Abstract
Previous functional magnetic resonance imaging (MRI) studies in healthy subjects with the apolipoprotein E 4 (APOE-4) genetic risk for Alzheimer’s disease have shown increased activation during memory task performance in broadly distributed cortical regions. These findings have been hypothesized to reflect compensatory recruitment of intact brain regions that presumably result from subtle neural dysfunction reflecting incipient disease. In this study, we used high-resolution functional MRI in APOE-4 carriers and non-carriers to measure activity in hippocampal subregions (CA fields 1, 2, 3; dentate gyrus [DG], and subiculum) and adjacent medial temporal lobe (parahippocampal and entorhinal) subregions. We found reduced left CA2, 3 and dentate gyrus (CA23DG) activity in cognitively intact APOE-4 carriers. These results suggest that reduced neural activity in hippocampal subregions may underlie the compensatory increase in extra hippocampal activity in people with a genetic risk for Alzheimer’s disease prior to the onset of cognitive deficits.
Keywords: Alzheimer’s Disease, ApoE, Hippocampus, MRI, fMRI, high-resolution imaging
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder affecting approximately 10% of individuals over the age of 65, with an estimated 18 million victims worldwide (Mount & Downton, 2006). The MTL structures (hippocampus and adjacent parahippocampal [PHC], entorhinal [ERC], and perirhinal cortices [PRC) are crucial for the formation of new declarative memories, including those for semantic (facts) and episodic (events), and are among the first affected in the progression of AD with atrophy that is markedly disproportionate to the rest of the brain (Dickson, 2001). Within the MTL, the first brain structures affected are the transentorhinal region and entorhinal region, the sources of major inputs into the hippocampus (Braak & Braak, 1991), making memory decline one of the earliest cognitive symptoms of the disease (Bélanger et al., 1994). Because current and future treatments are more likely to slow the disease’s effects rather than to reverse it, there is a strong need to develop reliable methods of identifying individuals who will go on to develop AD prior to symptoms of cognitive decline and significant brain atrophy.
The apolipoprotein E e4 (APOE-4) allele, is the primary genetic risk factor for late-onset AD; a single APOE-4 allele has been associated with a 20% risk of developing the disease (Corder et al., 1993; Pericak-Vance et al., 1988; Strittmatter & Roses, 1995). A growing body of neuroimaging studies have detected abnormalities in APOE-4 carriers before compromised function. To investigate metabolic changes over time, Small and colleagues (2000) used positron emission tomography (PET) imaging in combination with genotyping to reveal early alterations in cerebral metabolism among older, cognitively normal APOE-4 carriers. Several other groups have also identified changes in metabolism similar to AD patterns in genetically at-risk subjects who have yet to show cognitive symptoms (de Leon et al., 2001; Reiman et al., 2001). Magnetic resonance imaging (MRI) data have also shown changes in brain structure and function suggestive of AD in APOE-4 carriers with normal cognition (Chen et al., 2007; Juottonen et al., 1998; Reiman et al., 1998; Bookheimer et al., 2000; Burggren et al., 2008). Altogether, neuroimaging in combination with genetic risk may enhance the detection of early changes in AD prior to considerable brain alterations and memory impairment.
Investigating patterns of neural activation in cognitively intact, genetically at-risk subjects, Bookheimer and colleagues (2000) utilized a memory paradigm particularly sensitive to damage in the MTL (Rausch & Babb, 1993) during functional MRI. Despite equal performance on a challenging verbal paired-associates task, carriers of the APOE-4 allele showed increases in the magnitude and spatial extent of activation in frontal, parietal, and left MTL regions. Several other groups have confirmed this effect using multiple memory paradigms (Fleisher et al., 2005; Bondi et al., 2005; Dickerson et al., 2004; Han et al., 2007). As evidenced by the findings of Burggren and colleagues (2002), these results were specific to a memory challenge as genetically-defined groups showed no differences in functional MRI activation when performing another challenging task that did not include an episodic memory component. Taken together, these findings suggest that in cognitively intact, genetically at-risk subjects, increased activity in regions unaffected by AD progression serves a compensatory role to normalize memory-related performance during a cognitively challenging task (for review, see Bookheimer & Burggren, 2009).
To date, no study has used high-resolution functional MRI to investigate whether there are subtle hippocampal subregional differences associated with APOE-4 carrier status. Cortical compensatory changes would be expected to occur following neural changes elsewhere in the brain, presumably in the regions whose axonal projections connect to these cortical structures and show structural changes early in the progression of AD. Studies by Braak and Braak (1991, 1996) and others (Bobinksi et al., 1999; Gomez-Isla et al., 1996) have shown that subregions within the MTL, including the entorhinal cortex and hippocampal CA fields, are not only critical for memory encoding but are among the earliest regions to demonstrate structural changes in AD. Specifically, studies have shown decreased cortical thickness or volume in preclinical stages of the disease (Burggren et. al. 2008; Mueller et al., 2008). Traditional fMRI studies using typical slice thicknesses of approximately 5mm or greater would be ill-suited to findings subregional abnormalities in regions smaller than this. Our study used high-resolution functional MRI, combined with a cortical unfolding technique applied to the hippocampus and surrounding neocortex in order to investigate these small structures. To address this knowledge gap, the present study used a challenging verbal paired-associates task to identify early differences in activation in hippocampal subregions. We hypothesized that, in genetically at-risk subjects with normal memory, we would observe reduced activation in specific hippocampal subregions reflecting subtle neural dysfunction and cell loss.
Materials and methods
Subjects
A total of 69 subjects were recruited for functional MRI through the Semel Institute Memory & Aging Research Center at the University of California, Los Angeles wherein they participate in ongoing, comprehensive studies of aging and dementia. We excluded subjects that were left-handed, on medications that could potentially alter cognition, or have a history of neurological or psychiatric disorders. To identify both known and potential neuropsychological disorders, all subjects completed a standardized screening questionnaire prior to completing the longitudinal protocol required of this study: a battery of neuropsychological examinations, genotyping for the APOE allele, and both structural and functional MRI assessment. Of the volunteer pool (69), we identified 35 subjects who were cognitively normal (20 excluded with impaired cognition), right-handed (3 left-handers excluded), had no brain abnormalities as evident on the MRI scan (2 excluded), agreed to APOE genotyping (2 declined genotyping), and received the high-resolution functional verbal paired associates scan (7 excluded). One subject was later excluded based on excessive movement during the functional scan and two subjects were excluded based on their performance on the functional MRI paradigm (failed to learn any verbal-paired associates). Of these 32 subjects, 16 were APOE-4 carriers (15 heterozygous e3/4, 1 homozygote e4/4) and 16 were non-carriers (14 homozygous e3/3, 2 heterozygous e2/3), which we used in our analysis. However, excluding the e4/4 and e2/3 subjects did not change our overall results. Age-matched subjects were cognitively normal as assessed by two standardized neuropsychological memory tests: the Wechsler Memory Scale (Wechsler, 1945) and the Buschke-Fuld Selective Reminding test (Buschke & Fuld, 1974). Education was determined by the number of years in high school (12) plus the number of years thereafter. The criterion of one or more members of the immediate family was established in order to designate subjects with or without a family history of AD. The study was performed under the UCLA Institutional Review Board (IRB) testing protocols and approved by the UCLA Human Subjects Protection Committee. All subjects gave written informed consent to participate in this study.
DNA extraction
Supervised by Dr. Alarcon, APOE genotyping was performed in the laboratory of Dr. Daniel Geschwind at the University of California, Los Angeles. Following standard procedures, DNA was obtained from the blood samples of all subjects. After informed consent was acquired, 20 ml of blood was drawn from every subject. Leukocytes from 10 ml of each sample were frozen and stored at -80 C, and DNA was extracted from the remaining 10 ml as per usual lab protocol using the puregene kit (Gentra Systems). Approximately 200–400 μg of high quality genomic DNA was extracted from each 10 ml blood sample in the Geschwind laboratory, producing enough for thousands of PCR-based mutation detection assays, or microsatellite marker based genotypes. All investigators were blind to the APOE status of individuals and subjects are not informed of their APOE status.
Neuropsychological testing
Wechsler Memory Scale - Logical Memory (Wechsler, 1945). Two detailed story passages were read to subjects followed by a verbal recall assessment conducted immediately and after a 30-minute delay. We used the 30-minute delay score, which is highly sensitive in reflecting episodic memory and consolidation.
Buschke-Fuld Selective Reminding Test – Consistent Long-Term Retrieval (Buschke & Fuld, 1974). Subjects were presented with 16 pairs of unrelated words during 10 trials followed by an immediate verbal recall assessment of the word pairs. This test reflects verbal memory and has been shown to differentiate between AD-related memory changes, depression, and normal aging (Hart et al., 1987; Masur et al., 1989).
Experimental design
Subjects learned and recalled unrelated-word-pairs (e.g., jazz / beast, clock / green) during functional MRI scanning (Fig. 1A). The task consisted of six blocks of alternating encoding and retrieval separated by a baseline condition (Fig. 1B).
Fig. 1. Verbal paired associates paradigm.
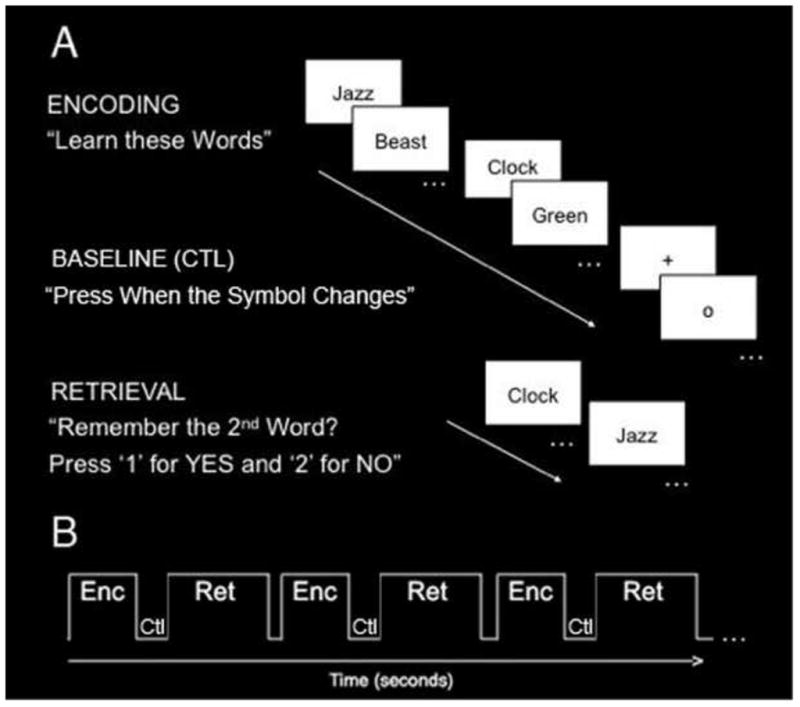
(A) Subjects learned (encoding) and recalled (retrieval) unrelated-word-pairs. During the encoding block, subjects saw and heard seven pairs of unrelated words and were instructed to learn the word pairs. During the baseline (ctl) block, subjects were instructed to fixate on a symbol (“+” or “o”) and press a button when the symbol changed. During the retrieval block, subjects saw and heard the first word and were asked to remember the second word. (B) The task consisted of six blocks of alternating encoding (Enc) and retrieval (Ret) separated by a baseline control (Ctl) task.
During the encoding block, subjects saw and heard seven pairs of unrelated words and were instructed to learn the word pairs. Immediately following the encoding block, subjects completed a baseline control task in which they were instructed to fixate on a symbol (“+” or “o”) centered on the screen and press a button each time it changed. While a typical fixation task activates the hippocampus, thereby providing an inaccurate baseline level (Stark & Squire, 2001), this fixation task activates non-mnemonic processes, thus reflecting a more optimal baseline level of activity. Immediately following the 30-second rest condition, subjects completed the retrieval block in which they saw and heard the first word of the pair and were asked whether or not they were able to silently recall the second word of the pair. Corresponding to one of two buttons, the subject’s behavioral response was recorded as recalled successfully or unsuccessfully. In order to prevent head motion while scanning, subjects were asked to silently recall the second word of the pair, but were then immediately tested again verbally in the scanner following the experimental session in order to ensure accurate recall. Prior to the scanning session, all subjects completed an identical task using alternate word pairs in order to ensure normal memory performance levels and to familiarize them with the task.
During all functional MRI scanning, word pairs were presented both visually and audibly using 512 × 512 resolution magnet-compatible 3-D goggles and headphones (Resonance Technologies, Inc.) from a Macintosh G4 Powerbook computer using Macstim Carbon software version 3.2.1 (WhiteAnt Occasional Publishing, West Melbourne, Australia). Key presses were recorded for behavioral analysis.
Imaging procedure
Both structural and functional MRIs were acquired using a head-only Siemens Allegra 3 Tesla scanner at the University of California, Los Angeles Ahmanson-Lovelace Brain Mapping Center. The following sequences were acquired in the oblique coronal plane, perpendicular to the long axis of the hippocampus, in order to achieve maximal resolution: a high in-plane resolution fast spin echo sequence (TR = 5200 ms, TE = 105 ms, matrix size = 512 × 512, 19 slices, contiguous spacing; voxel size: 0.391 × 0.391 × 3 mm), an echo-planar sequence with a matrix size of 128 × 128 (TR = 3000 ms, TE = 39 ms, 19 slices, contiguous spacing; voxel size = 1.6 × 1.6 × 3 mm), and a matched-bandwidth sequence (TR = 5000 ms, TE = 66 ms, contiguous spacing; voxel size = 1.6 × 1.6 × 3 mm). In order to account for steady-state equilibration, the first two volumes of each functional scan were excluded from our analysis. The high-resolution, coplanar structural and functional sequences were then registered with the matched-bandwidth sequence. See Zeineh et al. (2000) and Ekstrom et al. (2009) for further details on method.
Imaging and statistical analysis
To investigate differences in overall activity in contrasts of interest functional MRI analysis was completed using the FEAT (fMRI Expert Analysis Tool, version 5.98) tool of FMRIB Software Analysis (FSL version 4.0, www.fmrib.ox.ac.uk/fsl). Preprocessing included skull stripping, mathematical head motion correction, and image quality analysis prior to entering the data into statistical analysis. Motion correction to the medial volume was applied to functional images using MCFLIRT (Jenkinson et al., 2002) (FMRIB’s motion correction linear image registration tool); a normalized correlation ratio cost function and linear interpolation was used. Skulls were removed from brain images using BET (brain extraction tool) (Smith et al., 2002). Spatial smoothing was applied to images using a Gaussian kernel of FWHM 3mm for high-resolution functional images. Images were high-pass temporal filtered using a Gaussian-weighted least-squares straight-line fitting (sigma = 100.0s). High-resolution functional images were aligned using FLIRT (FMRIB’s Linear Image Registration Tool) to high-resolution coplanar images with an affine transformation with 6 degrees of freedom (DOF). The high-resolution coplanar images were then registered to the subject’s high-resolution structural images with 6 degrees of freedom (affine transformation). FILM (FMRIB’s improved linear model) with local autocorrelation correction (Woolrich et al., 2001) was used for all time-series statistical analysis. To create regressors of interest a delta function with trial onset times was convolved with a canonical (double gamma) hemodynamic response function, along with the temporal derivative. A cluster corrected threshold at Z > 2.4 and p < 0.05 was applied to all contrasts of interest.
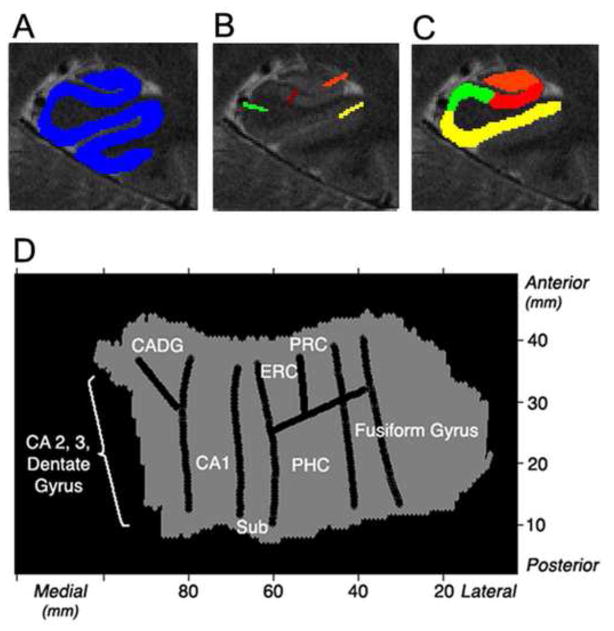
For slices acquired in high-resolution functional scanning, the 3D gray matter of MTL subregions (Fig. 2A) was created by manually segmenting the white matter and CSF using mrGray segmentation software (Teo et al., 1997). Using mrUnfold software, gray matter was then computationally unfolded (Fig. 2D) with an iterative algorithm based on metric multidimensional scaling. To improve segmentation along the long-axis of the hippocampus (Engel et al., 1997) gray matter was interpolated by a factor of 7, yielding a final voxel size of 0.391 × 0.391 × 0.429 mm. The position of the various CA (Cornu Ammonis) fields, subiculum (sub), entorhinal cortex (ERC), parahippocampal cortex (PHC), and fusiform gyrus on the 2D map were found by mapping pixels from known points from the structural images (Fig. 2B) using atlases by Amaral and Insausti (1990), and Duvernoy (1998). Raw functional images were aligned onto the unfolded hippocampus using identical transformation parameters as the matched bandwith images’ alignment to the structural. Activation images were then superimposed onto the structural images for precise localization of effects. Apriori, anatomical regions of interest (ROIs) were defined in 2-D space and then projected into 3-D space (Fig. 2C). ROIs included anterior and posterior CA2,3,DG, anterior and posterior CA1, anterior and posterior subiculum, ERC and PHC. FSL Featquery (FEAT version 5.98) was used to calculate the average percent signal change. For our ROI analysis perirhinal (PRC), anterior fusiform gyrus, and to a lesser extent ERC areas are associated with signal dropout. Therefore, like previous studies using this method, PRC and anterior fusiform activity were not included due to signal dropout associated with these areas (see Zeineh et al., 2000 for details).
Fig. 2. Unfolding method.
(A) Each subjects’ gray matter (blue) is created by segmenting white matter and cerebral spinal fluid. The gray matter is then computationally unfolded and boundaries (B) between regions are projected onto the unfolded flat map. (D) Averaged group flat maps (left side shown) are created showing regions CA2, 3, and dentate gyrus, CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus. (C) Voxels in 2D space are projected into 3D space to create anatomical regions of interests showing posterior regions (left): CA23DG (orange), CA1 (red), subiculum (green), and PHC (yellow).
Each subjects’ individual high-resolution functional activations were warped into an unfolded 2D group template, which was created based on the 16 (APOE-4) and 16 (APOE-3) individual subject anatomical images and boundaries. For each voxel, the fit between each individual subject’s activation (e.g., beta values) for contrasts of interest were then compared across subjects using a mixed-effects t-test (t ≥ 2.4, p < 0.05, corrected). See Thompson et al. (2000), Zeineh et al. (2001), and Ekstrom et al. (2009) for details on these methods. For ROI analysis, post hoc comparisons were made only if global analysis indicated a statistically significant (p < 0.05, corrected) effect of group (APOE-4 vs. APOE-3).
Cortical thickness measurements
Using the high-resolution structural images, cortical thickness values were obtained by doubling the maximum distance value (shortest distance between pixels of gray matter and pixels of non-gray matter) in a ROI, and then projected onto a 2-D flat map. We then averaged all values for 2-D voxels within a ROI to calculate the average cortical thickness. Each group mean (APOE-4 and APOE-3) thickness for a ROI was then averaged across subjects. Two sample t-tests (p < 0.05) were used to determine if cortical thickness differed significantly between APOE-4 carriers and non-carriers. See Burggren et al. (2008) for details on this method.
Results
The demographic and clinical characteristics of both genetic groups, APOE-4 carriers and non-carriers (APOE-3), were similar on multiple measures (Table 1). Memory-related performance scores of all subjects fell within the normal range for their age, reflecting normal cognitive ability and processing. More specifically, subjects completed the Wechsler Memory Scale test (Logical Memory Delayed Recall section) and the Buschke-Fuld Selective Reminding test (Consistent Long-Term Retrieval section); these were conducted as part of a standardized neuropsychological test battery, indicating that all subjects were cognitively intact with normal memory at the time of testing (Table 1). Additionally, there was no significant difference in mean scores on these tests according to genetic risk.
Table 1. Demographic and Clinical Characteristics of the APOE e3 and APOE e4 groups.
There were no significant differences between age-matched APOE e3 and APOE e4 groups in memory-related performance as assessed by the Wechsler Memory Scale, Buschke-Fuld Selective Reminding test, and verbal-paired-associates task. Age and neuropsychological test values are reported as mean ± s.e.m.
| Characteristic | Range of Possible Scores * | APOE e3 Carriers (N=16) | APOE e4 Carriers (N=16) |
|---|---|---|---|
| Female sex – no. (%) | 12 (75.0) | 9 (56.3) | |
| Family history of dementia – no. (%) | 6 (37.5) | 9 (56.3) | |
| Years of age | 60.3 ± 1.9 | 61.9 ± 2.8 | |
| Years of education | 15.3 ± 1 | 17.1 ± 1 | |
| Logical Memory Delayed Recall portion of the Wechsler Memory Scale ∫ | 0–50 | 30.2 ± 1.7 | 26.8 ± 2.0 |
| Consistent Long-Term Retrieval portion of the Buschke-Fuld Selective Reminding Test ψ | 0–144 | 75.4 ± 8.1 | 71.1 ± 8.8 |
| Verbal-paired-associates task ς | 0–42 | 31.6 ± 1.3 | 34.3 ± 1.5 |
For standardized rating scales and memory tests, lower scores reflect poorer performance.
p = n.s. for the difference between groups. A score of 18.1 ± 6.0 is considered normal in this age group.
A score of 60.2 ± 32.4 for men and 71.4 ± 36.8 for women is considered normal in this age group.
This test was performed before fMRI scanning; seven pairs of unrelated words with the degree of recall assessed after each presentation. The total score reflects the total number of correct responses during retrieval for the six blocks.
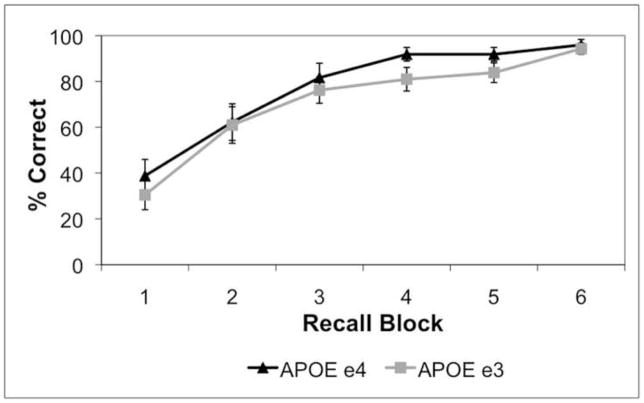
Performance on the experimental paradigm showed that subjects successfully encoded unrelated word pairs, as reflected in their ability to accurately recall words during retrieval (% correct: mean ± s.e.m., APOE-4: 76.87 ± 3.77; APOE-3: 68.91 ± 3.89; n.s.; Fig. 3). The genetic groups did not differ on the basis of their performance, as the percentage of word pairs correctly recalled was not significantly different.
Fig. 3. Behavioral Performance of APOE e4 and APOE e3 groups.
Both groups (APOE e4 and APOE e3) successfully encoded unrelated word pairs, as reflected in their ability to accurately recall words during retrieval. Shown is the mean % correct for each genetic group during recall blocks 1–6. Groups did not significantly differ in behavioral performance (n.s.).
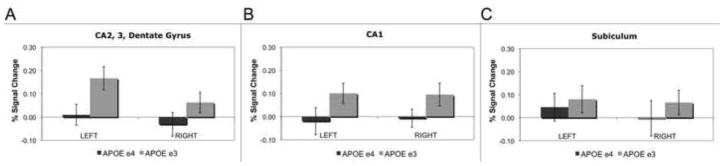
Based on previous findings (Bookheimer et al., 2000), we focused on the left MTL structures in our analysis. We performed a voxel-wise analysis to investigate changes during encoding of unrelated word-pairs. Both groups showed significant increases and decreases within MTL regions during encoding compared to baseline (Fig. 4). In a ROI analysis, mean percent signal change was calculated for each subregion. A 2 × 5 analysis of variance (ANOVA) in the left MTL revealed a significant main effect of group (APOE-3 vs. APOE-4), (F(1,170) = 6.29, p < 0.05)); no significant main effect of region or interaction between group and region was found. Post-hoc analyses yielded significant differences within the left CA23DG with APOE-3 individuals showing significantly great activity during learning compared to rest (learn > rest, t(16) = 3.52, p < 0.05, Fig. 5) and significantly greater activity compared to APOE-4 individuals (APOE-3 > APOE-4, t(30) = 2.47, p < 0.05, Fig. 5). No other regions (CA1, subiculum, ERC, and PHC) showed significant group differences in activity (Fig. 5; Supplementary fig. 1). Lastly, averaging across all hippocampal regions (CA23DG, CA1, and subiculum) showed no group differences in activity (Supplementary Fig. 2).
Fig. 4. Group Voxel-wise Analysis.
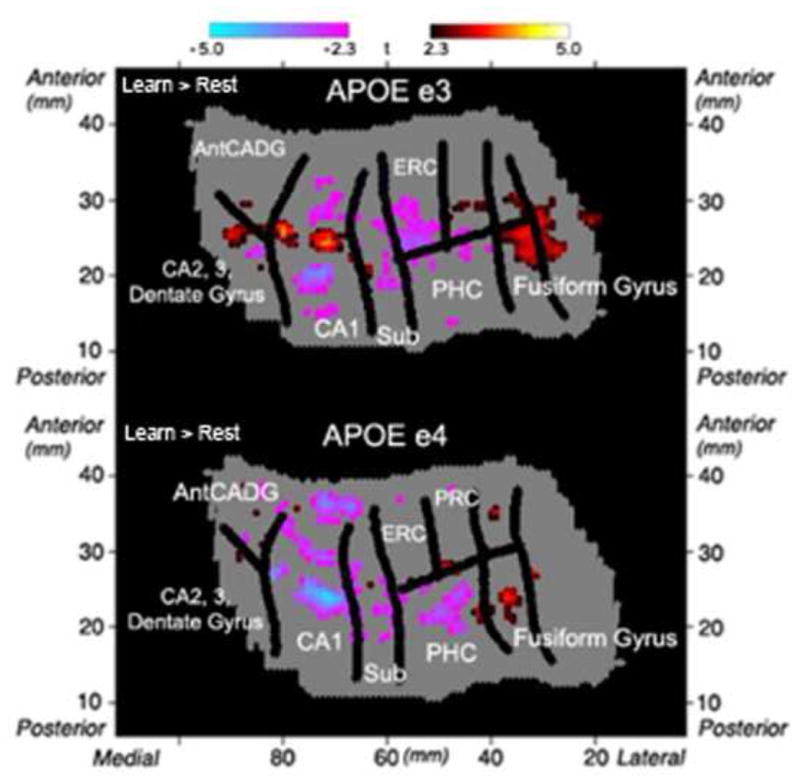
Group voxel based mixed-effects unfolded t-test maps (statistical maps of significantly activated and deactivated regions; learn > rest; −2.3 ≥ t ≥ 2.3, p < 0.05 corrected) in APOE e3 (n = 16) and APOE e4 (n = 16) carriers. Both groups showed significant increases and decreases compared to baseline within the left MTL regions during encoding of word-pairs. Regions shown include CA2,3 and dentate gyrus, anterior CA and dentate gyrus (AntCADG), CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus.
Fig. 5. Hippocampal Region of Interest Analysis.
Average % signal changes during encoding compared to baseline in (A) CA 2, 3, and dentate gyrus (CA23DG), (B) CA1, and (C) subiculum of genetic groups (APOE e3, n = 16; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. APOE e3 carriers showed a significant increase in the left CA23DG compared to baseline and significantly higher percent signal change than APOE e4 carriers (APOE e3 > APOE e4, t(30) = 2.22; p < 0.05). Neither CA1 nor subiculum showed significant group differences.
Using a novel cortical thickness measurement, we then investigated whether there were structural differences within CA23DG according to genetic risk. There was no overlap in participants within our study and those previously reported by Burggren and colleagues (2008). However, when using participants similar in age, education, and neuropsychological test scores, we find similar thickness differences based according to APOE-4 genetic risk. We see no significant differences in CA23DG thickness between APOE-4 carriers and non-carriers (t(30) = −0.0823, n.s.; Supplementary Fig. 3). However, we do see significant differences in thickness in other regions between APOE-4 carriers and non-carriers (Supplementary Fig. 4). Specifically, APOE-4 carriers show decreased thickness compared to non-carriers within the subiculum (e3 > e4; t(30) = 2.283, p < 0.05) and entorhinal cortex (e3 > e4; t(30) = 2.082, p < 0.05) as previously found by Burggren and colleagues (2008).
Discussion
Using high-resolution functional MRI in conjunction with computational cortical unfolding techniques, we investigated changes in activity within MTL subregions of cognitively intact, APOE-4 carriers and non-carriers. Analysis of activity during a cognitively challenging, verbal paired associates task revealed significant differences between groups in the left CA2, 3 and dentate gyrus.
It is important to emphasize that these early changes in activity are not accompanied by any memory decline, as evidenced by the scores on the neuropsychological test battery; all subjects were cognitively intact at the time of testing with no measurable memory impairment. These results extend previous findings of widespread increases in cortical activity in cognitively normal APOE-4 carriers compared to non-carriers. In this study, we investigated hippocampal subregions by utilizing a higher resolution technique than previously employed allowing for the detection of activity within separate hippocampal subregions. However, this high-resolution limits our field of view to the medial temporal structures and so we are unable to detect cortical changes in activity within our sample. Since we used the identical verbal paired associates paradigm to that of Bookheimer et al. (2000) we would expect to find similar cortical patterns of increases in activity in APOE-4 carriers compared to non-carriers. The previous study (Bookheimer et al. 2000) averaged across all MTL regions (hippocampus, ERC, PRC, PHC) and fusiform gyrus, and saw increased activation in APOE-4 carriers. The previous study used a lower resolution and larger field of view. The signal was likely dominated by extra-hippocampal regions, which may have masked subtle decreases within hippocampal subregions. Furthermore, in the current study due to the limited field of view we did not cover the very posterior medial temporal regions, which may have dominated the signal in the previous study. Thus it is difficult to compare the studies directly. Future studies using both whole-brain and high-resolution methods within the same participants will be needed to investigate the specific balance of activational increases and decreases present within the APOE-4 carriers, who are at risk for future cognitive decline.
Taken together, these results suggest that cognitive compensation wherein subjects use additional extrahippocampal regions to bring their memory-related performance to a normal level during early pathological AD changes may result from decreases in hippocampal CA23DG activity. Investigating the subregional activational changes between the two genetic groups suggests a possible relationship between a reduction in activity within areas first targeted by the disease (hippocampus) and compensatory increase in activity within spared cortical areas.
Due to the small and convoluted nature of the MTL subregions, visualization and localization of activity has proven to be a rather challenging task, making it difficult to detect subtle changes in activity without sufficient resolution. In order to detect such activity changes, previous studies commonly average activity patterns across subjects, thereby increasing signal detection. However, this may result in uncertainty in signal localization, as the hippocampus is increasingly vulnerable to misregistration between subjects. Therefore, previous studies demonstrating an increase in MTL activity within cognitively normal APOE-4 carriers through averaging across subregions may have masked subtle reductions in activity. Additionally, because subregions of the hippocampus serve functionally distinct and even opposite roles within the MTL, averaging across subregions would result in a misrepresentative signal. In fact, in the current study when we average across all hippocampal subregions (CA23DG, CA1 and subiculum) we lose the ability to detect significant group differences in activity. Here we deal with these challenges by using high-resolution functional MRI to detect subtle changes within hippocampal subregions to investigate functional changes associated with genetic risk for AD.
CA3 and dentate gyrus both receive synaptic input from the ERC (Amaral, 1990). Within the ERC, there is substantial evidence of cell loss and synaptic pathology apparent in even the earliest stages of AD (Gomez-Isla et al., 1996; Masiliah et al 1994). Gomez and colleagues (1996) have shown that single layers show severe atrophy very early in the progression of the disease; compared to controls, subjects with mild AD showed a 60% and 40% reduction in layers II and IV of the ERC, respectively. A recent study utilizing high-resolution MRI found APOE-4 effects on ERC within cognitively normal, APOE-4 carriers (Burggren et al., 2008). We have replicated these findings in the current study using this novel cortical thickness measurement, which Burggren and colleagues (2008) have shown to possibly be more sensitive to subtle differences in structure than volume measurements. We find reduced thickness in subiculum and ERC in APOE-4 carriers compared with non-carriers.
The blood-oxygenated-level-dependent (BOLD) signal has been shown to reflect both local field potentials in addition to neural firing rate (Logothetis et al. 2001). Within the hippocampus a recent study suggested the BOLD signal reflects local field potential (synaptic activity; Ekstrom et al., 2009). Therefore, since local field potentials contribute to the BOLD measurement, one possible interpretation of our findings could be that the activity increases are reflective of synaptic input from areas projecting to the hippocampus such as the ERC. It is possible that reductions in CA23DG BOLD activity in APOE-4 carriers compared with non-carriers reflect synaptic loss from ERC; however future studies are required to test this hypothesis. Additionally, CA1 region of the hippocampus receives input both from the CA3 pyramidal cells and the entorhinal cortex (Witter et al., 2000) and should thus be similarly affected. We do not see significant group differences within this region, although there is a trend showing decreased activity in APOE-4 carriers compared with non-carriers (Fig. 5, panel B). It may be the case that this particular cognitive task does not engage CA1 to the same extent as CA23DG making it more difficult to detect group differences. Future studies may find specific APOE differences in this region when using a task specific to CA1 (e.g., allocentric spatial encoding; Suthana et al. 2009).
We do not detect cortical thickness differences within CA23DG associated with APOE-4. However, it could be that gross structural changes occur later in the progression of the disease and that our functional differences occur prior to them. Animal and autopsy studies show structural differences in post-mortem CA3 and dentate and autopsies studies show structural changes in post-mortem CA3 and dentate gyrus subregions of the hippocampus associated with the APOE-4 allele (Cambon et al., 2000; Ji et al., 2003). These structural changes occurring at the level of neurons may be undetectable with the current in vivo resolution of human imaging techniques. However, A recent high-resolution MRI study detected reduced CA3 and dentate gyrus volume in older carriers (mean age: APOE-3 = 71.29; APOE-4 = 69.2) of the APOE-4 allele; however, this effect was not observed in younger carriers (Mueller et al., 2008). In this study, our groups’ average ages were 60.3 and 61.9 for APOE-3 and APOE-4 respectively. Therefore, it may be that functional changes in CA23DG are detectable prior to significant structural changes measurable with current techniques.
Previous studies have found interaction effects between APOE4 and family history outside of the hippocampus during a face recognition task (Xu et al. 2009). In our study, we did not find an interaction between APOE4 and family history in the CA3DG region of the hippocampus. However, our field of view limited us from reliably investigating other brain regions. Future studies are required to investigate the effects of family history on APOE more extensively.
Previous studies have shown correlations between changes in activity and cognitive decline two years later (Bookheimer et al., 2000). In our study, there is no way to know which of our subjects, if any, will develop AD symptoms. Therefore, longitudinal studies are required to determine whether effects of APOE-4 on CA23DG prior to symptoms of AD are associated with future cognitive deficits.
With the combination of high-resolution functional MRI and computational cortical unfolding techniques, we have demonstrated the feasibility of examining neural activity in subregions of the hippocampus, thus providing a more complete picture of the pattern of activational changes seen within AD at-risk individuals. We show a marked reduction in activity within the CA2, 3 and dentate gyrus regions of the hippocampus in older asymptomatic carriers of the APOE ε4 allele. These results support the use of these advanced technologies to study older, cognitively intact, genetically at-risk subjects already demonstrating pre-clinical AD changes, measurable by neuroimaging methods and thereby increasing the chance of identifying diseased individuals during the prodromal phase. These methods also hold the promise of identifying risk factors for brain changes reminiscent of AD before the onset of clinical symptoms and of further investigating risk factors beyond the APOE-4 allele. Overall, using advanced neuroimaging technologies in combination with genetics will greatly impact the future of diagnosis and prevention of AD as these methods will serve to create a composite picture of what is normal, or abnormal, for an individual further differentiating disease related pathology from normal aging. Combining activity pattern differences measurable by functional MRI, genetic risk information, and memory-related performance scores, may provide increased predictive power to better prevent and diagnose the onset of AD.
Supplementary Material
Average % signal changes during encoding compared to baseline in (A) entorhinal cortex (ERC) and (B) parahippocampal cortex (PHC) in genetic groups (APOE e3, n = 15; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences were found within ERC or PHC.
Average % signal changes during encoding compared to baseline averaged across all hippocampal subregions (CA 2, 3, and dentate gyrus (CA23DG), CA1, and subiculum) of genetic groups (APOE e3, n = 16; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences in hippocampal activity were found.
Average cortical thickness measurements (mm) in CA2, 3, and dentate gyrus (CA23DG) in genetic groups (APOE e3, n = 16; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences in cortical thickness were found within CA23DG (t(30) = −0.0823, n.s.).
Significant differences between the groups are indicated by ‘*’ (p<0.05, Student’s t-test). Cortical thickness was significantly decreased for APOE e4 vs. APOE e3 carriers within the subiculum (e3 > e4; t(30) = 2.283, p < 0.05) and entorhinal cortex (e3 > e4; t(30) = 2.082, p < 0.05). Regions shown include CA2,3 and dentate gyrus, CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), and parahippocampal cortex (PHC).
Acknowledgments
We thank Michael Zeineh and Paul Thompson for assistance with group unfolding scripts, and James Kyle and Brian Renner for technical assistance. The authors also thank Andrea Kaplan and Debbie Dorsey for their help in subject recruitment, data management, and study coordination. This work was supported by the NIMH T90 431587-BH-29793, NSF GK-12 0742410, and NIA 2R01 AG013308, P01 AG025831, P01-AG024831, P50 AG 16570, and M01-RR00865, General Clinical Research Centers Program; and the Fran and Ray Stark Foundation Fund for Alzheimer’s Disease Research; and the Larry L. Hillblom Foundation. No company provided support of any kind for this study. Finally, we also wish to thank all of the subjects for their participation in this study.
Footnotes
FINANCIAL DISCLOSURE: Dr. Small reports having served as a consultant and/or having received lecture fees from Abbott, Brainstorming Co., Dakim, Eisai, Forest, Medivation, Novartis, Ortho-McNeil, Pfizer, Radica, Siemens and Wyeth. Dr. Small also reports having received stock options from Dakim.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Insausti R. The human nervous system. San Diego: Academic Press; 1990. The hippocampal formation; pp. 711–755. [Google Scholar]
- Bélanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. The Journal of Clinical Endocrinology and Metabolism. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Saint Louis LA, Wisniewski HM. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet Neurology. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. The New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annual Review of Clinical Psychology. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurologica Scandinavica Supplementum. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:44–51. [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, et al. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage. 2008;41:1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of aged human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:685–692. doi: 10.1016/s0306-4522(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. The Journal of Neuroscience. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, Domb A, Osborne D, Fox N, Crum WR, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry. 2007;164:916–21. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of Alzheimer’s disease and other dementias. Clinics in Geriatric Medicine. 2001;17:209–228. doi: 10.1016/s0749-0690(05)70066-5. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. Berlin: Springer; 1998. [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation Between BOLD fMRI and Theta-Band Local Field Potentials in the Human Hippocampal Area. Journal of Neurophysiology. 2009;101:2668–2678. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, et al. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Archives of Neurology. 2005;62:1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. Journal of Neuroscience. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging. 2007;28:238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Hamer RM, Taylor JR. Selective reminding procedure in depression and dementia. Psychology and Aging. 1987;2:111–115. doi: 10.1037//0882-7974.2.2.111. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Hedley-Whyte ET, Rebeck GW, Vonsattel JP, West HL, Growdon JH. Apolipoprotein E epsilon4/4 in a neuropathologically normal very elderly individual. Archives of Neurology. 1996;53:215. doi: 10.1001/archneur.1996.00550030017010. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer’s disease carrying the apolipoprotein E epsilon4 allele. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;65:322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proceedings of the National Academy of Sciences. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. Journal of Clinical and Experimental Neuropsychology. 1989;11:615–630. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Mount C, Downton C. Alzheimer disease: progress or profit? Nature Medicine. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. NeuroImage. 2008;42:42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericak-Vance MA, Yamaoka LH, Haynes CS, Speer MC, Haines JL, Gaskell PC, et al. Genetic linkage studies in Alzheimer’s disease families. Experimental Neurology. 1988;102:271–279. doi: 10.1016/0014-4886(88)90220-8. [DOI] [PubMed] [Google Scholar]
- Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Archives of Neurology. 1993;50:812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Annals of Neurology. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurology. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2000;97:6037–42. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Women at risk for AD show increased parietal activation during a fluency task. Neurology. 2002;58:1197–1202. doi: 10.1212/wnl.58.8.1197. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. Proceedings of the National Academy of Sciences. 1995;92:4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Computational Biology. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. Journal of Neuroscience. 2009;29:10512–9. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo PC, Sapiro G, Wandell BA. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Transactions on Medical Imaging. 1997;16:852–863. doi: 10.1109/42.650881. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Woods RP, Mega MS, Toga AW. Mathematical/computational challenges in creating deformable and probabilistic atlases of the human brain. Human Brain Mapping. 2000;9:81–92. doi: 10.1002/(SICI)1097-0193(200002)9:2<81::AID-HBM3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Standardized memory scale for clinical use. J Psychol. 1945;19:87–95. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SE, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11:668–8. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Unfolding the human hippocampus with high resolution structural and functional MRI. The Anatomical Record. 2001;265:111–120. doi: 10.1002/ar.1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average % signal changes during encoding compared to baseline in (A) entorhinal cortex (ERC) and (B) parahippocampal cortex (PHC) in genetic groups (APOE e3, n = 15; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences were found within ERC or PHC.
Average % signal changes during encoding compared to baseline averaged across all hippocampal subregions (CA 2, 3, and dentate gyrus (CA23DG), CA1, and subiculum) of genetic groups (APOE e3, n = 16; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences in hippocampal activity were found.
Average cortical thickness measurements (mm) in CA2, 3, and dentate gyrus (CA23DG) in genetic groups (APOE e3, n = 16; APOE e4, n = 16). Error bars correspond to the standard error across subjects for each group. No significant group differences in cortical thickness were found within CA23DG (t(30) = −0.0823, n.s.).
Significant differences between the groups are indicated by ‘*’ (p<0.05, Student’s t-test). Cortical thickness was significantly decreased for APOE e4 vs. APOE e3 carriers within the subiculum (e3 > e4; t(30) = 2.283, p < 0.05) and entorhinal cortex (e3 > e4; t(30) = 2.082, p < 0.05). Regions shown include CA2,3 and dentate gyrus, CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), and parahippocampal cortex (PHC).