Abstract
Objective
This review will summarize current indications, limitations and outcomes of bariatric surgery in adolescents, as well as provide an overview of the physiologic effects of bariatric surgery on enteric hormones involved in regulating appetite, satiation and maintenance of weight.
Results
Extreme obesity (BMI ≥ 99 percentile) now affects 4% of children and adolescents in the United States. Traditional dietary and behavioral weight management methods have no demonstrated efficacy for extremely obese children and adolescents, in contrast with bariatric surgery which has produced significant and sustainable weight loss and associated improvements in comorbid diseases for the extremely obese. Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB) are the most commonly performed bariatric surgical procedures in adolescents, but vertical sleeve gastrectomy may be a promising new option for selected extremely obese adolescents. A mean weight loss of 37–40% is achieved in adolescents after RYGB, with LAGB showing similar results, albeit attained at a slower rate.
Conclusion
Alterations in the enteric hormones involved in the gut–brain axis that regulates appetite and energy expenditure may play a role in both the anorexigenic and weight-reducing effects of certain bariatric surgical procedures. In particular, RYGB induces a rise in both fasting and post-prandial peptide tyrosine–tyrosine which could contribute to the more rapid and greater degree of weight loss than is seen with LAGB. Limitations of bariatric surgery however include the potential for post-operative morbidity and mortality, as well as possible weight regain in a small proportion of patients.
Keywords: Obesity, Adolescent obesity, Bariatric surgery, Gastric bypass, Ghrelin, PYY, GLP-1
1. Introduction
Obesity has reached epidemic proportions in the United States and worldwide. In the last three decades, obesity prevalence in adults (defined as BMI ≥ 30 kg/m2) has doubled [1,2], while obesity prevalence (defined as BMI ≥ 95th percentile for age and gender) has tripled in adolescents age 12–19 [3]. Alarmingly, not only the prevalence but the severity of obesity has increased in both adults and children. Seven percent of women and three percent of men now have a BMI ≥ 40 kg/m2 [1,2], while four percent of adolescents are extremely obese (defined as BMI ≥ 99th percentile) [4]. Ninety-four percent of children with a BMI ≥ 99th percentile have excess adiposity and thirty-three percent have three or more cardiovascular disease risk factors [4]. Longitudinal analysis of children with a BMI ≥ 99th percentile indicate that 100% remain extremely obese, with an average adult BMI of 43 kg/m2 [4]. Though multidisciplinary behavioral programs have resulted in modest and sustained decreases in BMI and health risks in less severely obese children, behavioral or dietary interventions have not resulted in significant or sustained improvements in BMI for extremely obese children and adolescents [5,6].
Over the past decade, bariatric surgery has gained increasing acceptance as a treatment modality for adolescents with extreme obesity and significant comorbid diseases who have failed conventional dietary or behavioral interventions. This review will summarize the indications for, and outcome of bariatric surgery in adolescents and highlight the physiologic changes occurring post-operatively that may contribute to decreases in appetite and food intake, leading to durable reduction in weight.
2. Overview of bariatric surgery in adolescents
Bariatric surgery has been performed in adolescents since the 1970s and 1980s [7]. From 1996 to 2000 the annual rate of bariatric surgery in adolescents remained constant with approximately 200 cases per year [8]. However, from 2000 to 2003, the annual volume of cases increased threefold, reflecting a growing recognition of both the increasing prevalence and severity of adolescent extreme obesity and acceptance of bariatric surgery as a safe and effective weight loss tool for selected extremely obese adolescents.
2.1. Indications
Indications and contraindications for considering bariatric surgery in adolescence have been extensively reviewed elsewhere [7,9]. Briefly, because of the unique psychosocial, physical, behavioral and emotional needs of adolescents and their families, adolescent bariatric surgery should take place within the context of a pediatric multidisciplinary center and more conservative patient selection criteria are advocated. Typically, bariatric surgery has been recommended only for adolescents with a BMI ≥ 40 kg/m2 with a major comorbidity (diabetes, obstructive, pseudotumor cerebri) or BMI ≥ 50 kg/m2 with or without major comorbidities [10]. Prior attempts at weight loss through behavior or dietary changes must be well documented. Multidisciplinary evaluation by pediatric specialists is advised to ensure that the patient and family understand the risks and benefits and meet selection criteria [11]. Guidelines based on strict BMI cut-point of 40 or 50 kg/m2 may however eventually be replaced by BMI guidelines based on the 99th percentile for BMI, as this may be a more precise cut-point to identify extremely obese teens with high likelihood of having significant cardiovascular and dysmetabolic risk factors, as well as persistence of extreme obesity into adulthood [4,12].
2.2. Surgical options for adolescents
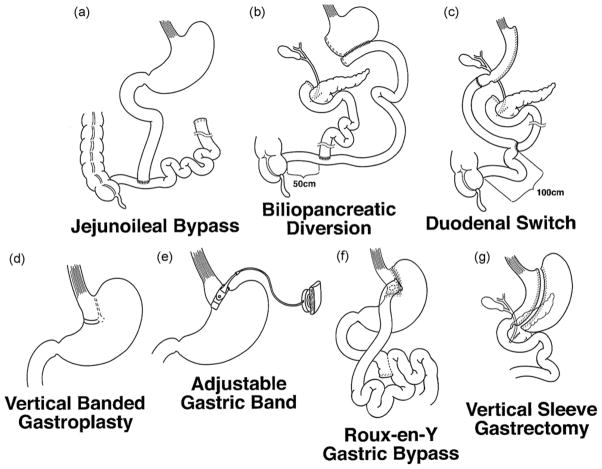
Historically, both the jejunoileal bypass (JIB) (Fig. 1a) and the biliopancreatic diversion (BPD) (Fig. 1b) were performed in adolescents in the 1970s and 1980s [13,14]. Both have since been abandoned, due to the large malabsorptive component of these procedures and high risk of nutritional complications, morbidity and mortality that resulted from bypassing the majority of the small intestine [14–16]. The modified biliopancreatic diversion with duodenal switch (BPD-DS) (Fig. 1c) which preserves a cuff of duodenum and lengthens the common small intestinal channel, has also been performed in a small number of adolescents [17]. However, the BPD-DS also carries an increased risk of malabsorptive complications and requires life-long nutritional supplementation. It is therefore only rarely performed, typically in adolescents at the highest extremes of BMI. A small number of adolescents also received vertical banded gastroplasty (VBG) (Fig. 1d) in the 1980–1990s [18]. However, due to modest weight loss and higher risk of post-surgical complications, this procedure is also no longer performed in adolescents.
Fig. 1.
Operations for surgical weight loss: (a) jejunoileal bypass, (b) biliopancreatic diversion, (c) biliopancreatic diversion with duodenal switch, (d) vertical banded gastroplasty, (e) Roux-en-Y gastric bypass, (f) laparoscopic adjustable gastric band and (g) vertical sleeve gastrectomy.
The two main surgical options for adolescents at present include the Roux-en-Y gastric bypass (RYGB) and the laparoscopic adjustable gastric banding (LAGB). The RYGB remains the most commonly performed procedure, comprising 90% of adolescent bariatric surgery cases in the United States in 2003 [8]. However, the first stage of the BPD-DS, the vertical sleeve gastrectomy (VSG) is gaining increasing interest due to a predictably lower risk of nutritional complications and weight loss performance that is potentially comparable to other procedures (discussed below) [17].
RYGB (Fig. 1e) has been used for surgical weight loss in adolescents since the 1980s and remains the most common bariatric procedure performed in obese adolescents [8,19,20]. Technically, a small 20–30 ml gastric pouch is fashioned just below the gastroesophogeal junction. This excludes the fundus and body of the stomach, which may have important physiologic considerations, as discussed in Section 3. The roux limb is typically 75–150 cm in length and is anastomosed to the gastric pouch. The surgery therefore includes both a restrictive, as well as a mildly malabsorptive component by excluding the body of the stomach and duodenum from the flow of ingested nutrients. In addition, following this procedure, there is little to no gastric phase of digestion, gastric emptying is accelerated, and ingested nutrients more quickly transit to the intestine and colon.
The LAGB (Fig. 1f) procedure in contrast involves only a restrictive component. A device consisting of a synthetic band with an inner inflatable balloon is placed around the proximal stomach, just below the gastroesophageal junction. The inner balloon diameter can be modified by injection of saline into a subcutaneous port to adjust the degree of gastric restriction. Thus, once the band is inflated, the device partitions the stomach into a small gastric pouch above the band with the larger gastric fundus, body, and antrum below the band. Once nutrients have passed through the partition, there is no alteration in the physiology of digestion. The LAGB is completely reversible as no part of the intestinal tract is divided.
The VSG (Fig. 1g) is the first stage of the BPD-DS, and involves a vertical gastrectomy, using a 12–16mm diameter internal bougie (a soft flexible cylindrical instrument temporarily inserted within the stomach) to form a narrow lesser curve gastric tube. No portion of the intestinal tract is bypassed, but the fundus and greater curve of the stomach is removed which may have important physiological effects on appetite and satiety as will be discussed in this review (Section 3). Vertical sleeve gastrectomy has been attracting much interest recently, due to the several reports demonstrating excellent weight loss results and comorbidity resolution. Most agree that there is a much reduced risk of nutritional complications, even without the need for life-long supplementation with vitamins and minerals, as prescribed with other procedures [17]. It has been performed in only a few adolescents to date, but is likely to be increasingly applied, given that adolescents have very poor reported adherence to nutritional supplementation [19], and may be at higher risk of nutritional deficiencies after more malabsorptive procedures [21].
2.3. Outcomes of bariatric surgery in adolescents
Long-term outcomes in adolescents after RYGB indicate that, in general, a mean weight loss of 37–40% is achieved [22,23]. The longest follow-up to date was provided by a retrospective analysis of 33 adolescents followed for up to 21 years after various modifications of gastric bypass surgery [24]. In this cohort with mean BMI 52 kg/m2 and mean age of 16 years, an average BMI of 38 kg/m2 was maintained at a mean of 14 years after surgery. However 5 (15%) patients regained all or most of their lost weight. In general, it is difficult to ascertain the degree of weight regain in adolescent patients after RYGB from data reported in the literature, in part due to the bias represented by the variable number of patients who are lost to follow-up. The prevalence of weight regain may be as high as 20% [22–24].
Significant improvements in comorbidities after gastric bypass have been well documented for obstructive sleep apnea [23,25], hypertension [23], and diabetes [22]. Interestingly, as in adults, glycemic control in patients with diabetes improves almost immediately after surgery, preceding any significant weight loss. This suggests that alterations in gastric hormones that augment insulin secretion (the incretins) may be altered by gastric bypass, as will be reviewed below (Section 3.3). Importantly for the patient, preliminary data suggest that quality of life and depressive symptoms after RYGB in adolescents dramatically improve, close to the level of normal controls [7].
As the LABG is not yet FDA approved in the U.S., the largest series of outcome data come from Australia [26,27]. In the largest Australian series of 41 patients, mean BMI was lower (42 kg/m2) than mean BMI seen typically in gastric bypass cohorts (typically >50 kg/m2) [26]. Also, only five patients had a significant comorbidity such as type 2 diabetes mellitus, obstructive sleep apnea or hypertension. Three-year follow-up data was available for 18 patients in this series and a weight loss of 30% was documented. All five patients with significant comorbidities demonstrated improvement in their disease severity. Two complications were reported including band slippage, requiring repositioning and one tubing leak requiring revision.
A recent study of 24 adolescents (mean BMI 49 kg/m2 and age range 14–20 years old) undergoing LAGB in the United States reported a sustained 42% excess weight loss at 2 and 3 years post-surgery [28]. However the complication rate was 29% with 25% of adolescents having gastric pouch enlargement. Two patients required band repositioning. Comparisons of LAGB and RYGB indicate that they achieve similar degree of weight loss at longer time intervals [29], but short-term results are greater in gastric bypass in the first 1–3 years [30]. Despite good short-term results of LAGB, there remains concern about the longevity of the band device in adolescents who can be expected to live much longer with the device in place [7].
There are no short or long-term safety and efficacy outcomes for VSG in adolescents. However, short-term outcomes (up to 3 years) and safety profile of VSG are excellent for adults [31,32]. Resolution of comorbidities, including diabetes, hypertension, dyslipidemia and sleep apnea are similar to those seen with other restrictive procedures. In particular, the higher degree of weight loss (range of reported excess weight loss of 33–83%) and reduced complexity of surgery make this an attractive option for the super-super obese patient, in whom a Roux-en-Y gastric bypass may be more technically challenging due to severe abdominal adiposity and limited intraabdominal surgical domain.
2.4. Complications of bariatric surgery in adolescents
The true incidence of morbidity and mortality after bariatric surgery in adolescents is difficult to calculate due to the small numbers of patients who have been studied. In adults, a recent large systematic review of the literature calculated an overall early mortality (≤30 days post-operative) of 0.3–1% for RYGB, compared with 0.02–0.4% for LAGB [8,33]. Reassuringly, however, no in-hospital perioperative deaths were found for adolescents when a large U.S. database was queried; length of stay for adolescents was also significantly shorter compared with adults [8].
Nonfatal complication rates in adolescents may also be less common and less severe than reported in adults. In a series of 36 adolescents undergoing RYGB, 2 (6%) had severe and 4 (11%) had moderate complications [22]. Severe complications were defined as sequelae lasting more than 30 days. These included a case of beriberi (vitaminB1 deficiency) with persistent neurological symptoms and a late death secondary to dehydration from C. difficile colitis, several months after surgery. LAGB complications in adolescents appear comparable to those in adults, occurring in about 10–29% of adolescents undergoing LAGB. The most common are gastric erosion, band slippage or dilated gastric pouch [28].
The main long-term risks of bariatric surgery in adolescents are nutritional in nature. With LAGB, these are related to reduced intake of food and with RYGB, both reduced intake and mild malabsorption occurring as a result of bypassing the stomach (diminishing gastric digestion) and the duodenum (a main site for calcium and iron absorption) [21]. Therefore, the most commonly reported nutritional deficiencies after RYGB are vitamin B12 [34], iron [34,35], and vitamin D [36], if adherence to daily vitamin supplementation is not maintained. Currently, recommended supplementation includes a high potency chewable multivitamin, calcium citrate with vitamin D, vitamin B12, and iron in menstruating females. Vitamin B1 deficiency leading to beriberi has also been reported in several adolescents [37], therefore many programs now supplement with vitamin B1 in the first several months after surgery, when the risk appears to be highest due to low intake and occasional post-operative nausea and vomiting. Adolescents have a variable but low rate of adherence to supplementation and therefore may potentially be at higher risk for development of nutritional deficiencies [19]. Annual monitoring for iron deficiency anemia, calcium, vitamin B12, vitamin D and parathyroid hormone is recommended.
3. Impact of bariatric surgery on gastrointestinal mediators of appetite and satiation
The gastrointestinal (GI) system plays a critical role not only in the digestion and absorption of nutrients, but also in alerting the central nervous system of the influx of nutrients after ingestion of meals. This occurs both through vagal nerve stimulation as well as via hormonal mediators, some of which are also expressed in the CNS [38–40].
Stimuli received from the gastrointestinal tract predominantly function as short-term satiation signals, causing termination of meals. These mediators include primarily cholecystokinin, insulin, PYY3–36 and the incretins, gastric inhibitory peptide (GIP) and glucagon-like peptide-1. The notable exception is ghrelin, an orexigenic hormone secreted by the stomach, which causes increased ingestion of food when administered prior to meals [41]. The primary organs secreting these short-term mediators of satiety are the stomach, pancreas, small and large intestine. Within the intestine, specialized enteroendocrine cells monitor the content of the chyme as it proceeds along the intestine, reacting to specific types of nutrients (carbohydrates, fat or proteins) to synthesize and secrete specific GI peptides. Long-term signaling of excess adiposity, including leptin and circulating insulin, can modulate the sensitivity of the vagal and hindbrain responses to these short-term gastrointestinal signals [42].
Bariatric surgical procedures, in particular those which bypass portions of the alimentary tract, have the potential to cause favorable alterations in these mediators of appetite and satiety. A growing body of the literature has also explored the alterations in GI hormone secretion following bariatric surgery in humans. However, results are very diverse and comparisons across groups are complicated by different bariatric procedures performed, and different research methodologies, including varying sample sizes and hormone assays. Further, the majority of these studies have been performed in adults and very scant data is available in adolescents. This review will summarize the available data on the impact of the most common bariatric surgical procedures on the gastro-enteric hormonal axis.
3.1. Ghrelin
Ghrelin is a 28 amino acid acylated peptide synthesized predominantly in endocrine A/X cells located in the gastric fundus [43]. Ghrelin is expressed to a lesser degree in numerous other tissues, particularly the upper small intestine [44]. However, the stomach appears to be the most significant source of ghrelin, as circulating levels fall up to 65% after gastrectomy [45]. Up to 45% of total ghrelin levels can be maintained after total gastrectomy in humans, presumably due to secretion by other sites in the gut, such as the upper small intestine [44–46].
Ghrelin is cleaved from a precursor peptide, preproghrelin and undergoes post-translational modification by acylation of the serine-3 residue at the amino terminus [47]. This acylation is required for ghrelin to bind to its growth hormone secretagogue receptor (GHS-R1a). GHS-R1a is expressed in a multitude of tissues, including the hypothalamus, pituitary, stomach, small intestine, pancreas, colon, liver, kidney, adipose tissue, myocardium [48]. Not surprisingly, ghrelin has been linked to a diverse array of activities, including intestinal motility, regulation of pancreatic secretions, glucose metabolism, immunity, inflammation, and cardiac function [49–51].
The orexigenic potential of ghrelin was first suggested by a preprandial rise in plasma ghrelin levels during fasting, and a rapid decrease post-prandially [52,53]. Administration of ghrelin directly increases food intake in both rodents and humans [41,54,55]. Blocking the action of ghrelin in animal models induces an expected anorexigenic effect [56,57].
Ghrelin is believed to exert its orexigenic effects in large part by stimulating the GHS-R on the neuropeptide Y (NPY) and Agouti-related protein (AgRP)-expressing neurons in the hypothalamic arcuate nucleus [58–60]. Interestingly, weight gain persists when ghrelin is given to NPY knockout mice, suggesting that additional mechanisms for the ghrelin-related increase in weight may be present [41]. Intact vagal nerve function appears to be required for the orexigenic action, as vagotomised rodents fail to demonstrate an increase in feeding when stimulated by ghrelin [61]. Ghrelin may decrease PYY3–36 and glucagon-like peptide 1 (GLP-1)-mediated inhibition of food intake and gastric emptying [62]. Ghrelin also directly increases gastric emptying, intestinal motility and acid secretion in rodents [63,64]. In addition to stimulating food intake, ghrelin may also directly promote development of excess adiposity. This may be due in part to direct stimulation of adipogenesis [65], or suppression of adiponectin expression, as shown in vitro in brown adipocyte tissue [66].
Ghrelin levels are lower in obese individuals preprandially [67], but suppression after food intake may be decreased [68]. The effect of weight loss on ghrelin secretory profiles remains unclear. After dietary weight loss, ghrelin levels have been reported to increase [69,70] or remain unchanged [71,72]. Similarly, the effect of gastric bypass on ghrelin levels is inconclusive, with numerous reports of increases, decreases, or variable changes in ghrelin profiles post-operatively. Studies are not always performed in a standardized fashion, complicating direct comparisons. Variation often occurs in the use of different assays measuring total serum ghrelin versus the biologically active acylated form of ghrelin or variable timepoints of blood collection after surgery, with some studies measuring fasting morning values and others measuring post-prandial secretion profiles.
Several authors have reported decreases in ghrelin secretion after RYGB. Cummings et al. first reported a 77% decrease in 24 h ghrelin secretion profiles in five subjects following RYGB compared with five normal weight controls (p < 0.001) and 72% decrease compared with five matched obese controls (p = 0.01) [69]. The RYGB patients were studied 9–31 months after surgery when they had reached a stable weight plateau. Total bioactive plasma ghrelin levels, measuring the acylated form of the hormone, were measured. Based on this suppression of ghrelin, it was postulated that ghrelin might contribute to the objective reduction in appetite as well as weight loss following gastric bypass.
Fruhbeck et al. confirmed an early decrease in fasting morning ghrelin levels in obese patients, occurring only 24 h after RYGB compared to an increase in ghrelin levels in matched obese patients 24 h after LAGB or Nissen fundoplication [73]. At 6 months, they likewise reported a significant decrease in fasting ghrelin levels in patients after RYGB, but not after LAGB or conventional dietary weight loss [74]. The decrease in ghrelin was independent of change in weight or insulin levels, and similar in magnitude to decreases seen in control patients after total gastrectomy, suggesting that the decrease in ghrelin was related to loss of contact of nutrients with the gastric fundus.
Korner et al. also reported that fasting levels of both total and bioactive ghrelin were decreased in RYGB recipients and obese matched controls, compared with lean controls [75]. However, suppression of the bioactive form of ghrelin after a standard test meal was similar among all groups (44–47%). Other studies however have shown that the suppression of ghrelin after meal or glucose challenge increases after RYGB compared with non-operative obese controls or patients undergoing LAGB [76,77].
Only a single study has evaluated change in ghrelin after RYGB in an adolescent. A steady decrease in peak and basal active ghrelin and insulin levels was observed from baseline to 14 months after RYGB in an adolescent with hypothalamic obesity secondary to radiation therapy for craniopharyngeoma [78]. The patient also reported significantly reduced food cravings as measured using visual analogue scales [78]. However, RYGB in this patient did not affect overall ghrelin profiles. This patient also underwent an anterior hemi-vagotomy in an attempt to limit vagally mediated insulin secretion.
Holdstock et al. in contrast reported significant increases in morning plasma ghrelin levels at 6 and 12 months after RYGB in 66 adults subjects (meanBMI45 kg/m2) [79]. However, secretion profiles after standard meal intake were not measured. Other groups have also reported a rise in fasting ghrelin levels after RYGB [80], or an increase during the first 6 months of active weight loss, followed by a return to pre-operative baseline levels [81].
Finally, some studies have shown no difference in ghrelin levels pre or post-operatively. Le Roux et al. evaluated 6 patients after RYGB compared to 15 lean and 12 matched obese controls. They found that there was no difference between baseline or post-operative fasting ghrelin levels 6–36 months after RYGB. Ghrelin levels remained significantly lower compared to the lean controls. Although ghrelin levels were collected at various time points after a test meal, only the fasting results were reported. Others have reported an increase during the first 6 months during active weight loss, but a return to baseline pre-operative levels after weight stabilization occurred [81]. No control groups of lean or non-operative obese patients were included in this particular study.
Effects of LAGB, a purely restrictive procedure, on ghrelin are likewise inconclusive, with several reporting an increase in fasting levels of ghrelin post-operatively [82,83] and others reporting no changes in ghrelin compared to baseline levels [84]. When compared directly with patients undergoing procedures that bypass or resect the fundus of the stomach (RYGB or VSG), LAGB appears to be consistently associated with an increase in fasting ghrelin levels [82,85]. Some studies have also reported an increase in suppression of ghrelin after meal challenges in patients following LAGB, but to a lesser degree than reported after RYGB [77].
Some data suggest that the vertical sleeve gastrectomy also results in a significant and sustained decrease in fasting plasma ghrelin. Langer et al. compared fasting plasma ghrelin levels in 10 patients after VSG and 10 patients after LAGB [85]. VSG significantly reduced plasma ghrelin levels at day 1, as well as 1 and 6 months after surgery, compared with no change in plasma ghrelin at day 1 and significant increases at 1 and 6 months after LAGB. Weight loss during the first 6 months was significantly greater in the VSG (61±16%) compared with LAGB (30±13%). Longer-term data following weight stabilization was not reported. The decreased in post-operative ghrelin levels in VSG compared with increase in LAGB has been confirmed by other studies [86].
In summary, the effect of RYGB, LAGB and VSG on ghrelin secretion and ghrelin-mediated changes in appetite after surgery remain inconclusive. However, RYGB may result in greater suppression of post-prandial ghrelin levels than LAGB. Initial reports in patients after VSG also suggest that ghrelin undergoes a sustained decrease in plasma levels. While it is tempting to speculate that VSG and RYGB result in superior weight loss results due to a decrease in the secretion of ghrelin or an increase in post-prandial suppression, which could reduce hunger and food intake, this has not been conclusively demonstrated [87]. Also, it is unknown whether changes in ghrelin levels precede or accompany significant post-operative weight regain which can occur in up to 10–20% of patients after bariatric surgery. A single study has reported a 7 kg/m2 increase in BMI in an adult 1 year following VSG, in spite of stably decreased plasma ghrelin levels [86].
3.2. The pancreatic polypeptide family: PYY3–36 and PP
Peptide tyrosine–tyrosine (PYY) is a 36 amino acid peptide, which together with NPY and pancreatic polypeptide (PP) belongs to a family of pancreatic polypeptides implicated in regulation of appetite. PYY is synthesized and released from endocrine L cells in distal ileum and colon in response to ingestion of carbohydrates and lipids, and proportionally in response to caloric intake [88]. Increases in PYY secretion occur before nutrients reach the distal intestine which implies additional neuroendocrine signaling from the proximal gut [89]. PYY is secreted as PYY1–36 and then cleaved to PYY3–36 by dipeptidyl peptidase IV (DPP-IV) [90].
It is thought that PYY exerts its central effect on appetite, by crossing the blood–brain barrier to activate receptors in the arcuate nucleus of the brain. Four subtypes of hypothalamic receptors have been identified that bind to the pancreatic polypeptide family: Y1, Y2, Y4, Y5 [91]. PYY1–36 is believed to function as an orexigenic agonist of the Y1 and Y2 receptors when administered centrally, while PYY3–36 binds to Y2 receptors on neurons in the hypothalamus [92,93]. Stimulation of Y2 receptors may inhibit NPY-expressing neurons leading to anorexigenic downstream effects [94,95].
The effects of PYY, in particular PYY3–36, in regulating appetite remain somewhat controversial, as highlighted in a recent review [96]. When injected centrally into the paraventricular nucleus or cerebral ventricles in rodents, PYY and PYY3–36 are associated with an orexigenic hyperphagic response and gain of weight [97,98]. However, when administered peripherally to rodents and humans, PYY3–36 appears to reduce food intake, though results have not been replicated widely in rodents [93,99–101].
Nonetheless, intravenous infusion of PYY3–36 in 12 obese and 12 lean human subjects in a placebo-controlled double-blinded, crossover study let to a 30% decrease in food intake in both obese and lean subjects at a single meal and a significant decrease in cumulative 24 h caloric intake [99]. Moreover, PYY3–36 infusion reduced the plasma level of ghrelin as well, which may have enhanced its anorexigenic effect. Endogenous fasting and post-prandial levels of PYY have also been shown to be significantly lower in obese subjects, correlating negatively with BMI (r = −0.84, p < 0.001) [99].
Numerous studies have reported increases in post-prandial total PYY and PYY3–36 responses after RYGB compared with lean or obese non-operative controls [75,102]. Korner et al. also demonstrated that post-prandial PYY and PYY3–36 response in the RYGB group was also increased two- to four fold compared with LAGB, in addition to lean and obese non-operative controls (all weight stable) [77]. Further, patients reported significantly greater satiety scores after RYGB, than LAGB patients or non-operative controls. In contrast, LAGB did not result in an increase in PYY response or satiety scores following the test meal and was indistinguishable from the obese and lean controls. These results were independently confirmed by le Roux et al. [84]. Thus, this post-operative increase in PYY secretion may be unique to RYGB, and not induced by the purely restrictive LAGB procedure. However, a single series of 12 patients undergoing VBG (also purely restrictive) reported an increase in fasting and post-prandial PYY after surgery to levels comparable to those measured lean controls [103].
An increase in total PYY AUC after meal challenge has also been confirmed during the first 6 months of acute weight loss period after RYGB, suggesting that the PYY increase occurs independently of weight loss after RYGB [104]. Fasting morning plasma PYY as well as the area under the curve after a standardized meal challenge was observed to rise progressively at 1, 3 and 6 months after RYGB in six patients. Post-prandial satiety, measured by visual analogue scores, also increased significantly by 1 month after surgery and was maintained to 6 months post-operatively.
In summary, RYGB appears to result in a dramatic increase in post-prandial rise in PYY, which is not seen after LAGB. Based on animal models, it is speculated that a more rapid delivery of nutrients to the distal ileum may trigger the rapid rise in PYY after RYGB [105]. This could potentially contribute to a decrease in appetite and intake after RYGB, and potentially be a factor in the more rapid and greater degree of weight loss that is typically achieved with RYGB.
3.3. The incretins: glucagon-like peptide 1, gastric inhibitory peptide and oxyntomodulin
The incretins are peptides released by the intestine in response to nutrient intake that augment or enhance glucose-stimulated insulin secretion. Although they have generated intense interest as a potential therapeutic targets for diabetes, the incretin hormones also have been implicated in regulation of appetite, as extensively reviewed recently [106]. These hormones, which include GLP-1, GIP and oxyntomodulin, are produced by post-translational processing of preproglucagon by prohormone convertase enzymes in the pancreas, intestine and brain (brainstem and hypothalamus) [107].
GLP-1 is secreted by intestinal endocrine L cells, predominantly in the ileum and colon. GLP-1 is released into the circulation proportionally to calories ingested, in particular after meals rich in lipids and glucose or other carbohydrates [108]. Oral (but not intravenous) glucose administration stimulates a biphasic release of GLP-1 in humans, with an early rapid rise within 10–15 min, followed by a longer second rise 30–60 min after ingestion [109]. As secretion of GLP-1 from the distal intestine precedes direct contact with nutrients, stimulation by autonomic nervous system or earlier release of GIP from the proximal small intestine is likely to trigger the rapid early release of GLP-1 from the distal intestine [110–113]. In contrast, the delayed rise in GLP-1 is believed to be caused by direct contact with intraluminal nutrients [114].
The primary action of GLP-1 is to enhance pancreatic secretion of insulin in response to oral glucose ingestion [115]. However, recent studies have also implicated GLP-1 in regulation of body weight and appetite. Plasma GLP-1 is reduced in obese subjects while diet-induced weight loss increases GLP-1 levels [116]. Although leptin stimulates release of GLP-1, obese individuals are often leptin resistant, which may account for lower levels of GLP-1 in obese individuals [117]. Antagonism of GLP-1 receptor increases food intake, while administration of GLP-1 in rodents peripherally or centrally inhibits food intake [106]. Inhibition of food intake by GLP-1 has been demonstrated in both lean and overweight humans [118], and the anorexogenic effects are preserved in obesity [119]. This appears to be mediated through activation of proopiomelanocortin neurons in the arcuate nucleus [120].
RYGB induces an exaggerated rise in post-prandial GLP-1 plasma levels, compared with both lean and obese controls [84]. In contrast, no increase in GLP-1 following LAGB has been reported [84]. As with PYY, it is speculated that a more rapid delivery of nutrients to the distal ileum may trigger the rapid rise in GLP-1 after RYGB [105]. Fasting GLP-1 levels also have been documented to rise significantly in comparison to baseline levels, when sampled during the phase of acute weight loss at 1, 3 and 6 months after RYGP in a small series of six patients with no controls [104]. This study also documented an exaggerated rise in GLP-1 after a standardized test meal, but did not include lean or obese controls. In addition to potential anorexic effects, the rise in GLP-1 levels after meal challenge may increase post-prandial insulin release, contributing to the rapid improvement in glycemic control seen in diabetic patients, typically occurring before significant weight loss has occurred.
Gastric inhibitory peptide is secreted by K cells in the proximal small intestine, mainly duodenum and jejunum [121]. Like GLP-1, GIP is secreted in response to meals, particularly those rich in fat or glucose [106]. The GIP receptor is widely expressed in the intestinal tract, adipose tissue, several areas of the brain and numerous other organs [106]. GIP functions similarly to GLP-1 in the pancreas, increasing insulin secretion in response to glucose stimulation. However, GIP may also have unique functions related to anabolic effects and increased lipolysis. GIP receptor knockout mice resist both high fat diet-induced obesity and hyperphagia-induced adiposity (when crossbred with leptin deficient ob/ob mice) [122]. However, energy expenditure in GIP receptor knockout mice was also increased and fat was preferentially oxidized [122].
A limited number of studies have examined the effect of bariatric surgery on gastric inhibitory peptide. These have generally reported a decrease in fasting and post-stimulatory GIP profiles after RYGB [123,124], although at 3 weeks post-operative, the decrease in GIP was only seen in patients with diabetes mellitus [125].
Like GLP1 and GIP, oxyntomodulin is produced by post-translational processing of preproglucagon. Oxyntomodulin is a 37 amino acid peptide, with close sequence homology to glucagon, but with an 8 amino acid c-terminal extension. Oxyntomodulin is released from intestinal L cells, also in proportion to nutrients ingested [126]. Given centrally or peripherally, oxyntomodulin results in decreased weight gain in rodents [127,128]. This reduction in caloric intake after oxyntomodulin administration has been replicated in normal weight humans [129]. Oxyntomodulin has also resulted in weight loss in overweight humans, when administered for 4 weeks subcutaneously three times a day [130]. This may occur in part due to suppression of ghrelin [128,129]. Oxyntomodulin may also have an effect on weight reduction by increasing spontaneous physical activity in both humans and rodents [128,131]. No studies of bariatric surgery to date have directly examined the potential effect of altered oxyntomodulin activity.
3.4. Cholecystokinin
Cholecystokinin (CCK) is rapidly released from gastrointestinal tract after nutrient ingestion, in particular after meals rich in fat and protein [126]. One of the most studied of the GI hormones, CCK has many physiologic functions that cumulatively promote digestion, terminate meals and reduce appetite [132,133]. CCK stimulates gall bladder contractions and secretion of pancreatic enzymes and delays gastric emptying [134,135]. When administered to humans and animals, CCK inhibits food intake by reducing meal size and duration [136]. It is believed to exert its anorexigenic effect primarily through the CCK-A receptor, found in the pancreas, on vagal afferents and enteric neurons as well in the nucleus of the solitary tract, area postrema and dorsomedial hypothalamus in the brain [137]. CCK-A receptor antagonists increase caloric intake and reduce satiety [138].
Limited data is available on changes in CCK after RYGB or the purely restrictive procedures, LAGB or VBG. Kellum et al. evaluated changes in CCK after RYGB and VBG [139]. Serum levels of CCK were measured after a standard glucose or protein–fat meal, but were not found to be altered by either procedure. This was confirmed by another recent study in 2004, evaluating changes in fasting CCK after RYGB [125]. Therefore it does not appear that CCK mediates any anorexigenic effects after RYGB.
4. Conclusion
The epidemic rise in pediatric obesity, in particular extreme obesity, has led to an increasing recognition and acceptance of bariatric surgery which has been demonstrated to be a safe and effective tool for weight loss in selected extremely obese adolescents. Both RYGB and LAGB achieve comparable long-term sustained weight reduction in adolescents, although RYGB appears to achieve greater and more rapid weight loss in the short and intermediate term (1–3 years). Bariatric surgery appears to induce alterations in several of the enteric hormones involved in the gut–brain axis that regulates appetite and energy expenditure. Fasting and post-prandial increases in PYY and GLP-1 may potentially contribute to the more rapid degree of weight loss seen in RYGB than LAGB. The role of ghrelin in modulating appetite suppression and weight loss after gastric bypass remains inconclusive. Well-designed prospective studies with appropriate control groups (including controlled comparisons of different bariatric procedures) and more objective quantification of appetite suppression and energy expenditure are necessary to better delineate the role of ghrelin and the other enteric hormones in the physiologic effects of bariatric surgery. VSG may be a promising surgical option for extremely obese adults and adolescents that may provide a similar degree of weight loss and comorbidity resolution as RYGB but without malabsorptive risk. However, the effects of VSG on appetite and satiation-regulating enteric hormones remain to be determined in prospective studies. Similarly, a proportion of adult and adolescent patients do regain weight after bariatric surgery and it remains unclear if differences in baseline or post-operative enteric hormones predict long-term response to bariatric surgery. Likewise, no prospective comparative studies of enteric hormone changes after bariatric surgery in adolescents have been performed to date.
Acknowledgments
The author would like to acknowledge Dr. Thomas Inge for his critical review and helpful comments and Ms. Jan Warren for creating the illustrations.
References
- 1.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Zeller M, Kirk S, Claytor R, Khoury P, Grieme J, Santangelo M, Daniels S. Predictors of attrition from a pediatric weight management program. J Pediatr. 2004;144:466–470. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30:318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- 7.Inge TH, Xanthakos SA, Zeller MH. Bariatric surgery for pediatric extreme obesity: now or later? Int J Obes (Lond) 2007;31:1–14. doi: 10.1038/sj.ijo.0803525. [DOI] [PubMed] [Google Scholar]
- 8.Tsai W, Inge T, Burd R. Adolescent bariatric surgery: recent national trends in utilization and in hospital outcomes. Arch Pediatr Adolesc Med. 2007;161:217–221. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 9.Xanthakos SA, Daniels SR, Inge TH. Bariatric surgery in adolescents: an update. Adolesc Med Clin. 2006;17:589–612. doi: 10.1016/j.admecli.2006.06.001. (abstract x) [DOI] [PubMed] [Google Scholar]
- 10.Inge TH, Krebs NF, Garcia VF, Skelton JA, Guice KS, Strauss RS, Albanese CT, Brandt ML, Hammer LD, Harmon CM, Kane TD, Klish WJ, Oldham KT, Rudolph CD, Helmrath MA, Donovan E, Daniels SR. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 11.Inge TH, Garcia V, Daniels S, Langford L, Kirk S, Roehrig H, Amin R, Zeller M, Higa K. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004;39:442–447. doi: 10.1016/j.jpedsurg.2003.11.025. (discussion 446–447) [DOI] [PubMed] [Google Scholar]
- 12.Xanthakos SA, Inge TH. Extreme pediatric obesity: weighing the health dangers. J Pediatr. 2007;150:3–5. doi: 10.1016/j.jpeds.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Randolph JG, Weintraub WH, Rigg A. Jejunoileal bypass for morbid obesity in adolescents. J Pediatr Surg. 1974;9:341–345. doi: 10.1016/s0022-3468(74)80290-3. [DOI] [PubMed] [Google Scholar]
- 14.Organ CH, Jr, Kessler E, Lane M. Long-term results of jejunoileal bypass in the young. Am Surg. 1984;50:589–593. [PubMed] [Google Scholar]
- 15.Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoileal bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308:995–999. doi: 10.1056/NEJM198304283081703. [DOI] [PubMed] [Google Scholar]
- 16.Silber T, Randolph J, Robbins S. Long-term morbidity and mortality in morbidly obese adolescents after jejunoileal bypass. J Pediatr. 1986;108:318–322. doi: 10.1016/s0022-3476(86)81012-5. [DOI] [PubMed] [Google Scholar]
- 17.Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI > or =50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244:611–619. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason EE, Scott DH, Doherty C, Cullen JJ, Rodriguez EM, Maher JW, Soper RT. Vertical banded gastroplasty in the severely obese under age twenty-one. Obes Surg. 1995;5:23–33. doi: 10.1381/096089295765558114. [DOI] [PubMed] [Google Scholar]
- 19.Rand CS, Macgregor AM. Adolescents having obesity surgery: a 6-year follow-up. South Med J. 1994;87:1208–1213. doi: 10.1097/00007611-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Anderson AE, Soper RT, Scott DH. Gastric bypass for morbid obesity in children and adolescents. J Pediatr Surg. 1980;15:876–881. doi: 10.1016/s0022-3468(80)80297-1. [DOI] [PubMed] [Google Scholar]
- 21.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–496. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 22.Lawson ML, Kirk S, Mitchell T, Chen MK, Loux TJ, Daniels SR, Harmon CM, Clements RH, Garcia VF, Inge TH. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41:137–143. doi: 10.1016/j.jpedsurg.2005.10.017. (discussion 137–143) [DOI] [PubMed] [Google Scholar]
- 23.Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001;138:499–504. doi: 10.1067/mpd.2001.113043. [DOI] [PubMed] [Google Scholar]
- 24.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–107. doi: 10.1016/S1091-255X(02)00125-7. (discussion 107–108) [DOI] [PubMed] [Google Scholar]
- 25.Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti R, Cohen A, Amin R. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–1179. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 26.Fielding GA, Duncombe JE. Laparoscopic adjustable gastric banding in severely obese adolescents. Surg Obes Relat Dis. 2005;1:399–405. doi: 10.1016/j.soard.2005.04.006. (discussion 405–397) [DOI] [PubMed] [Google Scholar]
- 27.Dolan K, Fielding G. A comparison of laparoscopic adjustable gastric banding in adolescents and adults. Surg Endosc. 2004;18:45–47. doi: 10.1007/s00464-003-8805-6. [DOI] [PubMed] [Google Scholar]
- 28.Dillard BE, 3rd, Gorodner V, Galvani C, Holterman M, Browne A, Gallo A, Horgan S, Le Holterman AX. Initial experience with the adjustable gastric band in morbidly obese US adolescents and recommendations for further investigation. J Pediatr Gastroenterol Nutr. 2007;45:240–246. doi: 10.1097/MPG.0b013e31805b82fb. [DOI] [PubMed] [Google Scholar]
- 29.Chapman AE, Kiroff G, Game P, Foster B, O’Brien P, Ham J, Maddern GJ. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery. 2004;135:326–351. doi: 10.1016/S0039-6060(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 30.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 31.Lee CM, Cirangle PT, Jossart GH. Vertical gastrectomy for morbid obesity in 216 patients: report of two-year results. Surg Endosc. 2007;21:1810–1816. doi: 10.1007/s00464-007-9276-y. [DOI] [PubMed] [Google Scholar]
- 32.Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 33.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 34.Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, Cody RP. Are vitamin B12 and folate deficiency clinically important after Roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–442. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]
- 35.Rabkin RA, Rabkin JM, Metcalf B, Lazo M, Rossi M, Lehman-Becker LB. Nutritional markers following duodenal switch for morbid obesity. Obes Surg. 2004;14:84–90. doi: 10.1381/096089204772787347. [DOI] [PubMed] [Google Scholar]
- 36.Fujioka K. Follow-up of nutritional and metabolic problems after bariatric surgery. Diabetes Care. 2005;28:481–484. doi: 10.2337/diacare.28.2.481. [DOI] [PubMed] [Google Scholar]
- 37.Towbin A, Inge TH, Garcia VF, Roehrig HR, Clements RH, Harmon CM, Daniels SR. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004;145:263–267. doi: 10.1016/j.jpeds.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol. 1989;3:71–100. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- 39.Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- 40.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 41.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 42.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dornonville de la Cour C, Bjorkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Hakanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- 44.Wierup N, Bjorkqvist M, Westrom B, Pierzynowski S, Sundler F, Sjolund K. Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab. 2007;92:3573–3581. doi: 10.1210/jc.2006-2756. [DOI] [PubMed] [Google Scholar]
- 45.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 46.Popovic V, Miljic D, Pekic S, Pesko P, Djurovic M, Doknic M, Damjanovic S, Micic D, Cvijovic G, Glodic J, Dieguez C, Casanueva FF. Low plasma ghrelin level in gastrectomized patients is accompanied by enhanced sensitivity to the ghrelin-induced growth hormone release. J Clin Endocrinol Metab. 2005;90:2187–2191. doi: 10.1210/jc.2004-1888. [DOI] [PubMed] [Google Scholar]
- 47.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 48.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 49.Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol. 2002;14:555–560. doi: 10.1046/j.1365-2826.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- 50.Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 51.Dixit VD, Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40:900–910. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 53.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 54.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 55.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 56.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 57.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 59.Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- 60.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 61.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 62.Chelikani PK, Haver AC, Reidelberger RD. Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3–36) on food intake and gastric emptying in rats. Diabetes. 2006;55:3038–3046. doi: 10.2337/db06-0730. [DOI] [PubMed] [Google Scholar]
- 63.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 64.Levin F, Edholm T, Ehrstrom M, Wallin B, Schmidt PT, Kirchgessner AM, Hilsted LM, Hellstrom PM, Naslund E. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul Pept. 2005;131:59–65. doi: 10.1016/j.regpep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 66.Ott V, Fasshauer M, Dalski A, Meier B, Perwitz N, Klein HH, Tschop M, Klein J. Direct peripheral effects of ghrelin include suppression of adiponectin expression. Horm Metab Res. 2002;34:640–645. doi: 10.1055/s-2002-38261. [DOI] [PubMed] [Google Scholar]
- 67.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 68.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 69.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 70.Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 71.Reinehr T, de Sousa G, Roth CL. Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight. Clin Endocrinol. 2008;68:304–310. doi: 10.1111/j.1365-2265.2007.03042.x. [DOI] [PubMed] [Google Scholar]
- 72.Reinehr T, Roth CL, Alexy U, Kersting M, Kiess W, Andler W. Ghrelin levels before and after reduction of overweight due to a low fat high-carbohydrate diet in obese children and adolescents. Int J Obes (Lond) 2005;29:362–368. doi: 10.1038/sj.ijo.0802913. [DOI] [PubMed] [Google Scholar]
- 73.Fruhbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–309. doi: 10.1056/NEJM200401153500323. [DOI] [PubMed] [Google Scholar]
- 74.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, Gil MJ, Gomez-Ambrosi J, Salvador J, Cienfuegos JA. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–1215. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 75.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 76.Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]
- 77.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 78.Inge TH, Pfluger P, Zeller M, Rose SR, Burget L, Sundararajan S, Daniels SR, Tschop MH. Gastric bypass surgery for treatment of hypothalamic obesity after craniopharyngioma therapy. Nat Clin Pract Endocrinol Metab. 2007;3:606–609. doi: 10.1038/ncpendmet0579. [DOI] [PubMed] [Google Scholar]
- 79.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 80.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 81.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 82.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 83.Nijhuis J, van Dielen FM, Buurman WA, Greve JW. Ghrelin, leptin and insulin levels after restrictive surgery: a 2-year follow-up study. Obes Surg. 2004;14:783–787. doi: 10.1381/0960892041590980. [DOI] [PubMed] [Google Scholar]
- 84.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 86.Cohen R, Uzzan B, Bihan H, Khochtali I, Reach G, Catheline JM. Ghrelin levels and sleeve gastrectomy in super-super-obesity. Obes Surg. 2005;15:1501–1502. doi: 10.1381/096089205774859335. [DOI] [PubMed] [Google Scholar]
- 87.Christou NV, Look D, McLean AP. Pre- and post-prandial plasma ghrelin levels do not correlate with satiety or failure to achieve a successful outcome after Roux-en-Y gastric bypass. Obes Surg. 2005;15:1017–1023. doi: 10.1381/0960892054621071. [DOI] [PubMed] [Google Scholar]
- 88.Adrian TE, Bacarese-Hamilton AJ, Smith HA, Chohan P, Manolas KJ, Bloom SR. Distribution and postprandial release of porcine peptide YY. J Endocrinol. 1987;113:11–14. doi: 10.1677/joe.0.1130011. [DOI] [PubMed] [Google Scholar]
- 89.Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roz C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch. 1999;438:299–306. doi: 10.1007/s004240050913. [DOI] [PubMed] [Google Scholar]
- 90.Mentlein R, Dahms P, Grandt D, Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 91.Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 92.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 93.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 94.Chen X, DiMaggio DA, Han SP, Westfall TC. Autoreceptor-induced inhibition of neuropeptide Y release from PC-12 cells is mediated by Y2 receptors. Am J Physiol. 1997;273:H1737–H1744. doi: 10.1152/ajpheart.1997.273.4.H1737. [DOI] [PubMed] [Google Scholar]
- 95.Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- 96.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 97.Morley JE, Levine AS, Grace M, Kneip J. Peptide YY (PYY), a potent orexigenic agent. Brain Res. 1985;341:200–203. doi: 10.1016/0006-8993(85)91490-8. [DOI] [PubMed] [Google Scholar]
- 98.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 99.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 100.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88:3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 101.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature. 2004;430:1–165. doi: 10.1038/nature02665. discussion 162 p following 165. [DOI] [PubMed] [Google Scholar]
- 102.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 103.Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernandez C, Cabrerizo L, Fernandez-Represa JA. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 104.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 105.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 106.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 107.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- 108.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 110.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 111.Balks HJ, Holst JJ, von zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82:786–790. doi: 10.1210/jcem.82.3.3816. [DOI] [PubMed] [Google Scholar]
- 112.Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420–2426. doi: 10.1210/endo.143.6.8840. [DOI] [PubMed] [Google Scholar]
- 113.Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology. 2003;144:3244–3250. doi: 10.1210/en.2003-0143. [DOI] [PubMed] [Google Scholar]
- 114.Roberge JN, Brubaker PL. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology. 1991;128:3169–3174. doi: 10.1210/endo-128-6-3169. [DOI] [PubMed] [Google Scholar]
- 115.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 116.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 117.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 118.Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 119.Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, Hellstrom PM. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91:439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 120.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 122.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 123.Sirinek KR, O’Dorisio TM, Hill D, McFee AS. Hyperinsulinism, glucose-dependent insulinotropic polypeptide, and the enteroinsular axis in morbidly obese patients before and after gastric bypass. Surgery. 1986;100:781–787. [PubMed] [Google Scholar]
- 124.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. (discussion 4–5) [PubMed] [Google Scholar]
- 125.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 127.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 128.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 129.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 130.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 131.Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 2006;30:1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 132.Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005;6:297–306. doi: 10.1111/j.1467-789X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 133.Stacher G, Bauer H, Steinringer H. Cholecystokinin decreases appetite and activation evoked by stimuli arising from the preparation of a meal in man. Physiol Behav. 1979;23:325–331. doi: 10.1016/0031-9384(79)90374-3. [DOI] [PubMed] [Google Scholar]
- 134.Burckhardt B, Delco F, Ensinck JW, Meier R, Bauerfeind P, Aufderhaar U, Ketterer S, Gyr K, Beglinger C. Cholecystokinin is a physiological regulator of gastric acid secretion in man. Eur J Clin Invest. 1994;24:370–376. doi: 10.1111/j.1365-2362.1994.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 135.Fried M, Erlacher U, Schwizer W, Lochner C, Koerfer J, Beglinger C, Jansen JB, Lamers CB, Harder F, Bischof-Delaloye A, et al. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991;101:503–511. doi: 10.1016/0016-5085(91)90031-f. [DOI] [PubMed] [Google Scholar]
- 136.Beglinger C. Overview. Cholecystokinin and eating. Curr Opin Investig Drugs. 2002;3:587–588. [PubMed] [Google Scholar]
- 137.Asin KE, Gore PA, Jr, Bednarz L, Holladay M, Nadzan AM. Effects of selective CCK receptor agonists on food intake after central or peripheral administration in rats. Brain Res. 1992;571:169–174. doi: 10.1016/0006-8993(92)90527-g. [DOI] [PubMed] [Google Scholar]
- 138.Beglinger C, Degen L, Matzinger D, D’Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1149–R1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 139.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770. doi: 10.1097/00000658-199006000-00016. (discussion 770–761) [DOI] [PMC free article] [PubMed] [Google Scholar]