Abstract
Objective
Ten percent of ovarian cancer is attributed to hereditary syndromes, most commonly to mutations in the BRCA1 or BRCA2 genes. These cancers are characterized by a prolonged sensitivity to platinum agents in spite of presentation at advanced stages. We hypothesized that women with BRCA-associated ovarian cancer would also show a high response rate to pegylated liposomal doxorubicin (Doxil).
Methods
A retrospective cohort study was conducted to compare the response rate, progression-free, and overall survival among women with BRCA-associated or sporadic ovarian cancer who were treated with Doxil.
Results
A response to Doxil was seen in 13 of 23 patients with BRCA mutations (56.5%; 3 by RECIST criteria and 10 by CA125 levels) compared with only 8 of 41 women with non-hereditary cancers (19.5%; 2 by RECIST criteria and 6 by CA125 levels; p=0.004). This was associated with an improved progression-free and overall survival as measured from the time of Doxil administration. Notably, platinum sensitivity did not directly correlate with a response to Doxil.
Conclusions
Women with BRCA-associated ovarian tumors demonstrate a greater sensitivity to cytotoxic therapy with Doxil than has previously been reported in unselected cases.
Introduction
More than 20,000 women are diagnosed with ovarian cancer annually in the United States, and nearly 14,000 women succumbed to this disease in 2010. Approximately ten percent of all ovarian cancer cases are attributed to hereditary syndromes, most commonly the result of mutations in the breast cancer susceptibility genes, BRCA1 and BRCA2 (1). In addition to an elevated risk of breast cancer, BRCA mutation carriers have a 16–68% lifetime risk of developing epithelial ovarian carcinoma (2–4).
BRCA-associated ovarian tumors are characterized by presentation at advanced stages, with high grade serous histology and a propensity for visceral metastasis (5–6). Despite these aggressive features, women with BRCA mutations have an improved response to chemotherapy and prolonged treatment-free intervals when compared with cases of sporadic disease (5). In describing a “BRCAness” phenotype, Tan et al. attribute this survival advantage to the persistent sensitivity of BRCA−/− tumor cells to platinum-induced DNA damage (7). In support of this observation, acquired platinum resistance has been directly associated with reversions of germline BRCA mutations in both ovarian cancer cell lines and tumor specimens from patients with platinum-resistant disease (8–10).
Like platinum agents, one mechanism of action for the cytotoxic effect of anthracyclines is direct DNA damage. Doxorubicin has been shown to cause single- and double-stranded DNA breaks through several mechanisms, including direct intercalation and inhibition of DNA polymerase, interference with the function of topoisomerase II and the formation of intracellular free radicals.(11). Fortunately women who demonstrate resistance to platinum agents often do not demonstrate cross-resistance to doxorubicin. As a result, a well-tolerated liposomal formulation of doxorubicin (Doxil) has become the preferred second-line agent for platinum-resistant disease with reported response rates that range from 9–16% (12–13). Because BRCA mutations disable double-stranded DNA repair mechanisms, BRCA-associated cancers would be expected to demonstrate a preferential sensitivity to any DNA-damaging therapy, including Doxil.
We have observed an excellent response to Doxil among women with hereditary cancer treated in our clinical practice and hypothesized that the response to Doxil by women with BRCA mutations may more closely approximate the higher response rate seen in platinum-sensitive cases(12). Here we present a retrospective cohort study designed to determine whether the response rate and survival following Doxil therapy is significantly different in women with BRCA-deleterious or sporadic ovarian cancer undergoing treatment for advanced or recurrent disease.
Methods
Patient Selection
Patient information was retrieved from a pharmacy database at the University of Pennsylvania to identify women who underwent treatment with Doxil between 2001 and 2007. Ovarian cancer cases were confirmed using treatment records from the Division of Gynecologic Oncology and these patients were cross-referenced with data from the Cancer Genetics Program of the Abramson Cancer Center. At two collaborating institutions, Cedars-Sinai Medical Center in Los Angeles and Fox Chase Cancer Center in Philadelphia, women who had undergone testing for BRCA mutations were identified, and those with a diagnosis of ovarian cancer were included if they had received Doxil chemotherapy.
Patients who were confirmed to carry a BRCA1 or BRCA2 mutation were evaluated as the experimental (exposed) cohort. An unexposed group was comprised of women who were tested and did not carry a BRCA gene mutation, and women with a thoroughly documented family history and no first- or second-degree relatives with breast or ovarian cancer who were unlikely to carry a genetic mutation, the “presumed negative” cases. Women were excluded from evaluation if they were still undergoing treatment with Doxil at the time of data collection, if family history or follow-up data was unavailable, or if they had a personal history of breast cancer or a concurrent diagnosis of a non-ovarian malignancy.
Patient data
After obtaining approval from each Institutional Review Board, charts were abstracted to collect basic demographic information, medical and oncologic history, and clinical course following Doxil therapy. Response rate was calculated based on either a decrease in tumor size or a fall in the CA125 during or following treatment (14–15). Progression-free survival was measured as the time from initiation of Doxil treatment until the first evidence of progression using either a rise in CA125 based on the Gynecologic Cancer Intergroup criteria or radiographic documentation of tumor growth based on RECIST criteria (Response Evaluation Criteria in Solid Tumors)(15–16). Overall survival was measured as the time from initiation of Doxil treatment until death or the date of last follow-up.
Data was collected regarding platinum sensitivity at the time that Doxil treatment was initiated: patients were considered to be platinum-sensitive if they had a 6 month treatment-free interval after their most recent course of platinum-based therapy. Patients who relapsed within 6 months of prior platinum therapy or who failed to respond to platinum therapy were considered platinum-resistant.
Statistical methods and sample size calculations
Our target sample size was calculated assuming a type I alpha error of 0.05, two-sided, and 80 percent power. We estimated that approximately 80–90% of all patients who received Doxil for recurrent disease would experience progression within one year (17). Furthermore, we anticipated that this fraction would be lower among patients with hereditary disease than among those with sporadic ovarian cancer. For these calculations we assumed that approximately 10% of all enrolled patients would be progression-free at one year, and that the ratio (BRCA−/− vs. sporadic disease) would range from 1.4 to 2.9. Our target was to identify 25 women with a mutated BRCA genotype among 50 patients undergoing BRCA testing to detect a 60% relative improvement in progression-free survival over non-hereditary cases (i.e. a hazard ratio=0.40).
Initial bivariate comparisons between the groups were performed using Fisher’s exact test or Chi-square tests. Log-rank tests were used to evaluate differences in survival between sporadic disease and BRCA-associated cancers, and Cox proportional hazards models were used to identify predictors of overall and progression-free survival. Univariate Cox models were fit to determine initial associations between predictors of individual and progression-free survival, and multivariate models were fit with marginally significant predictors (p<0.10) from univariate models. Statistical modeling was done using STATA version 11 (Stata Corp, College Station TX). All reported p-values are 2-sided with p<0.05 considered to be statistically significant.
Results
Patient characteristics
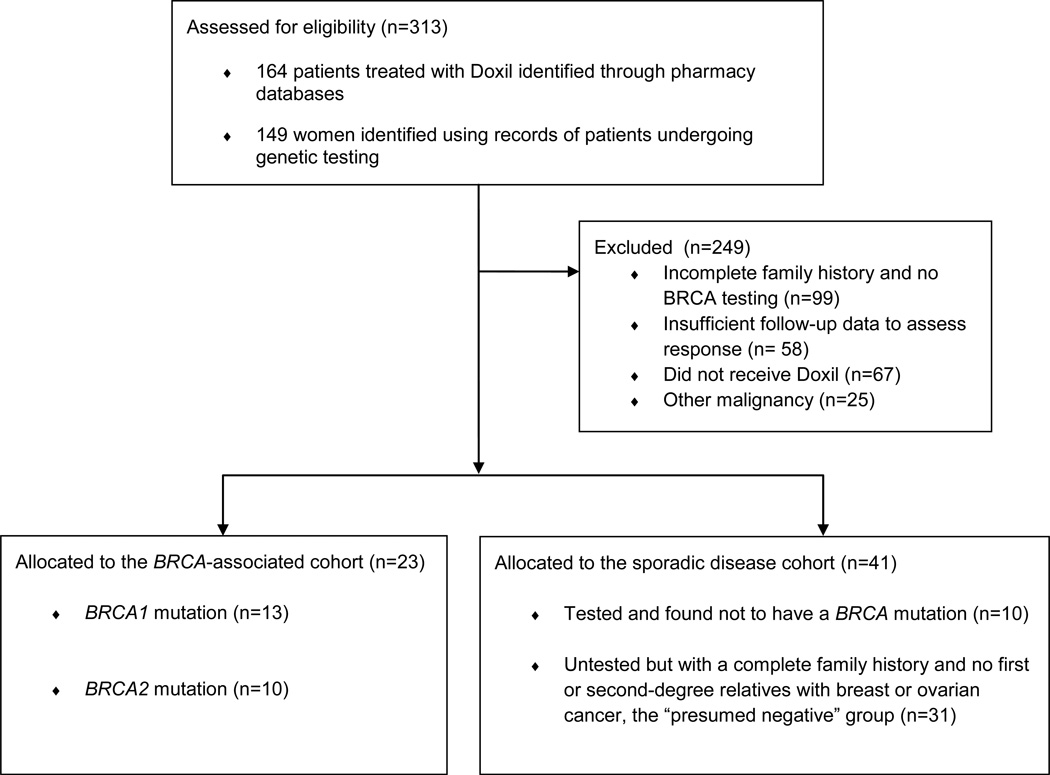
One hundred and sixty-four cases of women undergoing treatment with Doxil were identified using pharmacy databases and 149 cases of women with ovarian cancer were identified from records of women undergoing genetic testing. Details of the patient selection process are provided in Figure 1. From these, 23 women with epithelial ovarian cancer and a documented BRCA1 or BRCA2 mutation who received Doxil chemotherapy were identified and comprised the experimental cohort. An additional 31 patients who had no personal or family history of hereditary cancers, along with 10 women who underwent genetic testing but were found not to harbor a BRCA mutation, were considered the control group with sporadic disease.
Figure 1. Flow diagram for patient selection.
Women in the BRCA-associated and sporadic disease cohorts did not differ significantly in age, stage at presentation, or grade or histologic subtypes of their tumors. The majority of patients in both groups had high grade tumors that presented at an advanced stage. All patients with BRCA mutations had papillary serous cancers except for one patient with a poorly differentiated tumor of mixed histology, and two for whom histology data was unavailable. Among the non161 hereditary cases, 70% were papillary serous, 7% were poorly differentiated, and histology was unavailable for 7%. One patient in the sporadic cohort had an endometroid cancer, and one had a clear cell cancer. The majority of patients were Caucasian (85% overall; 96% of the BRCA cohort and 80% of the sporadic cohort), with African American patients comprising 6% and Asian/Pacific Islanders comprising 3% of the total population. Ethnicity data was unavailable for two patients. Patient characteristics are compared in Table 1.
Table 1. Patient Demographics.
Demographic data for women in the sporadic disease or BRCA-associated cohorts. No significant difference was seen when comparing the age, stage or tumor grade in the two groups. Women with BRCA mutations underwent a greater number of chemotherapy regimens prior to starting Doxil (p=0.004)
| Sporadic Disease Number (%) |
BRCA-associated Number (%) |
p-value | |
|---|---|---|---|
| Age <55 | 19 (46.3) | 13 (56.5) | 0.434 |
| ≥ 55 | 22 (53.6) | 10(43.5) | |
| Stage I–II | 4 (10) | 3 (14.3) | 0.683 |
| III–IV | 36 (90) | 18 (85.7) | |
| Grade 1–2 | 4 (14.3) | 1 (5.3) | 0.635 |
| Grade 3* | 24 (85.7) | 18 (94.7) | |
| Mean prior chemotherapy regimens | 2.34 | 3.39 | 0.004 |
Two patients were unstaged and grade was unknown for 17 cases
Response to Doxil treatment
A response to Doxil was seen in 56.5% of women with BRCA-associated cancer compared with only 19.5% of controls (p=0.004). Response was measured based on a decrease in the CA125 level by the third cycle of Doxil administration in 16 women, and by a reduction in the size of tumor implants on imaging studies using RECIST criteria in five cases (15). Women with stable disease were not counted among responders. In univariate logistic regression analysis, women with a BRCA mutation were 5.36 times more likely to experience a response than women with sporadic disease (95% CI [1.73, 16.59], p=0.004, Table 2).
Table 2. Response Rate.
A comparison of the response rate using a univariate model or a multivariate model which controlled for the number of prior treatment regimens.
| Univariate Logistic Model |
Multivariate Logistic Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | n | OR | p-value | 95% CI | OR | p-value | 95% CI | |
| Sporadic disease | 41 | 1 | 1 | |||||
| BRCA-associated | 23 | 5.36 | 0.004 | [1.73, 16.59] | 12.85 | 0.001 | [3.0, 54.92] | |
Progression-free and overall survival
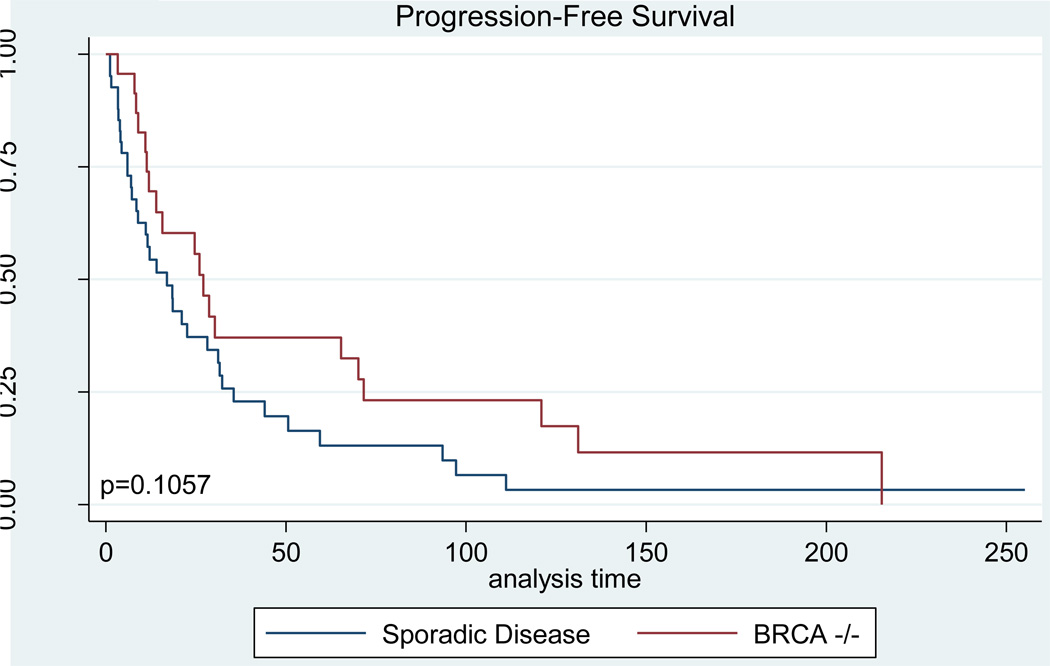
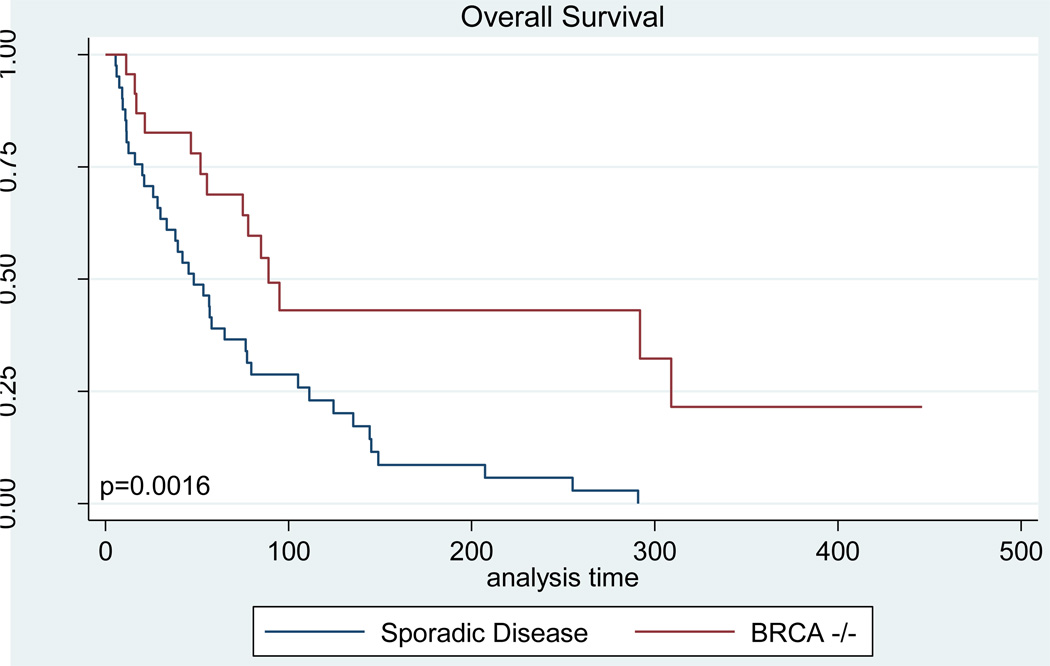
Progression-free and overall survival analyses were performed by measuring survival from the date of first Doxil administration, not from the time of diagnosis. Because women in the BRCA cohort received on average one additional course of chemotherapy, and because in general they would be expected to have longer treatment-free intervals between regimens, the survival data for these cases begins at a later time-point in the disease course. Despite this, both progression-free and overall survival was improved among BRCA mutation carriers (Figures 2, 3). The median disease-free interval for women with BRCA-associated cancer was 27.1 weeks compared with only 17 weeks among women with sporadic disease (p=0.109, Table 3). Overall survival in the BRCA cohort was significantly higher than that seen among controls, with a median survival of 89.1 weeks in women with BRCA mutations versus 48.3 weeks among women with non-hereditary cancers (p=0.002, Table 3).
Figure 2. Progression-Free Survival.
Kaplan-Meier curves demonstrating the disease-free survival time from the first dose of Doxil until evidence of disease progression for women in the BRCA−/− and sporadic disease cohorts.
Figure 3. Overall Survival.
Kaplan-Meier curves demonstrating the overall survival time from the first dose of Doxil until death from any cause for women in the BRCA−/− and sporadic disease cohorts.
Table 3. Platinum sensitivity at the time of Doxil administration.
Demographic data, tumor histology, platinum-sensitivity at the time of Doxil administration, response rate and progression-free and overall survival of women with documented BRCA mutations treated with Doxil. PFS = progression-free survival; OS= overall survival; CR = complete response; PR= partial response; PD= progressive disease. Data regarding platinum sensitivity was unavailable for 4 cases.
| Patient | Age |
BRCA mutation |
Stage | Histology | Number of prior regimens |
Response to platinum prior to starting Doxil |
Response to Doxil |
Measure of Response |
PFS (weeks) |
OS (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 1 | 4 | Papillary serous | 2 | Resistant | PD | CA125 | 11.4 | 33.3 |
| 2 | 49 | 1 | 3 | Papillary serous | 5 | Sensitive | PD | CA125 | 3.3 | 21.6 |
| 3 | 51 | 1 | 1 | Papillary serous | 4 | Sensitive | CR | CA125 | 71.6 | 118.1 |
| 4 | 73 | 2 | 3 | Papillary serous | 1 | Resistant | PR | CA125 | 30.3 | 83.2 |
| 5 | 32 | 2 | 3 | Papillary serous | 1 | Resistant | CR | RECIST | 131.1 | 316 |
| 6 | 64 | 2 | 3 | Unknown | 6 | Sensitive | PD | RECIST | 14 | 55.5 |
| 7 | 50 | 1 | 3 | Papillary serous | 2 | Sensitive | CR | CA125 | 132.3 | 132.3 |
| 8 | 55 | 2 | 3 | Papillary serous | 6 | Resistant | CR | CA125 | 28.7 | 309 |
| 9 | 44 | 2 | 4 | Papillary serous | 3 | Resistant | PD | CA125 | 8 | 46.7 |
| 10 | 46 | 1 | 3 | Papillary serous | 1 | Resistant | CR | RECIST | 215.4 | N/A |
| 11 | 61 | 2 | 3 | Papillary serous | 2 | Sensitive | PR | CA125 | 26 | 85 |
| 12 | 55 | 1 | 3 | Papillary serous | 2 | Resistant | PR | CA125 | 120.9 | N/A |
| 13 | 62 | 1 | 3 | Papillary serous | 4 | Resistant | CR | RECIST | 24.7 | 51.8 |
| 14 | 52 | 1 | 3 | Papillary serous | 3 | Resistant | PR | CA125 | 27.1 | N/A |
| 15 | 52 | 2 | 3 | Papillary serous | 4 | Resistant | PD | CA125 | 8.4 | 11.3 |
| 16 | 64 | 1 | 1 | Papillary serous | 4 | Resistant | PD | CA125 | N/A | 16.9 |
| 17 | 49 | 1 | 3 | Papillary serous | 1 | Resistant | CR | CA125 | 70.1 | 291.9 |
| 18 | 33 | 1 | 3 | Papillary serous | 2 | Resistant | PR | CA125 | 15.7 | 89.1 |
| 19 | 43 | 1 | 3 | Papillary serous | 4 | Resistant | CR | RECIST | 65.3 | N/A |
| 20 | 71 | 2 | 1 | Papillary serous | 7 | Unknown | PD | RECIST | 12 | 75 |
| 21 | 61 | 2 | 3 | Mixed histology | 4 | Unknown | PD | CA125 | 5 | 95 |
| 22 | 43 | 1 | 3 | Unknown | 7 | Unknown | PD | RECIST | 11 | 69 |
| 23 | 66 | 2 | U | Papillary serous | 4 | Unknown | PD | RECIST | 9 | 78 |
Prior treatment with chemotherapy
Patients in both groups were heavily pre-treated, undergoing an average of 2.7 prior chemotherapeutic regimens before receiving Doxil (range 0–6). Women with BRCA mutations underwent a significantly higher number of prior regimens compared to women with sporadic disease (mean 3.4 vs 2.3 respectively, p=0.004).
Prior treatment was a negative confounding factor in calculating the response rate as well as both disease-free and overall survival. Because women with BRCA mutations underwent a higher number of prior chemotherapy regimens before receiving Doxil, a multivariable logistic regression model controlling for the number of prior chemotherapy regimens was evaluated. In this model, the likelihood of response to Doxil was 12.85 times higher among the BRCA cohort compared to women with sporadic disease (95% CI [3.0, 54.92], p=0.001, Table 2).
As with the response rate, both progression-free and overall survival results were negatively affected by the number of prior chemotherapy regimens. After adjusting for BRCA mutation status, as the number of prior chemotherapy regimens increased, the relapse rate increased by 30.4% and the overall mortality rate increased by 22.5%. When controlling for prior treatment, the survival advantage associated with the presence of a BRCA mutation was statistically significant, with a hazard ratio of 0.508 (95% CI 0.28–0.93) for progression-free survival and 0.297 (95% CI 0.14, 0.59) for overall survival in the multivariate model (p=0.027 and 0.001, respectively,Table 3).
Specific data regarding platinum sensitivity at the time of Doxil treatment was available for 60 patients (93.7%). Among these, 14 of 19 (74%) BRCA-associated cases and 32 of 41(78%) sporadic cases demonstrated platinum resistance prior to Doxil treatment. Women who were platinum-resistant received fewer prior courses of chemotherapy compared with those demonstrating persistent platinum-sensitivity (2.1 vs 3.27 prior courses, respectively). Although the response rate overall was higher among platinum-sensitive patients (41.6% versus 33% for platinum-resistant patients; n=12 and 48, respectively), the response to Doxil among patients in this study did not directly correlate with platinum sensitivity. Of the women with BRCA-associated cancers who demonstrated a response to Doxil, only 3 out of 13 were platinum-sensitive (23%), while 77% were considered platinum-resistant when they began treatment with Doxil (Table 3). Similarly, among 8 patients with sporadic disease who demonstrated a response to Doxil, 2 (25%) were platinum-sensitive and 6 (75%) were platinum-resistant.
Discussion
Our results demonstrate a significantly improved response rate of 56.5% among women with BRCA mutations treated with Doxil, which is markedly higher than has been reported in studies evaluating second-line therapies in unselected cases of ovarian cancer (12, 17–18). BRCA mutation status was also associated with an improved progression-free and overall survival as measured from the date of the first cycle of Doxil, despite the fact that patients in the BRCA cohort received more chemotherapy cycles prior to initiating Doxil treatment. When controlling for the number of prior regimens, both the progression-free and overall survival advantage associated with BRCA mutation status were statistically significant.
The 19.5% response rate we describe among the cohort of women with sporadic disease is consistent with previously reported rates of 17–26%, and we observed a median progression free survival of 17 weeks in this group, which is similar to the 16 week median survival in prior studies of platinum-resistant patients (12, 17–19). Based on this relatively high response rate for a second-line therapy and the low toxicity profile of Doxil compared with other agents, an expert panel of oncologists has recommended that Doxil be the first-choice agent for platinum-resistant disease, and that a role for Doxil as maintenance or combination therapy for primary adjuvant treatment be considered (18).
Although a minority of patients in either cohort remained platinum-sensitive at the start of Doxil therapy, a high proportion of platinum-sensitive patients demonstrated a response to Doxil (41.6% overall, 60% among BRCA-associated cases, n=12). A difference in response based on platinum sensitivity has been associated with long term survival in large prospective studies: a follow-up report of a phase III study evaluating Doxil therapy for women with recurrent ovarian cancer documented significantly improved survival rates among platinum-sensitive patients treated with Doxil (74.1%, 51.2%, and 28.4% at 1, 3, and 5 years) compared with platinum refractory patients (41.5%, 21.1%, and 13.8 %, respectively) (17). Because both Doxil and platinum drugs induce direct DNA damage by intercalation, it is not surprising that platinum-sensitivity is associated with an improved response to Doxil. Similarly, the persistent platinum-sensitivity of BRCA−/− tumors, resulting from an inability to repair double-stranded DNA breaks, would also be expected to render them vulnerable to Doxil-induced DNA damage (7–9).
It is notable, then, that our results do not show a direct correlation between platinum-sensitivity and response to Doxil among women with BRCA mutations. Among platinum-resistant patients, who have historically shown a response in only 9–16% of cases, the advantage associated with BRCA mutation status was more pronounced: 77% of platinum-resistant BRCA mutation carriers responded to Doxil treatment, compared with only 17% of women with platinum-resistant sporadic disease (n=48) (12–13). This would suggest that BRCA status cannot be used as a surrogate marker for platinum sensitivity to explain the high response rate in these cases.
Accumulating evidence has demonstrated that platinum resistance in some BRCA−/− tumors may be the result of secondary mutations that restore wild-type BRCA function and the capacity to repair double-stranded DNA breaks (8–9). Although platinum-resistant BRCA−/− tumors may lose their selective vulnerability to DNA damage, alternate mechanisms of action may be responsible for the enhanced cytotoxic effects of Doxil in these cases. Recently, an immunomodulatory role for Doxil has been demonstrated which may enhance host anti-tumor immune responses (20–23). In particular, Alagkiozidis et al. demonstrated that Doxil, in a dose-dependent fashion, alters the immunophenotype of non-apoptotic ovarian tumor cells, rendering them more susceptible to T cell-mediated tumor killing (20). Because BRCA1- cancers have been shown to recruit high numbers of T cells to tumor implants, these cancers may be especially vulnerable to therapies that enhance anti-tumor immunity (24), possibly contributing to the improved response rate even among platinum-resistant BRCA−/− cases.
Limitations of this study include the retrospective design and the small sample size that lacked power to detect a significant difference in progression-free survival in univariate analysis. Although our results are comparable with historic response rates in unselected groups, a large proportion of patients were excluded due to a lack of sufficient data to consider them among the presumed negative group for analysis. Finally, because BRCA mutation carriers received more chemotherapy before beginning Doxil treatment, our decision to analyze survival outcomes starting at the time of Doxil administration may underestimate the survival benefit associated with BRCA mutation status and the potential benefit of Doxil for early recurrences in these patients. Prospective data evaluating Doxil therapy for BRCA-associated ovarian cancer are expected as results mature from a phase II trial comparing Doxil with PARP-inhibitors in women with hereditary ovarian cancer (Kaye et al, ESMO, 2010).
Overall, our results support the use of Doxil for advanced or recurrent ovarian cancer, particularly in women with hereditary disease. Together with data suggesting that Doxil treatment may prolong platinum-free intervals, and evidence that carboplatin combined with Doxil was not inferior to carboplatin/taxol therapy, our findings support a wider role for Doxil in the treatment of advanced ovarian cancer (25–26).
Research Highlights.
56.5% of women with BRCA-associated ovarian cancer demonstrated a response to Doxil compared with 19.5% with sporadic disease
BRCA mutation carriers had a longer progression-free and overall survival following Doxil treatment.
The response to Doxil in these cases did not correlate with platinum sensitivity.
Table 4. Progression-Free and Overall Survival.
A comparison of the hazard ratios for progression-free and overall survival using a univariate model or a multivariate model controlling for the number of prior treatment regimens.
| Median | Range | Univariate Cox Model | Multivariate Cox Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | n | (weeks) | (weeks) | HR | p-value | 95% CI | HR | p-value | 95% CI | |
| Progression Free Survival | ||||||||||
| Sporadic disease | 41 | 17 | (1.2 – 255.1) | 1 | 1 | |||||
| BRCA-associated | 23 | 27.1 | (3.3 – 215.4) | 0.632 | 0.109 | [0.36, 1.11] | 0.508 | 0.027 | [0.28–0.93] | |
| Overall Survival | ||||||||||
| Sporadic disease | 41 | 48.3 | (5.6 – 290.9) | 1 | 1 | |||||
| BRCA-associated | 23 | 89.1 | (11.3 – 445.9) | 0.361 | 0.002 | [0.36, 1.11] | 0.297 | 0.001 | [0.14, 0.59] | |
Acknowledgments
National Cancer Institute grant number T32 CA93283.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Rubin SC. BRCA-related ovarian carcinoma. Cancer. 2003 May 1;97(9):2127–2129. doi: 10.1002/cncr.11341. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009 Jan 21;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001 Mar;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domchek SM, Rebbeck TR. Prophylactic oophorectomy in women at increased cancer risk. Curr Opin Obstet Gynecol. 2007 Feb;19(1):27–30. doi: 10.1097/GCO.0b013e32801195da. [DOI] [PubMed] [Google Scholar]
- 5.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003 May 1;97(9):2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 6.Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. 2010 May 20;28(15):2505–2511. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- 7.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. "BRCAness" syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008 Dec 1;26(34):5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 8.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008 Feb 28;451(7182):1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008 Apr 15;68(8):2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005 Apr 14;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 11.Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie. 1984 May;66(5):333–352. doi: 10.1016/0300-9084(84)90018-x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000 Sep;18(17):3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 13.Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Phase 2 trial of liposomal doxorubicin (40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000 Sep;78(3 Pt 1):369–372. doi: 10.1006/gyno.2000.5921. [DOI] [PubMed] [Google Scholar]
- 14.Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003 May 15;21(10) Suppl:187s–193s. doi: 10.1200/JCO.2003.01.223. [DOI] [PubMed] [Google Scholar]
- 15.Gossner G, Coleman RL, Mutch DG, Horowitz NS, Rader JS, Gibb RK, et al. CA-125 response in patients with recurrent ovarian or primary peritoneal cancer treated with pegylated liposomal doxorubicin or topotecan. Gynecol Oncol. 2006 Oct;103(1):212–218. doi: 10.1016/j.ygyno.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004 Oct;95(1):1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Thigpen JT, Aghajanian CA, Alberts DS, Campos SM, Gordon AN, Markman M, et al. Role of pegylated liposomal doxorubicin in ovarian cancer. Gynecol Oncol. 2005 Jan;96(1):10–18. doi: 10.1016/j.ygyno.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Gorumlu G, Kucukzeybek Y, Kemal-Gul M, Karaca B, Cosan-Terek M, Karabulut B, et al. Pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients. J BUON. 2008 Jul–Sep;13(3):349–352. [PubMed] [Google Scholar]
- 20.Alagkiozidis I, Facciabene A, Carpenito C, Benencia F, Jonak Z, Adams S, et al. Increased immunogenicity of surviving tumor cells enables cooperation between liposomal doxorubicin and IL-18. J Transl Med. 2009;7:104. doi: 10.1186/1479-5876-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005 Dec 19;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007 Jan;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 23.Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A. Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin 2. Clin Cancer Res. 1999 Mar;5(3):687–693. [PubMed] [Google Scholar]
- 24.Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009 Mar;22(3):393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 25.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010 Jul 10;28(20):3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletto MO, Falci C, Pianalto D, Artioli G, Azzoni P, De Masi G, et al. Phase II study of pegylated liposomal doxorubicin and oxaliplatin in relapsed advanced ovarian cancer. Gynecol Oncol. 2006 Feb;100(2):318–323. doi: 10.1016/j.ygyno.2005.08.020. [DOI] [PubMed] [Google Scholar]