucose uptake. Although the initial shift towards glucose metabolism at progressively more advanced stages of cardiac dysfunction is physiologically adaptive, the magnitude and impact of this adaption can be significantly limited by extracardiac factors, specifically the development of insulin resistance. Whole body insulin resistance can affect cardiac energy metabolism, even in structurally normal hearts. Type 2 diabetic patients who have otherwise normal cardiac function regenerate phosphocreatine at a significantly lower rate after exercise compared with non-diabetic controls.33 In patients with cardiomyopathy, the development of insulin resistance is probably due to increased sympathetic signalling leading to liberation of FFAs from adipose tissue into the bloodstream.34 Thus, in the failing myocardium, decreases in insulin sensitivity can lead to further reductions in glucose oxidation and deteriorations in cardiac function by depriving the heart of access to a more metabolically efficient substrate.
Attempts to augment glucose flux and overcome insulin resistance in order to improve cardiac function have had mixed results (Figure 2). Glucose–insulin–potassium (GIK) therapy was an early attempt to potentiate myocardial glucose uptake during ischaemia.35 Insulin and ischaemia increase GLUT4 translocation via independent but additive mechanisms, with ischaemia acting via AMPK and insulin via phosphatidylinositol 3-kinase (PI3K). Thus, exposure to insulin during episodes of ischaemia could, in theory, further increase myocardial glucose uptake.36 Infusion of insulin during the perimyocardial infarction period was presumed to decrease adverse cardiac events and improve mortality by increasing glucose uptake and metabolism and by increasing pro-survival signalling mechanisms. However, subsequent clinical trials of GIK therapy have shown conflicting results.37,38 The lack of benefit may reflect differences in revascularization or other aspects of acute coronary care in earlier vs. subsequent studies. New strategies to enhance glucose uptake by the myocardium will depend on further understanding of the connections between GLUT4 translocation and the signalling cascades involved in ischaemia, insulin resistance, and hypertrophy.
Interestingly, regulation of cardiac glucose transport and metabolism is not entirely mediated by insulin. Glucagon-like peptide 1 (GLP-1) is a pancreatic peptide that induces glucose-mediated release of insulin from pancreatic beta-cells (Figure 2). While its primary role is thought to be the regulation of beta-cell glucose sensitivity, recent studies have shown extrapancreatic effects on myocyte metabolism, including the discovery of GLP-1 receptors on cardiomyoctes.39 The mechanism of action appears to be independent of insulin signalling pathways. Administration of GLP-1 in a canine pacing-induced cardiomyopathy model resulted in increased GLUT1 expression and translocation to the sarcolemma independent of insulin/Akt-1 signalling.40 Animals treated with GLP-1 had significant improvement in cardiac function compared with untreated animals.41 Given the commercial availability of the GLP-1 analogues exenatide and liraglutide, currently approved for treatment of type 2 diabetes, clinical trials should be undertaken to test the efficacy of these compounds for improving systolic function in patients with HF.
Other strategies to augment glucose metabolism in HF have shown promising but limited results (Figure 2). Compounds inhibiting pyruvate dehydrogenase kinase (PDK), such as dichloroacetate, augment glucose and pyruvate metabolism and lead to improved ejection fraction and stroke volume in patients with moderate systolic HF.42 Compounds inhibiting mitochondrial fatty acid uptake increase glucose metabolism and have been shown to be beneficial in small clinical trials.43 Further work is needed to determine if augmenting glucose metabolism, through either augmentation of insulin signalling or inhibition of fatty acid oxidation, in patients with systolic dysfunction is beneficial or deleterious. Although glucose oxidation is more metabolically efficient than fatty acid oxidation, it is unknown whether primary reliance on glucose will be enough to meet the metabolic demands of the myocardium.
Free fatty acid metabolism in heart failure
Under physiological conditions, the primary substrates utilized by the myocardium are FFAs, glucose, and lactate, with amino acids and ketones playing a lesser role.44 In the fasted state, high plasma FFA levels lead to the preferential uptake and utilization of FFAs for oxidative metabolism, with concomitant reductions in glucose oxidation. In contrast, during the fed state, circulating glucose and insulin levels are high and FFA concentrations are low, so myocardial FFA uptake declines, the inhibitory effect of FFAs on glycolysis diminishes, and glucose oxidation increases.
Three interconnected pathways regulate FFA breakdown, ATP synthesis, and energy transfer from the mitochondria to the contractile apparatus: (i) substrate utilization; (ii) oxidative phosphorylation; and (iii) ATP transfer.45,46 A notable aspect of FFA oxidation is its lower thermodynamic yield compared with glucose, which leads to higher myocardial oxygen consumption (MVO2) and decreased mechanical efficiency.27,47 The inferior energy efficiency of FFA oxidation is thought to arise from several factors, such as the lower phosphate/oxygen ratio of FFA metabolism compared with glucose metabolism, which reflects the number of molecules of ATP produced per atom of oxygen reduced during oxidative phosphorylation;48 the uncoupling of oxidative phosphorylation, which leads to diversion of the electrochemical proton gradient away from ATP production;49 and the increase in futile cycling of FFA intermediates, which leads to the consumption of ATP for non-contractile purposes and energy waste.50
The onset and progression of HF significantly affect cardiac energy metabolism. The initial, physiologically adaptive response of the injured/failing myocardium aims to maximize efficiency and leads to: (i) a preferential use of glucose metabolism, mediated by a down-regulation of pyruvate dehydrogease isoforms (PDKs) (which exerts a negative control on glucose oxidation); (ii) lower expression of the PPARα/RXR2 complex and of the enzymes carnitine palmitoyltransferase-1 (CPT-1) and medium chain acyl-CoA dehydrogenase, leading to a decline in FFA metabolism; and (iii) down-regulation of uncoupling proteins.51 However, with progression of HF, the compensatory hyperadrenergic state leads to an elevation of blood FFA levels and to the onset of insulin resistance.52 This condition impairs the normal adaptive metabolic response of the myocyte and leads to up-regulation of FFA metabolism, increased oxygen consumption, and decreased cardiac efficiency, thereby creating a vicious cycle that may lead to a secondary metabolic cardiomyopathy.53 Thus, in early HF, utilization of FFAs is unchanged or slightly augmented, while utilization of glucose is increased. With further progression of the disease, a steady decline in utilization of both FFAs and glucose is invariably observed, with an increase in myocardial MVO2 and impaired energy delivery to the myofibrils. Whether these changes are simply a consequence of HF or whether they contribute to contractile dysfunction remains unclear.
A promising approach to metabolic therapies in patients with HF is to modulate substrate utilization.54 Carnitine palmitoyltransferase-1 is a key regulator of the uptake of fatty-acyl-CoA, the activated form of FFAs, in the mitochondria.55 Therefore, a reduction in its activity results in a shift in myocardial energy metabolism from FFA to glucose utilization. Etomoxir is a hypoglycaemic drug that exerts its effects by irreversibly inhibiting mitochondrial CPT-1 and long chain FFA oxidation.56 The decline in intracellular levels of acetyl-CoA and the reduced NADH/NAD ratio that develops secondary to the reduction in FFA oxidation are associated with increased activity of pyruvate dehydrogenase and phosphofructokinase, and enhanced glycolysis and glucose oxidation,57 as well as relief of the inhibitory effects of long-chain acyl-CoA on the mitochondrial adenine nucleotide carrier, with improved supply of ATP to the cytosol.58 Furthermore, in the myocyte, these metabolic changes appear to induce the expression of the sarcoendoplasmic calcium-ATPase (SERCA), which may lead to improved calcium (Ca2+) handling.59 Consistent with the in vitro data, in an initial study etomoxir appeared to improve myocardial function and clinical status in patients with HF.60 However, a subsequent trial in patients with moderate HF was stopped prematurely because the use of this agent was associated with elevation in liver function tests.61 Perhexiline is a potent inhibitor of CPT-1 and CPT-2.62 In a small study, HF patients treated with perhexiline had improved MVO2max, left ventricular ejection fraction (LVEF), symptoms, resting and peak stress myocardial function, and skeletal muscle energetics.54 However, further studies are needed to better characterize its efficacy and safety in HF patients.
Trimetazidine is a partial inhibitor of 3-ketoacyl CoA thiolase, the terminal enzyme of β-oxidation.63 It has been shown to improve glucose oxidation,64 reduce Ca2+ current,65 and prevent Ca2+ overload.65 Moreover, in a rat model of isoprenaline-induced myocardial injury, trimetazidine protected against myocardial damage by preserving the ATP pool, reducing lipid peroxidation, inhibiting the catecholamine-mediated increase of intracellular diastolic Ca2+ concentration, and preventing reductions in sarcoplasmic reticulum Ca2+ content, SERCA activity, and L-type Ca2+ channel density in cardiomyocytes.66 Since Ca2+ transients may impair mitochondrial Ca2+ uptake and the bioenergetic feedback response, the improvement of Ca2+ handling by trimetazidine may positively affect the state of energy starvation in failing hearts.67 In HF patients, trimetazidine ameliorated symptoms and increased LVEF, independent of changes in FFA uptake, myocardial perfusion, oxidative metabolism, and work efficiency.68 Thus, trimetazidine appears to be a potential treatment for HF.
Ranolazine is similar in structure to trimetazidine.69 Even if it inhibits FFA metabolism at high serum concentrations,70 its main mechanism of action in the myocyte appears to be related to inhibition of the late inward sodium (Na+) channel.71 In the failing myocyte, the mechanisms of inactivation of this channel are abnormal and lead to Na+-triggered Ca2+ overload, with subsequent major contractile and electrophysiological disturbances.72 Furthermore, recent evidence indicates that Na+-mediated Ca2+ overload plays an important role in the mismatch between excitation/contraction coupling and oxidative phosphorylation present in the failing myocardium.67 Consistent with the in vitro evidence, in a dog model of HF, acute infusion of ranolazine improved LVEF and mechanical efficiency without increasing MVO2. Also, positive haemodynamic effects were observed after 3 months of oral treatment with ranolazine, alone or in combination with metoprolol or enalapril. Additionally, ranolazine exerted beneficial effects on myocardial hypertrophy, fibrosis, and capillary density. This animal evidence is complemented by ex vivo data conducted on isometrically contracting ventricular muscle strips from end-stage failing human hearts, in which ranolazine reduced the frequency-dependent increase in diastolic tension without exerting negative inotropic effects. However, in the TIMI-36 trial, treatment with ranolazine did not affect the rates of hospitalization for HF in patients with non ST-elevation acute coronary syndromes.73
Dichloroacetate (DCA) inhibits FFA oxidation, thus increasing myocardial glucose utilization. Its mechanism of action involves the inhibition of PDK, which leads to increased activity of the mitochondrial pyruvate dehydrogenase complex. This, in turn, promotes conversion of pyruvate to acetyl-CoA, which can then enter oxidative phosphorylation.50 In ex vivo studies, DCA enhanced the post-ischaemic recovery of cardiac function and increased cardiac efficiency by improving coupling between glycolysis and glucose oxidation.74 In nine patients with CAD, DCA increased LV stroke volume and myocardial efficiency.75 Further animal and human studies are needed to better characterize the therapeutic potential and safety profile of DCA.
A decrease in FFA oxidation with a parallel increase in pyruvate oxidation may be achieved by increasing myocardial levels of malonyl-CoA through the inhibition of malonyl-CoA decarboxylase (MCD), the main enzyme involved in the degradation of cardiac malonyl-CoA. Animal studies suggest that MCD inhibition exerts positive effects on cardiac function and cardiac efficiency, suggesting that this approach could be an effective means to modulate myocardial metabolism in HF.76
A note of caution should be added regarding the use of metabolic modulators to inhibit the availability of FFA, given the observation that depletion of FFA with acipimox leads to decreased myocardial efficiency in patients with idiopathic dilated cardiomyopathy.77
Non-pharmacological treatments have also shown potential as modulators of myocardial metabolism, thus improving myocardial performance.77 In particular, 4-weeks of treadmill training in a post-infarct rabbit model of HF induced an up-regulation of heart-type fatty acid-binding protein (h-FABP), a mediator of FFA transport across the sarcolemma of the cardiomyocytes.44 This protein appeared to be down-regulated 12 weeks after ligation of the anterior descending coronary artery in rabbits kept at rest; however, treadmill training elevated h-FABP expression, suggesting that exercise may elevate myocardial lipid uptake.
AMP-activated protein kinase
AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme expressed in nearly all mammalian tissues, including the myocardium. It is a key regulator of the metabolic changes that occur in myocardial cells, lies at the crossroads of multiple intracellular metabolic pathways, and responds to a variety of stressors and signals, ultimately decreasing ATP-consuming pathways (Figure 3). AMPK is highly sensitive to changes in AMP levels and is thus affected by changes in enzymes involved in phosphoryl transfers. The transfer of high-energy phosphates couples mitochondrial ATP production to cytosolic demand and provides an efficient mechanism for distributing energy locally to myofibrils.78 Levels of key phosphoryl transfer enzymes are altered in myocardial dysfunction; specifically, decreases in creatine kinase activity result in reduced shuttling of high-energy phosphates, which is only partially offset by the increased activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 3-phosphoglycerate kinase (PGK). Interestingly, levels of adenylate kinase (AK), which catalyses the production of AMP and ATP from two molecules of ADP, are not greatly altered in murine models of HF.79 However, because of its role in AMP production, AK probably plays an important role in regulating the activity of AMPK. The lack of AK up-regulation in states of cardiac dysfunction and the consequences for AMPK activity are unknown.
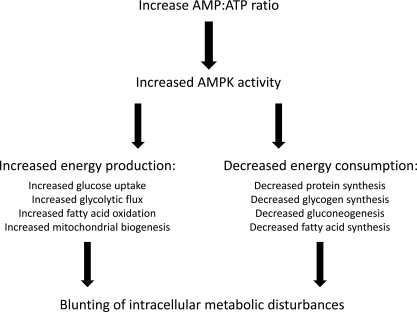
Figure 3.
Role of AMP-activated protein kinase (AMPK) in cellular energy homeostasis. Not all processes have been demonstrated in myocardial cells.
AMP-activated protein kinase is found in abundance in myocytes, but its role in normal myocyte function remains incompletely understood. In the heart, AMPK regulates fatty acid uptake and oxidative phosphorylation. It inactivates acetyl-CoA carboxylase (ACC), thereby reducing the production of malonyl-CoA, a known inhibitor of CPT-1.80 Therefore, through the inhibition of ACC, AMPK increases CPT-1-dependent fatty acid oxidation to increase energy production in cardiomyocytes. AMPK also stimulates glucose uptake by stimulating the translocation of GLUT4 transporters and increases glycolysis by activating phosphofructokinase. Thus AMPK activation is an important regulator of the cellular response to low energy states such as hypoxia and exercise.
The roles played by AMPK in cardiovascular diseases are multiple and varied. Dysregulated AMPK activation has been postulated to contribute to the development of diabetes and the metabolic syndrome, as downstream targets include key components of many metabolic pathways.81,82 AMPK also regulates intracellular pathways that promote normal endothelial function.83 Further, AMPK plays an important role in the response to myocardial ischaemia and the development of post-ischaemic LV dysfunction.84
Alterations in AMPK function play a role in the development and progression of HF. The failing heart undergoes numerous metabolic changes, among them a reduction in total cellular ATP levels, resulting in an increase in the AMP:ATP ratio.85 In animal models of hypertrophy-induced HF, there are notable decreases in high-energy phosphates, marked increases in the cellular AMP:ATP ratio, increased GLUT1 expression, and a significant increase in AMPK activity.86 Ultimately, this serves to increase ATP production and decrease ATP consumption. In the failing heart, the alterations induced by AMPK may not be enough to overcome the chronic underlying metabolic derangements or other pathophysiological abnormalities. However, these adaptive changes induced by AMPK may slow the progression of cardiac dysfunction.
Genetic studies from rare cardiomyopathies provide further insight into the importance of AMPK. Mutations in PRKAG2, the gene encoding the γ2 subunit of AMPK, result in a novel form of glycogen storage disease that mimics hypertrophic cardiomyopathy. These mutations cause inappropriate myocardial glycogen deposition, ventricular pre-excitation (Wolff–Parkinson–White syndrome), and hypertrophy.87 This leads to sudden cardiac death and progressive HF.88 This condition is due to the reduced or absent responsiveness of AMPK to AMP, which permits abnormally elevated AMPK activity that results in excess glycogen deposition.89,90
AMP-activated protein kinase is also an emerging therapeutic target. The only AMPK-modulating drugs presently on the market act indirectly. The antidiabetic drug metformin has been associated with reduced cardiovascular death and myocardial infarction in diabetics and is known to activate AMPK.91 In mouse models of ischaemia-induced HF, metformin treatment significantly improved survival and post-infarct LV systolic function,92 an effect lost in dominant negative AMPK α2-expressing mice. Metformin also reduces cardiomyocyte apoptosis in cell culture models and blunted the LV dysfunction associated with tachycardia-mediated HF in dogs.93 A recent paper suggests that metformin activates AMPK through inhibition of AMP deaminase.94 Thiazolidinediones and statins have also been shown to activate AMPK indirectly.95
Drugs that directly act on AMPK are not yet clinically available. Acadesine (AICAR) is an adenosine analogue that is a direct AMPK activator. It was initially developed in the 1980s and 1990s as an agent to reduce peri-operative ischaemia at the time of coronary artery bypass graft (CABG) surgery.96 It was thought to act by reducing adenosine uptake in the ischaemic heart. The drug has a short half-life, must be given intravenously, has the documented side effect of transient hyperuricaemia, and may be associated with significant hypoglycaemia and bradycardia. As such, AICAR has yet to be approved for routine clinical use.96
Summary
Heart failure is characterized by reduced myocardial performance, mainly due to the loss of myocardial tissue, reduced function of viable myocytes, or a combination of both. In this disorder, the heart is in a functionally impaired state characterized by the presence of viable, but dysfunctional, myocardium. The process that leads to impaired myocardial function is mediated through several pathways, including early metabolic derangements and increased adrenergic tone. These, in turn, may lead to further changes in metabolic dysregulation and the progression of myocardial dysfunction (Figure 4).
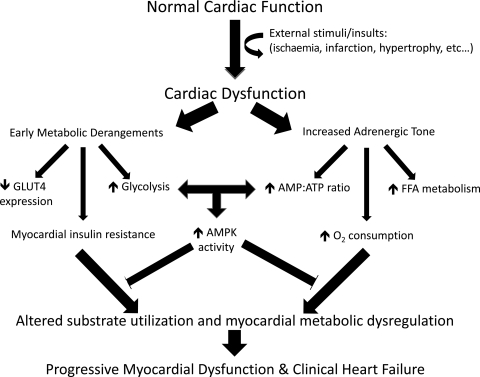
Figure 4.
Progressive metabolic derangements in heart failure. External insults or stimuli such as ischaemia/infarction or the development of cardiac hypertrophy are associated with early metabolic derangements that serve to increase glucose utilization and contribute to early insulin resistance. This cardiac dysfunction also leads to increased adrenergic tone, which has delayed effects on fatty acid metabolism and the cellular AMP:ATP ratio, ultimately compounding the early metabolic derangements and resulting in decreased myocardial energy efficiency and increased myocardial oxygen consumption. These metabolic derangements are partly blunted by the effects of the key metabolic regulator, AMP-activated protein kinase (AMPK). However the effects of AMPK cannot fully rectify these problems, and the alteration in substrate utilization and global myocardial metabolic dysregulation contribute to progressive myocardial dysfunction and overt heart failure.
Myocardial metabolism is altered in HF, and substrate utilization switches from mostly fatty acids to glucose. Despite these alterations, there are currently no drugs that directly target cardiac metabolism for the treatment of HF. Better characterization of the pathways that regulate energy production and metabolic function may potentially lead to new therapies for HF. In the last few years, a number of drugs targeting cardiac metabolism have been tested, and some of them appear to be promising potential therapies for HF. More research is needed to better elucidate the role of these agents in the management of HF.
Funding
The National Institutes of Health (grants K02 HL107448, R01 HL087149, R01 HL104181, and 1PO1 HL108795 to H.A.; grant PO1-HL074237-06 to H.N.S.; grant K12-HL083790to U.C.).
Conflict of interest: P.L.: grant support from Pfizer, Novartis, Amgen, and Roche. G.C.F.: Consultant, Novartis; Honorarium, Medtronic. M.G. has received research grants from the National Institutes of Health (NIH), Otsuka, Sigma Tau, Merck, and Scios, and is/has been a consultant for Abbott, Astellas, AstraZeneca, Bayer Schering Pharma, CorThera, Cytokinetics, DebioPharm, Errekappa Terapeutici, GlaxoSmithKline, Johnson & Johnson, Medtronic, Merck, Novartis, Otsuka, Pericor Therapeutics, Protein Design Laboratories, Sanofi Aventis, Sigma Tau, and Solvay. H.A.: Honorarium, Merck and Boehringer-Ingelheim. All other authors declare no conflict of interest.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 3.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, Mule JD, Vered Z, Lahiri A. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 5.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. doi: 10.1161/01.CIR.0000139340.88769.D5. [DOI] [PubMed] [Google Scholar]
- 6.Shen W, Vatner DE, Vatner SF, Ingwall JS. Progressive loss of creatine maintains a near normal DeltaG approximately (ATP) in transgenic mouse hearts with cardiomyopathy caused by overexpressing Gsalpha. J Mol Cell Cardiol. 2010;48:591–599. doi: 10.1016/j.yjmcc.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Taegtmeyer H, Harinstein ME, Gheorghiade M. More than bricks and mortar: comments on protein and amino acid metabolism in the heart. Am J Cardiol. 2008;101:3E–7E. doi: 10.1016/j.amjcard.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 11.van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 13.Tang WH, Francis GS. Neurohormonal upregulation in heart failure. Heart Fail Clin. 2005;1:1–9. doi: 10.1016/j.hfc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960;5:370–382. doi: 10.1016/0002-9149(60)90084-9. [DOI] [PubMed] [Google Scholar]
- 16.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer A, Klein G, Brand B, Lippolt P, Drexler H, Meyer GP. Evaluation of left ventricular diastolic function by pulsed Doppler tissue imaging in mice. J Am Soc Echocardiogr. 2003;16:1144–1149. doi: 10.1067/S0894-7317(03)00679-5. [DOI] [PubMed] [Google Scholar]
- 18.Christensen G, Chen J, Ross J, Chien KR. Mouse models of human cardiovascular disease. In: Chien KR, editor. Molecular Basis of Cardiovascular Disease: A Companion to Braunwald's Heart Disease. 2nd. Philadelphia: Saunders; 2004. pp. p72–106. [Google Scholar]
- 19.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 20.Toischer K, Rokita AG, Unsöld B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Krüger M, Linke WA, Backs J, Regitz-Zagrosek V, Schäfer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 22.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 23.Ichihara K, Abiko Y. Inhibition of endo- and epicardial glycogenolysis by propranolol in ischemic hearts. Am J Physiol. 1977;232:H349–H353. doi: 10.1152/ajpheart.1977.232.4.H349. [DOI] [PubMed] [Google Scholar]
- 24.Kantor PF, Robertson MA, Coe JY, Lopaschuk GD. Volume overload hypertrophy of the newborn heart slows the maturation of enzymes involved in the regulation of fatty acid metabolism. J Am Coll Cardiol. 1999;33:1724–1734. doi: 10.1016/s0735-1097(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, Givertz MM. Modulation of myocardial energetics: emerging evidence for a therapeutic target in cardiovascular disease. Circulation. 2005;112:3218–3221. doi: 10.1161/CIRCULATIONAHA.105.581819. [DOI] [PubMed] [Google Scholar]
- 26.Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. AmJ Physiol Heart Circ Physiol. 2001;280:H977–H983. doi: 10.1152/ajpheart.2001.280.3.H977. [DOI] [PubMed] [Google Scholar]
- 27.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–H1351. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 28.Chavez PN, Stanley WC, McElfresh TA, Huang H, Sterk JP, Chandler MP. Effect of hyperglycemia and fatty acid oxidation inhibition during aerobic conditions and demand-induced ischemia. Am J Physiol Heart Circ Physiol. 2003;284:H1521–H1527. doi: 10.1152/ajpheart.00974.2002. [DOI] [PubMed] [Google Scholar]
- 29.Abel ED. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 30.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–481. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 31.Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J, Delbridge LM. Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation. J Mol Cell Cardiol. 2010;48:663–672. doi: 10.1016/j.yjmcc.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42:246–253. doi: 10.1016/s0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 33.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Sodi-Pallares D, Testelli MR, Fishleder BL, Bisteni A, Medrano GA, Friedland C, De Micheli A. Effects of an intravenous infusion of a potassium–glucose–insulin solution on the electrocardiographic signs of myocardial infarction. A preliminary clinical report. Am J Cardiol. 1962;9:166–181. doi: 10.1016/0002-9149(62)90035-8. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- 37.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenstrom A. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 39.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 40.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via pp38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 42.Bersin RM, Stacpoole PW. Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J. 1997;134:841–855. doi: 10.1016/s0002-8703(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 43.Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005;91:161–165. doi: 10.1136/hrt.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 45.Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 46.Frayn KN, Arner P, Yki-Jarvinen H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006;42:89–103. doi: 10.1042/bse0420089. [DOI] [PubMed] [Google Scholar]
- 47.Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005;1706:1–11. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem. 2001;276:20240–20244. doi: 10.1074/jbc.M102540200. [DOI] [PubMed] [Google Scholar]
- 50.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 51.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. 2006;12:644–652. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 55.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 56.Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res. 1988;63:1036–1043. doi: 10.1161/01.res.63.6.1036. [DOI] [PubMed] [Google Scholar]
- 57.Selby PL, Sherratt HS. Substituted 2-oxiranecarboxylic acids: a new group of candidate hypoglycaemic drugs. Trends Pharmacol Sci. 1989;10:495–500. doi: 10.1016/0165-6147(89)90049-7. [DOI] [PubMed] [Google Scholar]
- 58.Paulson DJ, Noonan JJ, Ward KM, Stanley H, Sherratt A, Shug AL. Effects of POCA on metabolism and function in the ischemic rat heart. Basic Res Cardiol. 1986;81:180–187. doi: 10.1007/BF01907382. [DOI] [PubMed] [Google Scholar]
- 59.Vetter R, Rupp H. CPT-1 inhibition by etomoxir has a chamber-related action on cardiac sarcoplasmic reticulum and isomyosins. Am J Physiol. 1994;267:H2091–H2099. doi: 10.1152/ajpheart.1994.267.6.H2091. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000;99:27–35. [PubMed] [Google Scholar]
- 61.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy JA, Kiosoglous AJ, Murphy GA, Pelle MA, Horowitz JD. Effect of perhexiline and oxfenicine on myocardial function and metabolism during low-flow ischemia/reperfusion in the isolated rat heart. J Cardiovasc Pharmacol. 2000;36:794–801. doi: 10.1097/00005344-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Lopaschuk GD, Barr R, Thomas PD, Dyck JR. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2003;93:e33–e37. doi: 10.1161/01.RES.0000086964.07404.A5. [DOI] [PubMed] [Google Scholar]
- 64.Renaud JF. Internal pH, Na+, and Ca2+ regulation by trimetazidine during cardiac cell acidosis. Cardiovasc Drugs Ther. 1988;1:677–686. doi: 10.1007/BF02125756. [DOI] [PubMed] [Google Scholar]
- 65.Kiyosue T, Nakamura S, Arita M. Effects of trimetazidine on action potentials and membrane currents of guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1986;18:1301–1311. doi: 10.1016/s0022-2828(86)80433-3. [DOI] [PubMed] [Google Scholar]
- 66.Meng D, Feng L, Chen XJ, Yang D, Zhang JN. Trimetazidine improved Ca2+ handling in isoprenaline-mediated myocardial injury of rats. Exp Physiol. 2006;91:591–601. doi: 10.1113/expphysiol.2005.032615. [DOI] [PubMed] [Google Scholar]
- 67.Kohlhaas M, Maack C. Adverse bioenergetic consequences of Na+–Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation. 2010;122:2273–2280. doi: 10.1161/CIRCULATIONAHA.110.968057. [DOI] [PubMed] [Google Scholar]
- 68.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 69.Scirica BM, Morrow DA. Ranolazine in patients with angina and coronary artery disease. Curr Cardiol Rep. 2007;9:272–278. doi: 10.1007/BF02938375. [DOI] [PubMed] [Google Scholar]
- 70.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 71.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 Suppl 4:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 74.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–948. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 75.Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol. 1988;61:65–70. doi: 10.1016/0002-9149(88)91306-9. [DOI] [PubMed] [Google Scholar]
- 76.Lopaschuk GD, Stanley WC. Malonyl-CoA decarboxylase inhibition as a novel approach to treat ischemic heart disease. Cardiovasc Drugs Ther. 2006;20:433–439. doi: 10.1007/s10557-006-0634-0. [DOI] [PubMed] [Google Scholar]
- 77.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 78.Ingwall JS, Shen W. On energy circuits in the failing myocardium. Eur J Heart Fail. 2010;12:1268–1270. doi: 10.1093/eurjhf/hfq193. [DOI] [PubMed] [Google Scholar]
- 79.Aksentijevic D, Lygate CA, Makinen K, Zervou S, Sebag-Montefiore L, Medway D, Barnes H, Schneider JE, Neubauer S. High-energy phosphotransfer in the failing mouse heart: role of adenylate kinase and glycolytic enzymes. Eur J Heart Fail. 2010;12:1282–1289. doi: 10.1093/eurjhf/hfq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 82.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 83.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 84.Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes (Lond) 2008;32 Suppl 4:S29–S35. doi: 10.1038/ijo.2008.120. [DOI] [PubMed] [Google Scholar]
- 85.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 87.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 89.Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, Tian R, Goodyear LJ, Berul CI, Ingwall JS, Seidman CE, Seidman JG. Increased alpha2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation. 2005;112:3140–3148. doi: 10.1161/CIRCULATIONAHA.105.550806. [DOI] [PubMed] [Google Scholar]
- 90.Zou L, Shen M, Arad M, He H, Lofgren B, Ingwall JS, Seidman CE, Seidman JG, Tian R. N488I mutation of the gamma2-subunit results in bidirectional changes in AMP-activated protein kinase activity. Circ Res. 2005;97:323–328. doi: 10.1161/01.RES.0000179035.20319.c2. [DOI] [PubMed] [Google Scholar]
- 91.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 94.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 96.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116:607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]