Abstract
Down syndrome (DS) is the most common genetic cause of intellectual disability in children, and the number of adults with DS reaching old age is increasing. By the age of 40 years, virtually all people with DS have sufficient neuropathology for a postmortem diagnosis of Alzheimer disease (AD). Trisomy 21 in DS leads to an overexpression of many proteins, of which at least two are involved in oxidative stress and AD: superoxide dismutase 1 (SOD1) and amyloid precursor protein (APP). In this study, we tested the hypothesis that DS brains with neuropathological hallmarks of AD have more oxidative and nitrosative stress than those with DS but without significant AD pathology, as compared with similarly aged-matched non-DS controls. The frontal cortex was examined in 70 autopsy cases (n=29 control and n=41 DS). By ELISA, we quantified soluble and insoluble Aβ40 and Aβ42, as well as oligomers. Oxidative and nitrosative stress levels (protein carbonyls, HNE-bound proteins, and 3-nitrotyrosine) were measured by slot-blot. We found that soluble and insoluble Aβ and oligomers increase as a function of age in DS frontal cortex. Of the oxidative stress markers, HNE-bound proteins were increased overall in DS. Protein carbonyls were correlated with Aβ40 levels. These results suggest that oxidative damage, but not nitrosative stress, may contribute to the onset and progression of AD pathogenesis in DS. Conceivably, treatment with antioxidants may provide a point of intervention to slow pathological alterations in DS.
Keywords: Alzheimer disease, 4-hydroxy-2-nonenal, 3-nitrotyrosine, oligomers, protein carbonyl, trisomy 21
Introduction
One of the most common genetic abnormalities in live-born children in the United States (1 in 700-1000) is Down syndrome (DS) (CDC, 2006). DS is linked to an extra copy of chromosome 21 (Lejeune et al., 1959). In addition to intellectual disability, children often have cardiac and gastrointestinal congenital malformations, various types of leukemia, growth retardation, immune disorders and other clinical pathologies (Roizen and Patterson, 2003). A key concern in adults with DS is an increased vulnerability to the development of Alzheimer disease (AD), which typically has an age of onset between 40-60 years (Schupf, 2002).
A link between DS and AD has been established (Ball and Nuttall, 1980; Lott, 1982; Lott and Head, 2001; Bush and Beail, 2004). Virtually all DS adults over the age of 40 years show neuropathological hallmarks of AD, including senile plaques (SPs) and neurofibrillary tangles (NFTs) (Wisniewski et al., 1985; Mann and Esiri, 1989; Hof et al., 1995). SPs are primarily composed of amyloid beta peptide (Aβ) produced via sequential cleavage of the amyloid precursor protein (APP) by beta- and gamma-secretase (Shoji et al., 1992). Several peptides of varying length are produced, but the most actively studied are the 42 amino acid fragment (Aβ42) and the more soluble 40 amino acid peptide (Aβ40) (Selkoe, 1994). Aβ42 shows a higher propensity to adopt neurotoxic conformations, including oligomers (Pike et al., 1991; Li et al., 2009). Oligomers of Aβ have been increasingly implicated in the initiation and pathogenesis of AD, while monomeric forms of Aβ may be less harmful (Walsh et al., 2002; Giuffrida et al., 2009). In DS, the accumulation of Aβ42 in brain can be observed as young as between 8-12 years of age (Lemere et al., 1996; Leverenz, 1998). The extent of SP deposition increases markedly between 35-45 years, with NFTs developing after SPs (Wisniewski et al., 1985; Mann et al., 1988). Deposits of Aβ in DS are first seen in the frontal and entorhinal cortex and spread to other cortical regions and layers with increasing age (Azizeh et al., 2000). Interestingly, the incidence of dementia typically does not increase until adults with DS are over the age of 50 years (Lai, 1989; Lott and Head, 2001; Tyrrell et al., 2001; Schupf et al., 2007), suggesting a ~10 year prodromal phase where clinical signs are minimal or not detectable.
Similar to AD (Hensley et al., 1995; Smith et al., 1996; Markesbery, 1997; Aksenov et al., 2001; Butterfield et al.), Aβ accumulation in DS is associated with enhanced formation of reactive oxygen species (ROS) in neurons leading to premature neuronal dysfunction and death as a consequence of increased oxidative stress (Kedziora and Bartosz, 1988; Busciglio, 1995; Lott et al., 2006). Interestingly, intracellular Aβ accumulation is observed early in DS, prior to the accumulation of extracellular Aβ deposits (Cataldo, 2000; Cataldo et al., 2004). Subsequently high molecular weight aggregates of Aβ may accumulate, enhancing the deposition of plaques (Knauer et al., 1992; Head et al., 2001) and ROS production (Behl et al., 1994). Moreover, of many genes overexpressed due to trisomy 21, several are particularly relevant for the development of AD in DS. Among these, APP and cytoplasmic superoxide dismutase (Cu2+/Zn2+; SOD-1) play a pivotal role in the regulation of oxidative and nitrosative stress levels (Schuchmann and Heinemann, 2000; Butterfield et al., 2010b).
To characterize age- and AD-associated changes in oxidative and nitrosative stress in frontal cortex from DS autopsy cases, we quantified two markers of protein oxidation (protein carbonyls [PCs] and 3-nitrotyrosine [3-NT]) and a marker of protein modification that is a lipid peroxidation product (4-hydroxy-2-trans-nonenals [HNE]). In these samples, we also examined the levels of Aβ40 and Aβ42 and Aβ oligomers. We hypothesized that oxidative damage would be higher overall in DS as compared to non-DS, but further exacerbated with the development of AD neuropathology. In addition, we hypothesized that the extent of oxidative and nitrosative damage would be associated with levels of age-associated Aβ accumulation in DS brain.
Materials and methods
Subjects
DS and young or non-demented older control cases were obtained from the University of California-Irvine-ADRC Brain Tissue Repository, the Eunice Kennedy Shriver NICHD Brain and Tissue Bank for Developmental Disorders, and the University of Kentucky ADC. Table 1 shows the characteristics of the included cases. DS cases were divided into two groups, with or without sufficient pathology for a neuropathology diagnosis of AD. All cases with both DS and AD were over the age of 40 years. Thus for the current study, controls were split into two groups, either less than or equal to 40 years or older than 40 years at death. The post mortem interval (PMI) was different across groups, with the AD group overall having a lower PMI (F(3,66)=7.3 p<0.0005). A subset of these autopsy cases was used in a previous experiment measuring insoluble Aβ as a function of age in DS (Nistor et al., 2007). In the current study, additional cases were included, soluble Aβ was measured and the extent of oligomer accumulation was quantified.
Table 1.
Case Demographics
| Group | n | Gender (M/F) | Age (SEM) | PMI (SEM) |
|---|---|---|---|---|
| Young Control (YC) | 13 | 8/5 | 17.8 (3.7) | 16.2 (1.9) |
| Old Control (OC) | 16 | 7/9 | 50.8 (2.2) | 14.3 (2.0) |
| DS | 13 | 7/6 | 27.2 (5.1) | 16.0 (1.7) |
| DS+AD | 28 | 12/16 | 53.8 (2.5) | 6.91 (1.2) |
Sample preparation for oxidative stress measures
Brain tissue (frontal cortex) from non-DS controls, DS, and DS with AD were thawed in lysis buffer (pH 7.4) containing 320 mM sucrose, 1% of 1.0 M Tris-HCl (pH=8.8), 0.098 mM MgCl2, 0.076 mM EDTA, proteinase inhibitors leupeptin (0.5mg/mL), pepstatin (0.7μg/mL), aprotinin (0.5 mg/mL), and phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO). The brains were homogenized by 20 passes of a Wheaton tissue homogenizer, and the resulting homogenate was centrifuged at 14,000 × g for 10 min to remove cellular debris. The supernatant was extracted to determine the total protein concentration by the BCA method (Pierce, Rockford, IL).
Measurement of protein carbonyls (PCs)
Five μl of frontal cortex homogenate were derivatized with 10μl of 10mM 2,4-dinitrophenylhydrazine (DNPH) (OxyBlot™ Protein Oxidation Detection Kit, Chemicon-Millipore, Billerica, MA) in the presence of 5 μl of 10% sodium dodecyl sulfate (SDS) for 20 min at room temperature (25° C). The samples were then neutralized with 7.5 μl of 2M Tris in 30% glycerol. Protein samples (250 ng) were then loaded in each well on a nitrocellulose membrane with a slot-blot apparatus under vacuum. The membrane was blocked for 2 h with a solution of 3% (w/v) bovine serum albumin in PBS containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20. Membranes were incubated with rabbit polyclonal anti-DNP antibody (1:100 dilution, OxyBlot™ Protein Oxidation Detection Kit) for 2 h at room temperature. After washing with PBS, membranes were further incubated with alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (1:5000; Sigma Aldrich, St. Louis, MO) for 1 h at room temperature. Membranes were then washed with PBS three times for 5 min and developed using 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) color developing reagent for alkaline phosphatase activity (Sigma Aldrich). Blots were dried and scanned to TIF format using Adobe Photoshop on a Canoscan 8800F (Canon, Lake Success, NY). The images were quantified with Image Quant TL 1D version 7.0 software (GE Healthcare, Fairfield, CT).
Measurement of protein-bound 4-hydroxy-2-trans-nonenal (HNE-bound protein)
For the analysis of HNE-bound protein levels, 10 μl of frontal cortex homogenate were incubated with 10 μl of Laemmli buffer containing 0.125M Tris base pH 6.8, 4% (v/v) SDS, and 20% (v/v) glycerol. The resulting samples (250 ng per well) were loaded onto a nitrocellulose membrane with a slot-blot apparatus under vacuum pressure. The membrane was blocked as described above for 2 h and incubated with a rabbit polyclonal anti-4-hydroxynonenal antibody (1:3000; Alpha Diagnostics, San Antonio, TX) for 2 h at room temperature. Membranes were washed and incubated with anti-rabbit IgG alkaline phosphatase secondary antibody (1:5000; Sigma-Aldrich) for 1 h at room temperature. Membranes were then processed and quantified as described above.
Measurement of 3-nitrotyrosine (3-NT)
3-NT content was determined immunochemically as previously described (Butterfield et al., 2007). Briefly, 5 μL of frontal cortex homogenate were incubated with Laemmli sample buffer in a 1:2 ratio (0.125M Trizma base, pH 6.8, 4% SDS, 20% glycerol) for 20 min. Protein (250 ng per well) was then loaded onto the nitrocellulose membrane using the slot-blot apparatus as described above. Membranes were incubated with rabbit anti-nitrotyrosine antibody (1:1000; Sigma-Aldrich) for 2 h at room temperature. Membranes were then washed and incubated with alkaline phosphatase-linked anti-rabbit IgG secondary antibody (1:5000, Sigma-Aldrich) for 1 h at room temperature. Membranes were then processed and quantified as described above.
AβELISAs
Aβ was extracted from tissue measured as previously described (Beckett et al., 2010). Briefly, frozen cortical samples were extracted sequentially in ice cold phosphate buffered saline (PBS, pH 7.4) with a complete protease inhibitor cocktail (PIC) (Amresco, Solon, OH) and centrifuged at 20,800 × g for 30 min. at 4°C. Following centrifugation, the supernatant was collected and the pellets were sonicated (10 × 0.5 sec pulses at 100W, Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA) in 2% SDS with PIC and centrifuged at 20,800 × g for 30 min. at 14°C. The supernatant was again collected and the remaining pellets were sonicated in 70% formic acid (FA), followed by centrifugation at 20,800 × g for 1 hour at 4°C.
FA-extracted material was initially neutralized by a 1:20 dilution in TP buffer (1 M Tris base, 0.5 M Na2HPO4), followed by a further dilution as needed (1:100 to 1:400) in Antigen Capture buffer (AC) (20mM Na3PO4, 0.4% Block Ace (AbD Serotec), 0.05% NaN3, 2mM EDTA, 0.4M NaCl, 0.2% BSA, 0.05% CHAPS, pH 7) . SDS soluble fractions were diluted (1:20) in AC buffer alone. PBS fractions were diluted 1:4 in AC buffer alone.
Aβ was measured in tissue samples using a standard, well-characterized two-site sandwich ELISA as described previously [47]. Briefly, an Immulon 4HBX plate was coated with 0.5 ug antibody per well, incubated overnight at 4°C, and blocked with a solution of Synblock (AbD Serotec, Raleigh, NC), as per the manufacturer's instructions. Antigen capture was performed using monoclonal antibody Ab9 (against Human Aβ1-16). Antigen detection was performed using biotinylated antibodies 13.1.1 (specific for Aβ40) and 12F4 (specific for Aβ42; Covance, Princeton, NJ).
A peptide standard curve of Aβ was run on the same plate for comparison, and standards and samples were run at least in duplicate; Aβ values were determined by interpolation relative to the standard curve. Plates were washed between steps with standard PBS containing 0.05% Tween-20 (2-4x) followed by PBS (2-4x). Plates were developed with TMB reagent (KPL, Inc., Gaitherburg, MD), stopped with 6% o-phosphoric acid, and read at 450 nm using a multiwell plate reader (BioTek, Winooski, VT).
Oligomer assay
Aβ oligomers from the SDS-soluble fraction were measured using a single-site sandwich ELISA as described above, except the same antibody (4G8; Covance, Princeton, NJ) was used for capture and detection. SDS samples were diluted 1:50 in AC buffer. Synthetic Aβ42 oligomers were used to prepare a standard curve; oligomeric Aβ values were determined by interpolation relative to the standard curve.
Statistical analysis
A univariate analysis of covariance (ANCOVA) with PMI as the covariate was used to compare groups on different outcome measures reflecting oxidative stress. In these analyses, we compared DS v. non-DS. Further, we placed DS cases into two groups, either having insufficient pathology for a diagnosis of AD or having sufficient neuropathology for a diagnosis of AD. Since the majority of DS cases with AD neuropathology were over 40 years, the control cases were categorized as either ≤40 or >40 years old. For comparison of two groups, independent t-tests were used. Spearman rank correlations were calculated to test the association between age and oxidative damage, as well as oxidative damage and Aβ. All statistics were calculated using PASW (IBM, Chicago, IL) and evaluated using a p-value of <0.05.
Results
Effect of PMI and gender on oxidative and nitrosative stress markers levels in brain from control, DS, and DS with AD subjects
We first determined whether PMI was a significant contributor to the various measures of oxidative damage, given that DS with AD cases overall had shorter PMIs. The correlation between PMI and PCs (r=-0.34, p=0.004), 3-NT (r=-0.015, p=0.90) and HNE-bound proteins (r=-0.10, p=0.41) show that PCs are negatively correlated with PMI. Thus, for subsequent analyses, PMI was included as a covariate. We then examined whether gender (n=34 male and n=35 female) was a contributor to the outcomes. All samples were combined for this analysis and an independent t-test did not show gender effects on PCs (t(67)=0.58, p=0.57), 3-NT (t(67)=-0.42, p=0.68), or HNE-bound proteins (t(67)=0.0.54, p=0.59).
Oxidative and nitrosative marker levels in control, DS, and DS with AD subjects
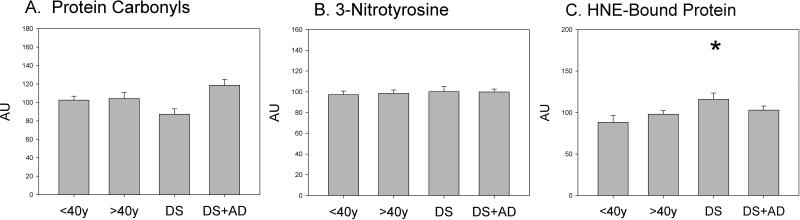
We tested the hypothesis that oxidative damage would be higher in DS overall relative to controls and higher still in DS cases with AD neuropathology. An ANCOVA was used with genotype (DS or Control) and age group (≤40y or >40y) as factors and PMI as a co-variate. No significant differences in PC were noted by genotype (F(1,69)=0.15, p=0.70), age group (F(1,69)=0.19, p=0.66), or interaction between genotype and age (F(1,69)=0.25 p=0.62) (Figure 1A), although there was a trend towards higher PC in DS cases with AD. Similarly, no significant differences in 3-NT were observed by genotype (F(1,69)=0.19 p=0.7), age group (F(1,69)=1.3 p=0.3), or interaction between genotype and age group (F(1,69)=0.4 p=0.5) (Figure 1B). Interestingly, HNE-bound proteins were significantly higher overall in DS cases ≤40 years of age (F(1,69)=5.6 p=0.02), but no significant differences were noted by age group (F(1,69)=0.02 p=0.9) or interaction between age and genotype (F(1,69)=1.59 p=0.2;Figure 1C).
Figure 1.
Levels of oxidative and nitrosative stress markers in control, DS, and DS with AD cases. Protein was extracted from frontal cortex and loaded on nitrocellulose membranes in a slot-blot apparatus. Membranes were probed with either anti-DNP protein adducts polyclonal antibody (Figure 1A), anti-nitrotyrosine polyclonal antibody (Figure 1B), or anti-HNE-bound protein polyclonal antibody (Figure 1C). Data are expressed as mean ± SEM (*p=0.02).
Aβ and oligomer accumulation in DS brain
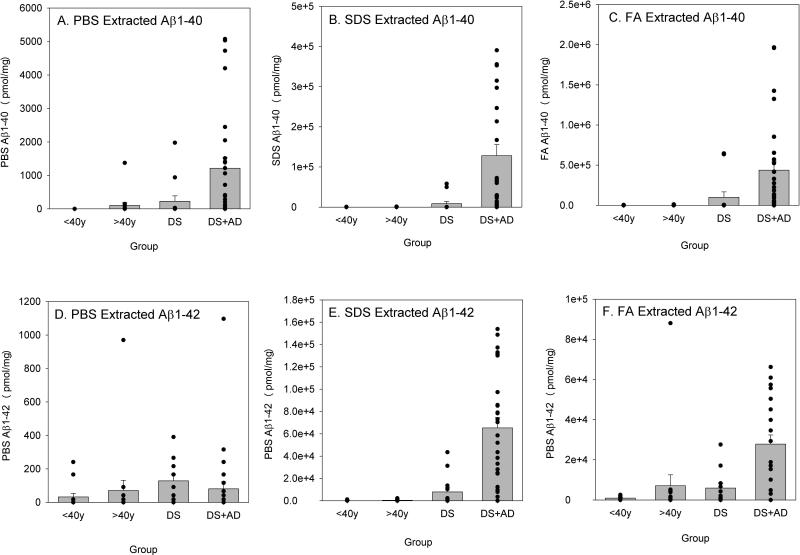
To determine if soluble or insoluble forms of Aβ as well as oligomers were differentially higher in individuals with DS ± AD relative to controls, a univariate analysis of variance was conducted. For virtually all Aβ outcome measures, there was individual variability, particularly in the older DS brains with significant AD neuropathology. PBS-soluble Aβ40 was significantly higher in individuals >40 years old (F(1,69)=4.21 p=0.044), but was not significant by genotype (F(1,69)=2.91 p=0.09) or interaction between age and genotype (F(1,69)=2.90 p=0.093;Figure 2A). SDS-extracted Aβ40 was significantly higher in DS (F(1,69)=4.79 p=0.032) and in cases >40 years old (F(1,69)=4.83 p=0.032). The interaction between the presence of DS and age >40 years was also significant (F(1,69)=4.81 p=0.032), with older DS individuals having the highest average amounts of SDS-extracted Aβ40 (Figure 2B). FA-extracted Aβ40 was significantly higher in individuals with DS (F(1,69)=3.9 p=0.05) and in individuals >40 years old (F(1,69)=3.92 p=0.05). Further, as with SDS-extracted Aβ40, FA-extracted Aβ40 was highest in adults with DS over the age of 40 years (F(1,69)=3.91 p=0.05) (Figure 2C).
Figure 2.
Soluble and insoluble Aβ40 and Aβ42 as a function of genotype and age group. PBS-, SDS-, and FA-extracted Aβ40 increased with age in both DS and controls (A, B, C). SDS- and FA-extracted Aβ40 were also significantly higher in DS (B, C) and highest in DS with AD. No genotype or age effects were noted for PBS-extracted Aβ42 (D). SDS-extracted Aβ42 was higher overall with age, with DS and DS with AD cases showing the highest levels overall (E). FA-extracted Aβ42 was higher in older cases with or without DS (F). Bars represent mean ± SEM. Closed circles indicate individual data points.
PBS-extracted Aβ42 did not differ across genotype groups or age groups; however, there was significant individual variability (Figure 2D). Similarly, older individuals had lower PBS Aβ42 than younger individuals (F(1,67)=3.76 p=0.57), regardless of genotype (Figure 3D). The interaction between genotype and age group was not significant. SDS-extracted Aβ42 was significantly higher overall in individuals with DS (F(1,69)=10.89 p=0.002) and in individuals >40 years old (F(1,69)=8.4 p=0.005). Further, adults with DS over the age of 40 years had the highest levels of SDS-extracted Aβ42 overall (F(1,69)=8.23 p=0.006;Figure 2E).
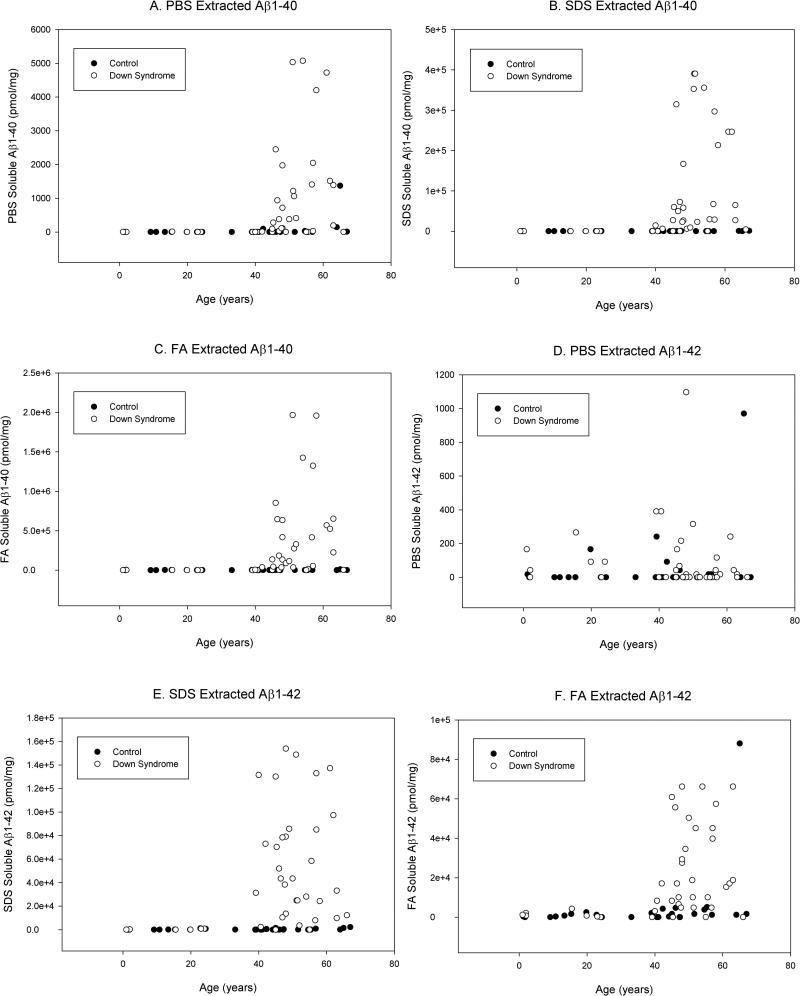
Figure 3.
Correlations between age and Aβ as a function of genotype. PBS-, SDS-, and FA-extracted Aβ40 increased with age in DS (A, B, C). However, in control cases, only SDS-extracted Aβ40 increased with age. SDS- and FA-extracted Aβ40 also increased with age in DS (E,F), although but PBS-extracted Aβ42 did not (D). As with SDS-extracted Aβ40, Aβ42 increased with age in control cases, although levels were not as high as in those with DS (E).
The effect of age on FA-extracted Aβ42 was similar to that of SDS-extracted Aβ42, with cases over 40 years having significantly higher levels of Aβ overall (F(1,69)=9.08 p=0.004). Although DS cases overall had higher levels of FA-extracted Aβ relative to controls, this difference only approached statistical significance due to large individual differences (F(1,69)=3.4 p=0.069) and the interaction between genotype and age was not significant (F(1,69)=3.22 p=0.078) (Figure 3F). The lack of significance for the interaction term was primarily due to one individual without DS over the age of 40 years with a substantially higher amount of FA-extracted Aβ42 than all the other cases (including DS). When this case was removed, FA-extracted Aβ42 was significantly different by both genotype (F(1,68)=7.9 p=0.006) and the interaction was also significant (F(1,68)=7.58 p=0.008), indicating that individuals with DS over the age of 40 years had higher levels of FA-extracted Aβ42 overall (Figure 2F).
As shown in Figure 4, all measures of Aβ except PBS-extracted Aβ42 were significantly correlated with age in DS cases (Table 2 shows correlation co-efficients). Interestingly, in control cases, SDS-extracted Aβ40 and Aβ42 were both correlated with age (Figure 3).
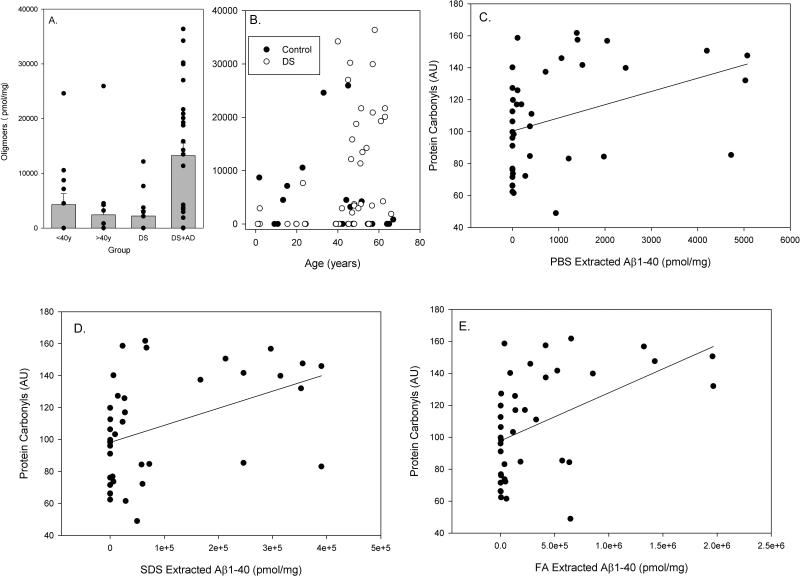
Figure 4.
Aβ Oligomer accumulation with age and DS and correlations between oxidative damage and Aβ. Oligomer accumulation was significantly higher in DS cases over the age of 40 years with significant AD neuropathology (A). Further, oligomers increased as a function of age in DS but not in control cases (B). Higher levels of SDS-extracted Aβ40 (D) and FA-extracted Aβ40 (E) were significantly correlated with higher levels of PCs. A similar trend was observed between PBS-extracted Aβ40 and PCs (C). Bars represent mean ± SEM. Closed circles indicate individual data points.
Table 2.
Correlations Between Aβ and Age in DS and Controls
| Genotype | n | PBS Extracted Aβ1-40 | SDS Extracted Aβ1-40 | FA Extracted Aβ1-40 |
|---|---|---|---|---|
| Control | 29 | 0.32 | 0.37* | 0.22 |
| DS | 40 | 0.37* | 0.38* | 0.37* |
| Genotype | n | PBS Extracted Aβ1-42 | SDS Extracted Aβ1-42 | FA Extracted Aβ1-42 |
|---|---|---|---|---|
| Control | 29 | 0.26 | 0.40* | 0.31 |
| DS | 40 | -0.08 | 0.37* | 0.42* |
p<.05
Oligomeric Aβ accumulation was not significantly different for genotype (F(1,69)=1.64 p=0.21), although and the main effect of age approached significance (F(1,69)=2.96 p=0.09). The interaction between genotype and age was significant (F(1,69)=6.08 p=0.02), which was a result of individuals with DS over the age of 40 years having significantly higher levels of Aβ oligomers (Figure 4A). The extent of PBS-extracted Aβ oligomers in DS (r=0.37 p=0.018) but not in control cases was correlated with age (Figure 4B).
Association between Aβ and oxidative damage in DS
In all Aβ measures, the DS cases and particularly those over the age of 40 years showed significant individual variability. Thus, we hypothesized that individual Aβ measures may reflect differences in the level of oxidative damage. A partial correlation co-efficient that controlled for PMI was calculated between Aβ measures and measures of oxidative damage. The amount of oligomeric Aβ was not correlated with PCs (r=0.17 p=0.16), NT (r=-0.07 p=0.55) or HNE (r=-0.097 p=0.43). Similarly, there were no correlations between any measure of Aβ42 and the extent of PCs (PBS Aβ42 r=-0.14 p=0.27; SDS Aβ42 r=0.02 p=0.89; FA Aβ42 r=0.13 p=0.31), HNE (PBS Aβ42 r=-0.03 p=0.0.83; SDS Aβ42 r=0.04 p=0.77; FA Aβ42 r=-0.10 p=0.43), or 3-NT (PBS Aβ42 r=-0.18 p=0.38; SDS Aβ42 r=-0.07 p=0.58; FA Aβ42 r=-0.14 p=0.91). There was a trend towards PBS-extracted Aβ40 being correlated with PCs, although the level of significance was marginal (r=0.310 p=0.058 n=36)(Figure 4C). SDS- (r=0.369 p=0.023 n=36) and FA- (r=0.39 p=0.016 p=36) extracted Aβ40 were correlated with significantly higher PC accumulation, but were not correlated with either HNE or 3-NT levels (Figure 4D, 4E).
Discussion
An imbalance between pro-oxidant stimuli and cellular antioxidant activity may lead to increased oxidative stress levels that may have an important role in the development of AD neuropathology in DS (Kedziora and Bartosz, 1988; Busciglio, 1995; Busciglio et al., 1998). Involvement of oxidative and nitrosative stress-induced neuronal damage is a well-established feature during the development of AD (Smith et al., 1996; Butterfield et al., 2010a; Butterfield et al., 2010b). In the current study, we provided new evidence of higher levels of oxidative damage in brains from individuals with DS, although measures of oxidative damage were not increased further with AD pathology. The frontal cortex of DS subjects had significantly increased HNE-bound proteins levels, a sensitive marker of lipid peroxidation, compared to non-DS controls. Lipid peroxidation leads to various aldehydic products, with 4-hydroxy-2-nonenal (HNE) being one of the most abundant (Uchida, 2003). HNE is a highly reactive alkenal responsible for the damaging effects of oxidative stress and linked to neurodegenerative diseases such as AD (Bradley et al.; Riahi et al.; Butterfield et al., 2010a).
This study extends earlier studies demonstrating that lipoperoxidation is enhanced in prenatal DS brains compared to non-DS controls (Brooksbank et al., 1985; Odetti et al., 1998). Pratico et al. also demonstrated that another marker of lipid peroxidation, 8,12-iso-iPF2α-VI, was significantly increased in the urine of young subjects with DS, as compared to age-matched controls (Pratico et al., 2000). Furthermore, cortical neurons cultured from prenatal DS cases exhibited the intracellular accumulation of ROS and increased lipid peroxidation, leading to neuronal apoptosis (Busciglio, 1995). A recent study showed that amniotic fluid from mothers carrying DS fetuses had significantly elevated markers of oxidation (Perluigi et al., 2011). HNE-bound proteins were also present in the embryonic brains of Ts1Cje mice, a transgenic animal model of DS (Ishihara et al., 2009). In combination, these data suggest that lipid peroxidation is an early and possibly chronic event in DS.
Increased lipid peroxidation in DS may be a result of the overexpression of one of the key enzymes in the regulation of oxidative stress, SOD1 (Groner et al., 1990; de Haan et al., 1997). Levels of SOD1 in cells from DS patients are approximately 50% greater than normal (Groner et al., 1994). In general, SOD1 is responsible for converting superoxide radical into hydrogen peroxide, which is subsequently neutralized by glutathione peroxidase or catalase (Iannello, 1999). If catalase or glutathione peroxidase activity in DS brains is not able to compensate for the increased superoxide dismutase level and activity, hydrogen peroxide may accumulate, leading to increased oxidative damage (Jovanovic, 1998; Pratico et al., 2000). Consistent with this hypothesis, gene transfection of SOD1 into two different cell lines leads to increased lipid peroxidation, elevated PCs, and a trend to toward increased levels of 8-hydroxyguanine and 3-NT (Lee et al., 2001). However, in the current study, 3-NT levels, a marker of protein nitration and nitrosative stress, was neither higher in DS nor further increased with AD neuropathology. Surprisingly, PCs, a marker of protein oxidation that typically shows robust increases in AD in the general population (Aksenov et al., 2001), showed a trend towards increasing levels in DS with AD neuropathology but did not reach statistical significance, most likely due to individual variability in DS cases.
The gene for APP, a key protein involved in AD, is located on chromosome 21 (Weidemann et al., 1989). The overexpression of APP in DS is associated with increased concentrations of Aβ in the brains of these individuals, and Aβ neuropathology has been well characterized in DS (Wisniewski et al., 1985; Mann et al., 1988; Hof et al., 1995). Aβ plaques have been observed as young as 8 years of age and consistently accumulate after 30 years of age (Lemere et al., 1996; Leverenz, 1998). The prevalence of dementia, however, does not increase substantially until after 50 years of age and some individuals with DS remain dementia-free (Tyrrell et al., 2001; Schupf, 2002). In an extension of previous work (Nistor et al., 2007), we now show that both soluble and insoluble Aβ40 and Aβ42 in frontal cortex increase with age in DS. The one exception appears to be PBS-soluble form of Aβ42 peptide, which did not show a consistent age or genotype association.
We provide novel information regarding oligomer accumulation in DS as a function of age and genotype in a large autopsy cohort. There were no significant group mean differences between cases with or without DS. However, individuals with DS over the age of 40 years with AD neuropathology have significantly higher levels of Aβ oligomers. Further, age and oligomer accumulation were significantly correlated in DS but not controls. These data confirm a previous case report in which immunohistochemistry was performed on the brain of a 46 year old subject with DS and AD, in which plaque-associated oligomer deposits were seen in the entorhinal and frontal cortex (Lott and Head, 2005). In addition, using an antibody that recognizes Aβ fibrils and soluble fibrillar oligomers, we previously demonstrated that DS individuals show an early age of fibrillar Aβ neuropathology onset in hippocampus and midfrontal gyrus, as compared to aged non-DS individuals (Sarsoza et al., 2009). In addition, age-dependent accumulation of Aβ fibrils increased as a function of age in DS, which was absent in non-DS controls (Sarsoza et al., 2009).
Once Aβ is cleaved from APP, it may appear first as a soluble isoform. Soluble isoforms of Aβ, including oligomers, protofibrils, and Aβ-derived diffusible ligands may initially accumulate inside neurons and may be more important in causing neuronal dysfunction than extracellular Aβ. These different assembly states of soluble Aβ show different toxicities (Deshpande et al., 2006; Lesne et al., 2006). For example, Aβ oligomers are toxic to neurons both in vitro (Lambert et al., 1998; Deshpande et al., 2006) and in vivo (Walsh et al., 2002; Lesne et al., 2006). Further, oligomeric Aβ may cause mitochondrial dysfunction through interactions dynamin-related protein 1, exacerbating the oxidative defect in DS (Manczak et al., 2011). Therefore, Aβ oligomers may be important in causing neuronal dysfunction before neuronal loss occurs during aging in DS (Karlsen and Pakkenberg, 2011). By studying the brains of individuals with DS that came to autopsy at a wide range of ages, it may be possible to determine temporal events associated with soluble and insoluble Aβ, oligomers, and intracellular Aβ that may precede the onset of clinical signs of dementia.
Aβ is associated with oxidative stress in vitro and in vivo (Hensley et al., 1994; Butterfield et al., 2001; Butterfield et al., 2010c). The presence of Aβ deposits in DS brain may be a critical factor in the generation of oxidative stress, similar to AD patients in the general population (Hensley et al., 1995; Smith et al., 1996; Markesbery, 1997). In turn, neuronal oxidative stress may lead to enhanced Aβ production (Nunomura, 2000; Pratico et al., 2001). Indeed, a previous report of oxidized Aβ in frontal and entorhinal cortex from AD and DS cases showed that this modification was associated with neuritic plaques, suggesting that oxidation of Aβ is an important event in plaque biogenesis (Head et al., 2001). Therefore, increased oxidative damage in DS may affect a variety of pathways.
Oxidative damage may lead to enhanced Aβ production (Nunomura, 2000; Pratico et al., 2001; Reddy, 2006) and vice versa, higher levels of Aβ may exacerbate ongoing oxidative damage (Behl et al., 1994; Huang et al., 1999; Varadarajan, 2000). In DS, abnormal APP processing may result from increases in reactive oxygen species production due to mitochondrial dysfunction (Busciglio et al., 2002). Increased oxidative damage reflecting overexpression of SOD-1 in DS may also impact degradation of Aβ as enzymes responsible for Aβ degradation and clearance, may themselves be vulnerable to oxidative damage. A rise in oxidized neprilysin, an enzyme responsible for degrading Aβ , in AD brain that may provide one mechanism underlying the accumulation of extracellular Aβ (Wang et al., 2003).
The data reported in this study demonstrate a selective positive correlation between one marker of oxidative stress (PCs) and the levels of Aβ40 peptide. Although Aβ42 is generally regarded as the more toxic of the two peptides, Aβ40 has been shown to be neurotoxic in neuronal cultures (Harris et al., 1995; Aksenov et al., 1998). Furthermore, Aβ40 may exert neurotoxic properties on neuronal progenitor cells, impairing the survival and differentiation of these cells by generating oxidative damage (Mazur-Kolecka et al., 2006). Lastly, Aβ40 fibrils are able to directly induce specific nitrosative and oxidative modifications such as oxidation, glycation, and 3-nitrotyrosination in cell proteins (Ill-Raga et al., 2010). However, it is surprising that we did not see an association between Aβ42 and oxidative damage. Indeed, Aβ42 is capable of causing oxidative stress through free radical reactions (Yatin et al., 1999). One possible explanation of these data could be related to the amount of Aβ42 and Aβ40 measured in frontal cortex. The amount of Aβ40 is consistently higher than Aβ42 in frontal cortex in DS, particularly in DS with AD. Therefore, we hypothesize that the oxidative stress observed in DS brains may be more strongly associated with Aβ40 than Aβ42. Interestingly, increasing plasma Aβ40 levels in adults with DS is associated with onset of dementia (Schupf et al., 2010). Interestingly, prior to Aβ deposition in DS, oxidized DNA/RNA rises with age but once Aβ42 is deposited in DS temporal cortex there appears to be a decrease in the level of oxidized DNA/RNA (Nunomura, 1999, 2000). It is possible that Aβ might serve an anti-oxidant role in DS and is consistent with the lack of further increase in oxidative damage noted in the current study in individuals with both DS and AD. We note, however, both measures of oxidative damage and Aβ were by immunohistochemical methods whereas the current study used bulk protein measures.
In summary, we demonstrate that oxidative stress, but not nitrosative stress, is present in DS brain. Specifically, markers of oxidative stress are significantly correlated with Aβ40 monomer levels. Lipid peroxidation appears to be an early event occurring in DS individuals, as it is present in both younger and older cases. Increased oxidative stress may leave the DS brain vulnerable to subsequent AD neuropathology. The oxidative and nitrosative protein modifications analyzed in the frontal cortex of DS, DS with AD, and control individuals can lead to dysfunctional proteins. Future experiments should focus on determining which proteins are modified by oxidative stress in DS. We also note that our measures represent bulk determinations of proteins, and not only will neuronal and glial cell populations contribute to these results but importantly, vascular cells will also contribute significantly. Proteomics analysis may be a useful approach for this type of investigation, resulting in a better understanding of which biological mechanisms are involved in the onset and progression of AD in DS, which and may then provide useful information for clinical trials in DS.
Highlights.
Older people with Down syndrome are at high risk for developing Alzheimer disease
Aβ and oligomers increase with age
Hydroxynonenal-bound proteins are increased in Down syndrome
Protein carbonyls were correlated with Aβ40 levels
Oxidative damage may contribute to Alzheimer disease pathogenesis in Down syndrome
Acknowledgements
This work was supported in part by grants from NIH to D.A.B [AG-05119], and to E.H. and F.S [HD-064993]. Additional funding was provided by NIH to the UCI ADRC (P50 AG16573) and to the UK ADC (P30 AG028383). Human tissue obtained from NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, was under contract HHSN275200900011C, Ref. No. N01-HD-9-0011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Aksenova MV, Markesbery WR, Butterfield DA. Amyloid beta-peptide (1-40)-mediated oxidative stress in cultured hippocampal neurons. Protein carbonyl formation, CK BB expression, and the level of Cu, Zn, and Mn SOD mRNA. Journal of molecular neuroscience : MN. 1998;10:181–192. doi: 10.1007/BF02761773. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Azizeh BY, Head E, Ibrahim MA, Torp R, Tenner AJ, Kim RC, Lott IT, Cotman CW. Molecular dating of senile plaques in the brains of individuals with Down syndrome and in aged dogs. Exp Neurol. 2000;163:111–122. doi: 10.1006/exnr.2000.7359. [DOI] [PubMed] [Google Scholar]
- Ball MJ, Nuttall K. Neurofibrillary tangles, granulovacuolar degeneration, and neuron loss in Down Syndrome: quantitative comparison with Alzheimer dementia. Ann Neurol. 1980;7:462–465. doi: 10.1002/ana.410070512. [DOI] [PubMed] [Google Scholar]
- Beckett TL, Niedowicz DM, Studzinski CM, Weidner AM, Webb RL, Holler CJ, Ahmed RR, LeVine H, 3rd, Murphy MP. Effects of nonsteroidal anti-inflammatory drugs on amyloid-beta pathology in mouse skeletal muscle. Neurobiology of disease. 2010;39:449–456. doi: 10.1016/j.nbd.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bradley MA, Xiong-Fister S, Markesbery WR, Lovell MA. Elevated 4-hydroxyhexenal in Alzheimer's disease (AD) progression. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooksbank BW, Martinez M, Balazs R. Altered composition of polyunsaturated fatty acyl groups in phosphoglycerides of Down's syndrome fetal brain. J Neurochem. 1985;44:869–874. doi: 10.1111/j.1471-4159.1985.tb12896.x. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Andersen JK, Schipper HM, Gilad GM, McCarty R, Marzatico F, Toussaint O. Stress, aging, and neurodegenerative disorders. Molecular mechanisms. Ann N Y Acad Sci. 1998;851:429–443. doi: 10.1111/j.1749-6632.1998.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid b precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Bush A, Beail N. Risk factors for dementia in people with down syndrome: issues in assessment and diagnosis. Am J Ment Retard. 2004;109:83–97. doi: 10.1352/0895-8017(2004)109<83:RFFDIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta. 2010a;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer's disease. Free Radic Res. 2010b;45:59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer's disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010c;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging. 2004;25:1263–1272. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Improved national prevalence estimates for 18 selected major birth defects--United States, 1999-2001. 2006. [PubMed]
- de Haan JB, Wolvetang EJ, Cristiano F, Iannello R, Bladier C, Kelner MJ, Kola I. Reactive oxygen species and their contribution to pathology in Down syndrome. Adv Pharmacol. 1997;38:379–402. doi: 10.1016/s1054-3589(08)60992-8. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner Y, Elroy-Stein O, Avraham KB, Yarom R, Schickler M, Knobler H, Rotman G. Down syndrome clinical symptoms are manifested in transfected cells and transgenic mice overexpressing the human Cu/Zn-superoxide dismutase gene. J Physiol (Paris) 1990;84:53–77. [PubMed] [Google Scholar]
- Groner Y, Elroy-Stein O, Avraham KB, Schickler M, Knobler H, Minc-Golomb D, Bar-Peled O, Yarom R, Rotshenker S. Cell damage by excess CuZnSOD and Down's syndrome. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1994;48:231–240. doi: 10.1016/0753-3322(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer's beta-amyloid peptide (1-40) in cultured hippocampal neurons. Experimental neurology. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Head E, Garzon-Rodriguez W, Johnson JK, Lott IT, Cotman CW, Glabe C. Oxidation of Abeta and plaque biogenesis in Alzheimer's disease and Down syndrome. Neurobiol Dis. 2001;8:792–806. doi: 10.1006/nbdi.2001.0431. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down's syndrome. Arch Neurol. 1995;52:379–391. doi: 10.1001/archneur.1995.00540280065020. [DOI] [PubMed] [Google Scholar]
- Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- Iannello RC, Crack PJ, de Haan JB, Kola I. Oxidative stress and neural dysfunction in Down syndrome. J Neural Transm. 1999;57:257–267. doi: 10.1007/978-3-7091-6380-1_17. [DOI] [PubMed] [Google Scholar]
- Ill-Raga G, Ramos-Fernandez E, Guix FX, Tajes M, Bosch-Morato M, Palomer E, Godoy J, Belmar S, Cerpa W, Simpkins JW, Inestrosa Nc, Munoz FJ. Amyloid-beta peptide fibrils induce nitro-oxidative stress in neuronal cells. Journal of Alzheimer's disease : JAD. 2010;22:641–652. doi: 10.3233/JAD-2010-100474. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Amano K, Takaki E, Ebrahim AS, Shimohata A, Shibazaki N, Inoue I, Takaki M, Ueda Y, Sago H, Epstein CJ, Yamakawa K. Increased lipid peroxidation in Down's syndrome mouse models. J Neurochem. 2009;110:1965–1976. doi: 10.1111/j.1471-4159.2009.06294.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radical Biology & Medicine. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Karlsen AS, Pakkenberg B. Total Numbers of Neurons and Glial Cells in Cortex and Basal Ganglia of Aged Brains with Down Syndrome--A Stereological Study. Cerebral cortex. 2011 doi: 10.1093/cercor/bhr033. [DOI] [PubMed] [Google Scholar]
- Kedziora J, Bartosz G. Down's syndrome: a pathology involving the lack of balance of reactive oxygen species. Free Radic Biol Med. 1988;4:317–330. doi: 10.1016/0891-5849(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992;89:7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Williams MD. A prospective study of Alzheimer Disease in Down Syndrome. Archives of Neurology. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusable, nonfibrillar ligands derived from Ab1-42 are potent central nervous system neurotoxins. PNAS. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Hyun D, Jenner P, Halliwell B. Effect of overexpression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative damage and antioxidant defences: relevance to Down's syndrome and familial amyotrophic lateral sclerosis. J Neurochem. 2001;76:957–965. doi: 10.1046/j.1471-4159.2001.00107.x. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Gautier M, Turpin R. Etude des chromosomes somatiques de neuf enfants mongoliens. Comptes Rendus Hebdomadaires des Seances de L'Academie des Sciences. 1959;248:1721–1722. [PubMed] [Google Scholar]
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APOE in Down Syndrome: Implications for initial events in amyloid plaque formation. Neurobiology of Disease. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: A regional quantitative analysis. Experimental Neurology. 1998;150:296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT. Down's syndrome, aging, and Alzheimer's disease: a clinical review. Ann N Y Acad Sci. 1982;396:15–27. doi: 10.1111/j.1749-6632.1982.tb26840.x. [DOI] [PubMed] [Google Scholar]
- Lott IT, Head E. Down syndrome and Alzheimer's disease: A link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- Lott IT, Head E. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lott IT, Head E, Doran E, Busciglio J. Beta-amyloid, oxidative stress and down syndrome. Curr Alzheimer Res. 2006;3:521–528. doi: 10.2174/156720506779025305. [DOI] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Marcyniuk B, Yates PO, Neary D, Snowden JS. The progression of the pathological changes of Alzheimer's disease in frontal and temporal neocortex examined both at biopsy and at autopsy. Neuropathol Appl Neurobiol. 1988;14:177–195. doi: 10.1111/j.1365-2990.1988.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J Neurol Sci. 1989;89:169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Mazur-Kolecka B, Golabek A, Nowicki K, Flory M, Frackowiak J. Amyloid-beta impairs development of neuronal progenitor cells by oxidative mechanisms. Neurobiology of aging. 2006;27:1181–1192. doi: 10.1016/j.neurobiolaging.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol Aging. 2007;28:1493–1506. doi: 10.1016/j.neurobiolaging.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Hirai K, Aliev G, Takeda A, Chiba S, Smith MA. Neuronal RNA oxidation in Alzheimer's Disease and Down's syndrome. Annals of NYAS. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla M, Friedland RP, Hirai K, Chiba S, Smith MA. Neuronal oxidative stress precedes amyloid-b deposition in Down syndrome. J Neuropath Exp Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- Odetti P, Angelini G, Dapino D, Zaccheo D, Garibaldi S, Dagna-Bricarelli F, Piombo G, Perry G, Smith M, Traverso N, Tabaton M. Early glycoxidation damage in brains from Down's syndrome. Biochem Biophys Res Commun. 1998;243:849–851. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Fiorini A, Cocciolo A, Giorgi A, Foppoli C, Butterfield DA, Giorlandino M, Giorlandino C, Schinina ME, Coccia R. Oxidative stress occurs early in Down syndrome pregnancy: a redox proteomics analysis of amniotic fluid. Proteomics-Clinical Applications. 2011 doi: 10.1002/prca.201000121. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain research. 1991;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased Lipid Peroxidation Precedes Amyloid Plaque Formation in an Animal Model of Alzheimer Amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Iuliano L, Amerio G, Tang LX, Rokach J, Sabatino G, Violi F. Down's syndrome is associated with increased 8,12-iso-iPF2alpha-VI levels: evidence for enhanced lipid peroxidation in vivo. Ann Neurol. 2000;48:795–798. [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer's disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab. 299:E879–886. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Patterson D. Down's syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Sarsoza F, Saing T, Kayed R, Dahlin R, Dick M, Broadwater-Hollifield C, Mobley S, Lott I, Doran E, Gillen D, Anderson-Bergman C, Cribbs DH, Glabe C, Head E. A fibril-specific, conformation-dependent antibody recognizes a subset of Abeta plaques in Alzheimer disease, Down syndrome and Tg2576 transgenic mouse brain. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Heinemann U. Increased mitochondrial superoxide generation in neurons from trisomy 16 mice: a model of Down's syndrome. Free Radic Biol Med. 2000;28:235–250. doi: 10.1016/s0891-5849(99)00226-9. [DOI] [PubMed] [Google Scholar]
- Schupf N, Patel B, Pang D, Zigman WB, Silverman W, Mehta PD, Mayeux R. Elevated plasma beta-amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64:1007–1013. doi: 10.1001/archneur.64.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupf N, Zigman WB, Tang MX, Pang D, Mayeux R, Mehta P, Silverman W. Change in plasma Ass peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75:1639–1644. doi: 10.1212/WNL.0b013e3181fb448b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down's syndrome. British journal of psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Tyrrell J, Cosgrave M, McCarron M, McPherson J, Calvert J, Kelly A, McLaughlin M, Gill M, Lawlor BA. Dementia in people with Down's syndrome. Int J Geriatr Psychiatry. 2001;16:1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid b-peptide-associated free radical oxidative stress and neurotoxicity. J Structural Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang D-S, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer's disease brains. Biochemical and biophysical research communications. 2003;310:236–241. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Wisniewski H, Wen G. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Link CD, Butterfield DA. In vitro and in vivo oxidative stress associated with Alzheimer's amyloid beta-peptide (1-42). Neurobiology of aging. 1999;20:325–330. doi: 10.1016/s0197-4580(99)00056-1. discussion 339-342. [DOI] [PubMed] [Google Scholar]