Abstract
Aberrant subcortical-prefrontal connectivity may contribute to insula hyper-reactivity to threat in generalized social anxiety disorder (gSAD). A novel PsychoPhysiological Interaction (PPI) analysis was used to examine functional ‘coupling’ between the insula and prefrontal cortex in gSAD patients and healthy controls (HCs). During fMRI, 29 gSAD and 26 HC volunteers performed an Emotional Face Matching Task, involving the processing of fear, angry, and happy expressions. As expected, compared with HCs, gSAD patients exhibited greater bilateral anterior insula (aINS) reactivity for fear vs. happy faces; this group difference was less robust for angry vs. happy faces. PPI of insula connectivity when processing fearful faces revealed the gSAD group had less right aINS-dorsal anterior cingulate coupling compared to HCs. Findings indicate that aINS hyper-reactivity for fear faces in gSAD, compared to controls, involves reduced connectivity with a prefrontal region implicated in cognitive control and emotion regulation.
Keywords: fMRI, emotion, faces, prefrontal, PsychoPhysiological, imaging
Generalized social anxiety disorder (gSAD) is a prevalent, debilitating disorder marked by social-evaluative fears (Kessler et al., 2005). Symptoms include negative beliefs (Heimberg, 1994, 2002) and autonomic changes in anticipation of, or during, feared situations (Cornwell et al., 2006; Davidson, 2000; McTeague et al., 2009), reflecting a heightened threat processing system. It has been generally established that the amygdala, a crucial region in fear circuitry (LeDoux, 2000), plays a role in gSAD and other types of anxiety (Freitas-Ferrari et al., 2010; Phan and Klumpp, 2010). Less often highlighted is the insula, shown to be hyper-reactive to salient stimuli in gSAD (Amir et al., 2005; Etkin and Wager, 2007; Evans et al., 2008; Shah et al., 2009; Straube et al., 2004, 2005) and anxiety-prone individuals (Simmons et al., 2006; Stein et al., 2007; Wright et al., 2003).
Broadly, the insula is proposed to link internal and external information to produce an awareness of mind-body experiences that aid in maintaining a context relevant homeostatic state (Craig, 2009; Jones et al., 2010). Notably, interoceptive awareness involves the anterior insula (aINS) (Craig, 2009), which is modulated via reciprocal connections with the prefrontal cortex (PFC) (Augustine, 1996; Craig, 2002, 2009; Mesulam and Mufson, 1982; Nelson et al., 2010; Seeley et al., 2007). Therefore, regulation of anxious responsiveness (Critchley, 2009) may involve intact aINS-PFC interactions, which if aberrant, has implications for anxiety disorders (Paulus and Stein, 2006, 2010). For example, patients with gSAD may experience fluctuations in bodily state (e.g., heart rate variability) when meeting someone new and in doing so engage in self-referential somatic-cognitive interpretations. Negative interpretations of internal state driven by exaggerated insula responsiveness and/or inefficient prefrontal regulation could lead to over-arching threat schemas that govern over-estimations of threat and associated anxiety (Beck and Clark, 1997). In social anxiety, evidence of frontal hypo-activation to threat when control functions are recruited and hyper-reactivity concerning threat perception (Freitas-Ferrari, et al., 2010) indicate the sensitivity of a task to engage more dorsal than ventral regions or vice versa effects direction of the observed frontal abnormality. Findings of increased dorsal and ventral ACC and medial PFC activity during threat processing suggest expression, appraisal and/or emotion regulation functions, sub-served by dorsal-caudal and ventral-rostral areas, respectively (Etkin et al., 2011) enhance threat processing in social anxiety.
We used functional connectivity based on task-related aINS activation to threat in patients with gSAD as a step towards elucidating the role of aINS-prefrontal interactions in the pathophysiology of gSAD. Based on evidence of deficient subcortical-PFC relationships in social anxiety (Danti et al., 2010; Phan et al., 2009), we hypothesized that patients would have enhanced insula reactivity and less insula-(pre)frontal connectivity when processing threat faces compared to healthy controls.
Methods
Participants
Twenty-nine gSAD patients and 26 HC participated. The Structured Clinical Interview for DSM-IV (First et al., 1995) was used for diagnosis and the Liebowitz Social Anxiety Scale (Liebowitz, 1987), as a complementary measure of social anxiety pervasiveness. See supplemental material for subject characteristics. All participants provided written informed consent, as approved by the local Institutional Review Board. See Table 1 for group characteristics.
Table 1.
Group Characteristics (Mean± Standard Deviation)
| gSAD (n = 29) | HC (n = 26) | t(df = 53) | p | |
|---|---|---|---|---|
| Age (years) | 24.7 ± 5.9 | 26.2 ± 6.3 | 0.96 | 0.34 |
| Gender | 17F/12M | 16F/10M | ||
| Race/Ethnicity | 5Asa/2AA/22C | 3Asa/1AA/22C | ||
| Social Anxiety Severity | 81.0 ± 15.2 | 8.2 ± 7.7 | 20.9 | <0.001 |
| State Anxiety Level | 39.6 ± 8.8 | 25.7 ± 6.8 | 6.2 | <0.001 |
| Trait Anxiety Level | 47.7 ± 9.4 | 27.4 ± 6.4 | 8.9 | <0.001 |
| Depression Level | 12.1 ± 7.7 | 0.79 ± 1.4 | 7.1 | <0.001 |
Note. gSAD, participants with Generalized Social Anxiety Disorder; HC, Healthy Control participants; F, Female; M, Male; Asa, Asian American; AA, African American; C, Caucasian; Social anxiety severity measured with the Liebowitz Social Anxiety Scale; State and trait anxiety levels measured with the Spielberger State-Trait Inventory; Depression level measured with the Beck Depression Inventory.
Design and Procedure
During fMRI participants performed a modified Emotional Face Matching Task (EFMT) involving a trio of faces from a validated set (Gur et al., 2002), in which was one of the two faces (bottom) that expressed the same emotion as the target face (top) was selected. Matching face blocks were interspersed with “baseline” blocks, in which participants matched geometric shapes (circles, rectangles, or triangles) similar to instructions above. See supplemental material for more details on the EFMT.
Functional imaging: acquisition and analysis
Using a 3T GE Signa System (General Electric; Milwaukee, Wisconsin, USA), whole-brain functional images (i.e., blood oxygenated level-dependent [BOLD]) were acquired from 30 axial, 5-mm-thick slices using a standard T2*-sensitive gradient echo reverse spiral acquisition sequence (repetition time, 2000 ms; echo time, 25 ms; 64 × 64 matrix; 24 cm field of view; flip angle, 77). A high-resolution, T1-weighted volumetric anatomical scan (3-dimensional magnetization-prepared rapid gradient echo) was also acquired for anatomical localization. High quality and scan stability with minimum motion corrections was set at < 2 mm displacement in any one direction. See supplemental material for details of preprocessing and first-level analysis methods.
To test a priori hypothesis, a region of interest (ROI) approach localized to anatomically-based left and right insula masks (Tzourio-Mazoyer et al., 2002; Walter et al., 2003) with the anterior portion demarcated as y-axis=0 and forward was used to examine group differences (see Figure 1). Significance was set at p<0.05, False Discovery Rate (FDR) corrected for multiple comparisons using a small volume correction (Nichols et al., 2005; Worsley et al., 1996). Each participant parameter estimates of activation were extracted from the ROI (β weights, arbitrary units [a.u.]) and averaged across all voxels to examine the direction and variance in gSAD and HC separately. For completeness, a whole brain analysis was conducted; significance was set at p < 0.005, uncorrected for multiple comparisons. Regarding functional connectivity, the time series from significant voxels were extracted within this same left and right aINS ROI using conventional steps of the PsychoPhysiological Interaction (PPI) analysis as implemented in SPM5 to measure aINS-prefrontal connectivity; see supplemental material for details.
Figure 1.
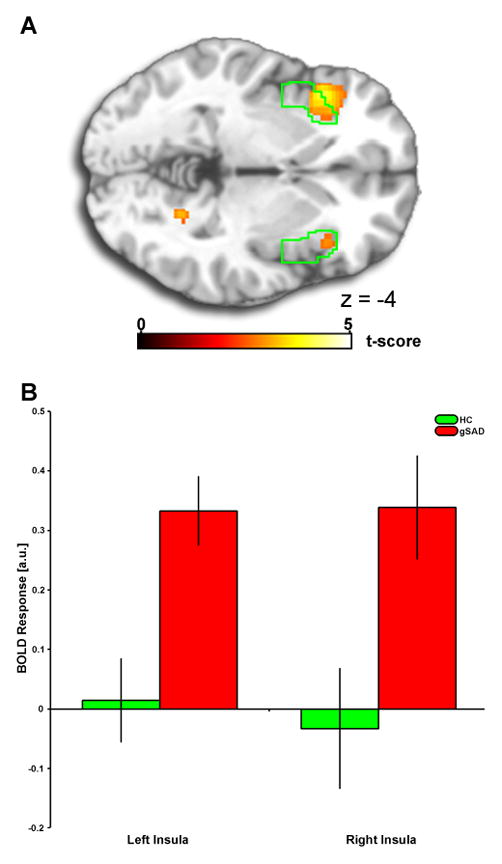
1A) Voxel-wise statistical t-map displayed on a canonical brain, compared to healthy controls, patients with generalized social anxiety disorder have greater bilateral anterior insula (aINS) reactivity to fear (>happy) faces; activation difference shown in relation to the aINS anatomically-derived region of interest (ROI), depicted in green. Color scale reflects t-score. gSAD, Generalized Social Anxiety Disorder; HC, Healthy Control. 1B) Bar graphs depicting extracted parameter estimates of activation from the anatomical aINS ROI within each group showing that gSAD exhibited positive anterior insula activation, whereas HCs showed no ‘activation’ to fear faces.
Results
Behavioral
For accuracy and reaction times for accurate trials, there was a significant main effect of Emotion but no main effect of Group or Emotion × Group interaction. See supplemental materials for these results.
fMRI
Voxel-wise between-groups t-test revealed gSAD patients exhibited greater activation to fear (>happy) faces than HC in the left aINS (peak MNI coordinates: [−40, 20, −4], k=294, Z-score=3.90; FDRsvc p<0.003) and right aINS [(38, 24, −4), k=37, Z-score=2.83; FDRsvc p<0.005) (Fig. 1A). Extracted β-weights of activation (BOLD response [a.u.]) from the aINS ROI revealed an exaggerated aINS response to fear (>happy) faces in gSAD, whereas HC showed no differences in aINS to fear (>happy) (Fig. 1B). See Table 2 for whole-brain results beyond insula. Regarding angry (>happy), group differences for left [(−40, 20, −4), k=87, Z-score=2.97] and right [(38, 26, −2), k=80, Z-score=2.19] insula only emerged at liberal significance threshold, puncorrected <0.05; therefore, subsequent PPI analysis was restricted to fear perception.
Table 2.
Whole-brain between-group comparison in activation to fearful (versus happy) faces
| Contrast Type | Region | MNI Coordinates | Voxels | Z-score | ||
|---|---|---|---|---|---|---|
| gSAD > HC
| ||||||
| Inferior frontal gyrus | 58 | 10 | 36 | 1126 | 3.58 | |
| −54 | 16 | 32 | 971 | 4.50 | ||
| Insula | −40 | 20 | −4 | 294 | 3.90 | |
| 38 | 24 | −4 | 37 | 2.83 | ||
| Parietal superior gyrus | −28 | −68 | 52 | 820 | 3.81 | |
| Caudate | −4 | 0 | 10 | 188 | 3.34 | |
| Postcentral gyrus | −52 | −22 | 36 | 104 | 3.17 | |
| Dorsal medial frontal cortex | −2 | 24 | 46 | 79 | 2.98 | |
| Parahippocampal gyrus | 24 | −54 | −8 | 50 | 3.32 | |
| Temporal inferior gyrus | 48 | −48 | −30 | 18 | 3.19 | |
| Amygdala* | −22 | −12 | −12 | 73 | 2.12 | |
| 14 | 2 | −20 | 11 | 2.05 | ||
|
| ||||||
| HC > gSAD | ||||||
| None | ||||||
Note. MNI, Montreal Neurological Institute; gSAD, generalized social anxiety disorder; HC, healthy controls; Results at Puncorrected< 0.005, 10 voxel minimum;
Results at Puncorrected< 0.05, 10 voxel minimum.
The only area showing a significant group difference in connectivity to the right aINS was the dorsal ACC (dACC) ([4, 32, 20], k=1339, Z-score=4.40) (Fig. 2A). Group differences in left aINS connectivity emerged in the dACC ([4, 38, 22], k=24, Z-score=2.89), albeit below our predetermined threshold for significance. Extracted β-weights of connectivity (parameter estimates, a.u.) showed HC had robust positive coupling whereas gSAD participants demonstrated an inverse coupling pattern (Fig. 2B).
Figure 2.
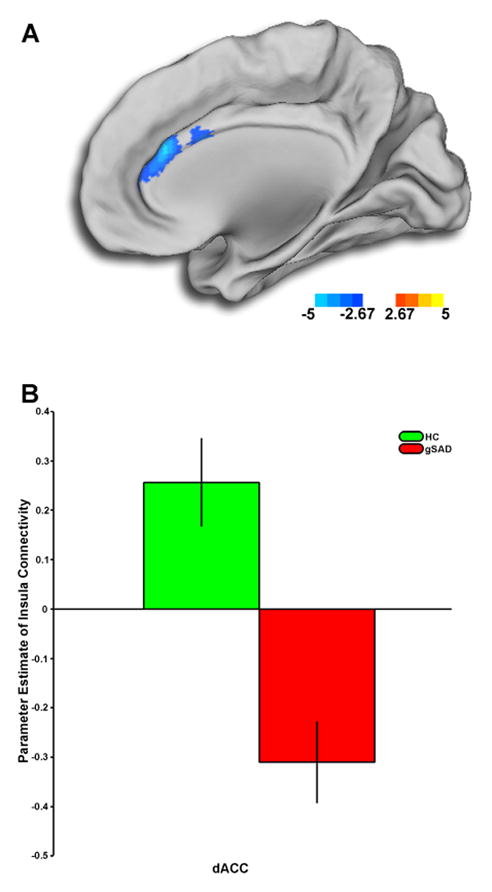
2A) Voxel-wise statistical t-map displayed on a canonical brain, compared to healthy controls, patients with generalized social anxiety disorder have less anterior insula (aINS) to anterior cingulate cortex (ACC) connectivity. Color scale reflects t-score. gSAD, Generalized Social Anxiety Disorder; HC, Healthy Control. 2B) Bar graphs shows extracted parameter estimates of aINS-ACC connectivity within each group showing HCs exhibited a positive connectivity, whereas gSAD subjects showed the reversed pattern of connectivity.
Discussion
As expected, gSAD versus HCs exhibited greater bilateral aINS reactivity for fear (>happy) faces. Similar results occurred for angry (>happy) though only at a more liberal significance thresholding procedure (Lieberman and Cunningham, 2009).
Fear-related connectivity analyses revealed gSAD had less right aINS-dACC connectivity than HCs. Right insula is a proposed interface between bodily arousal and its representation as a subjective feeling (Critchley et al., 2004). Significant connectivity results for right aINS support the notion that anxiety disorders are a consequence of altered interoceptive state (Paulus & Stein, 2006, 2010). The strong link between aINS and dACC, which help give rise to an awareness of mind-body interactions (Craig, 2009; Critchley, et al., 2004; Dosenbach et al., 2007), confirm that aINS and ACC interact when processing threat. Based on deficient subcortical-PFC relationships in social anxiety (Danti, et al., 2010; Phan, et al., 2009), we hypothesize the aINS exaggerated reactivity to threat in gSAD is due to deficiencies in cognitive control over threat, threat appraisal, and/or regulation of threat signals, functions of dACC (Bush et al., 2000; Etkin, et al., 2011; Ochsner and Gross, 2005). In support, healthy controls revealed a non-significant aINS response to fear faces yet robust aINS-dACC coupling.
Study limitations include PPI analysis, which does not permit interpretation of direction or causality of aINS-ACC associations and use of aINS as the only seed, which precludes identifying other brain networks relevant to gSAD pathophysiology outside of aINS.
In conclusion, gSAD is associated with an exaggerated aINS response, and altered aINS-dACC connectivity to salient threat suggests deficient subcortical-PFC coupling. Findings elucidate the role of insula reactivity and insula-ACC connectivity in the pathophysiology of gSAD.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health, National Institute of Mental Health Patient-Oriented Career Development Award K23MH076198 (KLP) and by a grant from the National Center for Research Resources (NCRR) UL1RR024 986 (HK). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or the National Institutes of Health.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heide Klumpp, Email: heidek@umich.edu.
Mike Angstadt, Email: mangstad@umich.edu.
K. Luan Phan, Email: luan@umich.edu.
References
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Beck AT, Clark DA. An information processing model of anxiety: automatic and strategic processes. Behaviour, Research, and Therapy. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson L, Berardi L, Grillon C. Anticipation of public speaking in virtual reality reveals a relationship between trait social anxiety and startle reactivity. Biological Psychiatry. 2006;59:664–666. doi: 10.1016/j.biopsych.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Danti S, Ricciardi E, Gentili C, Gobbini MI, Pietrini P, Guazzelli M. Is social phobia a “mis-communication” disorder? Brain functional connectivity during face perception differs between patients with Social Phobia and healthy control subjects. Frontiers in Systems Neuroscience. 2010;4:1–11. doi: 10.3389/fnsys.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heimberg RG. Cognitive assessment strategies and the measurement of outcome of treatment for social phobia. Behaviour, Research, and Therapy. 1994;32:269–280. doi: 10.1016/0005-7967(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Heimberg RG. Cognitive-behavioral therapy for social anxiety disorder: current status and future directions. Biological Psychiatry. 2002;51:101–108. doi: 10.1016/s0006-3223(01)01183-0. [DOI] [PubMed] [Google Scholar]
- Jones CL, Ward J, Critchley HD. The neuropsychological impact of insular cortex lesions. Journal of Neurology, Neurosurgery, & Psychiatry. 2010;81:611–618. doi: 10.1136/jnnp.2009.193672. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. Liebowitz Social Anxiety Scale. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey III: efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Structure and Function. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. The Journal of Psychiatry and Neuroscience. doi: 10.1503/jpn.100176. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenui I, Povpovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of Neuroscience. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Klumpp H. Neuroendocrinology and Neuroimaging Studies of Social Anxiety Disorder. In: Hofmann S, DiBartolo PM, editors. Social Anxiety: Clinical, Developmental, and Social Perspectives. 2. London: Elsevier; 2010. pp. 273–312. [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. Journal of Psychiatry & Neuroscience. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schlienle A, Stark R, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. Paper presented at the 9th International Conference on Functional Mapping of the Human Brain; New York, NY. 2003. [Google Scholar]
- Worsley KJ, Marrett P, Neelin AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biological Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


