Abstract
Social interactions in rodents are rewarding and motivating and social isolation is aversive. Accumulating evidence suggests that disruption of the social environment in adolescence has long-term effects on social interactions, on anxiety-like behavior and on stress reactivity. In previous work we showed that adolescent isolation produced increased reactivity to acute and to repeated stress in female rats, whereas lower corticosterone responses to acute stress and decreased anxiety-related behavior were noted in isolated males. These results indicate a sex specific impact on the effects of social stress in adolescence. However, little is known about whether social isolation impacts behaviors related to affect and whether it does so differently in male and female rats. The present study investigated the impact of adolescent social isolation from day 30-50 of age in male and female Sprague Dawley rats on behavior in the forced swim test at the end of adolescence and in adulthood and on behavior in the sucrose preference test in adulthood. Adult female rats that were isolated in adolescence exhibited increased climbing on the first and second day of the forced swim test and showed an increased preference for sucrose compared to adult females that were group-housed in adolescence. There were no effects in male rats. The results indicate that social isolation in adolescence produces a stable and active behavioral phenotype in adult female rats.
Keywords: adolescent stress, forced swim test, sucrose preference test, sex differences, social stress
1.1. Introduction
Adolescence is a period of life characterized by maturation of cognitive, reproductive and social skills and capacities in all mammals [1, 2]. These maturational processes are based in robust and widespread changes in neuronal structure and function [3]. In adolescence, peer relationships are the primary source of life stressors in boys and girls though there are striking sex differences [4]. Adolescent girls report higher levels of stress associated with their friendships, report more negative life events and experience more distress when such negative life events occur [4]. Understanding the impact of stress in adolescence is important because of the strong link between stress and affective and anxiety disorders [4, 5, 6]. Furthermore, the stress-responsive hypothalamic-pituitary-adrenal axis becomes sexually differentiated in adolescence suggesting that the impact of stress during adolescence may differ between males and females.
Rodent models of adolescent social stress have validity for understanding the impact of social stress in adolescent humans because adolescent rodents live in groups and exhibit higher levels of social behavior than either younger or older animals [7]. Social behavior in adolescence, including play and social grooming, is thought to facilitate normal cognitive and social development [8]. Social interactions in rodents are rewarding and motivating and social isolation is aversive [9, 10]. Accumulating evidence suggests that disruption of the social environment in adolescence has long-term effects on social interactions [11, 12, 13] and on anxiety-like behavior [14, 15] though evidence for effects on anxiety-like behavior is conflicting [16]. In previous work we showed that adolescent isolation produced increased reactivity to acute and to repeated stress in female rats, whereas lower corticosterone responses to acute stress and decreased anxiety-related behavior were observed in isolated males [15]. These results indicate a sex specific impact on the effects of social stress in adolescence, consistent with other findings [17]. However, little is known about whether social isolation impacts behaviors in the forced swim and sucrose preference tests and whether it does so differently in male and female rats. These tests examine important components of affective disorders, including behavioral despair and anheonia, respectively, and have been extensively validated with anti-depressant drugs [18, 19, 20, 21]. In studies in which anti-depressants are not administered, behavior in forced swim test provides indications of coping strategies. On the first day of the forced swim test, animals are typically more proactive (more time spent climbing and swimming than in immobility) and these active behaviors shift towards a more passive phenotype as indicated by increased time spent in immobility on the second day of the test [22]. Thus, the present study investigated the impact of adolescent social isolation on behavior in the forced swim test at the end of adolescence and in adulthood and on behavior in the sucrose preference test in adulthood.
1.2. Materials and Methods
1.2.1. Animals
Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA). Twenty female and male rats arrived at postnatal day 23, (P23) and were housed in same-sex groups of 3 to 4 per cage. Water was provided through one bag per cage and the capacity of each bag was approximately 450ml. Half of the males and females were assigned to the control group and the other half was assigned to the socially isolated, experimental group. On P30, the control rats were re-housed in groups of 2-3 and the isolated rats were re-housed one per cage. On P49-P50, all rats were tested in the two-day Porsolt forced swim test (FST). On P51, the rats in the control group were re-housed in new same-sex, same-treatment groups of 2-3 rats per cage. The isolated rats were housed in same-sex, same-treatment groups of 2-3 rats per cage. On P70 and 71, the FST was re-administered. Then at least 2 weeks later, all rats were singly housed in order to conduct the sucrose preference test. Rats were housed in larger cages for this test to allow fluid provisions through two bags. These studies were approved by the Children’s Hospital of Philadelphia Research Institute’s Institutional Animal Care and Use Committee. The experimental design is depicted in Figure 1.
Figure 1.
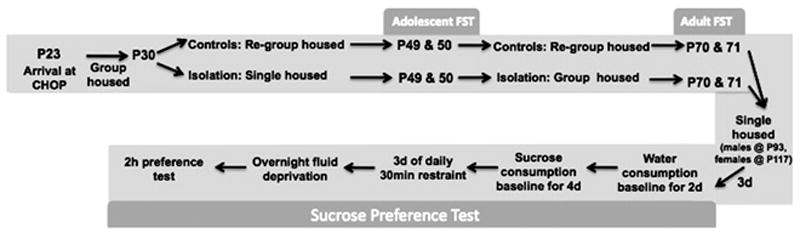
The timeline and experimental designs for the current studies.
We chose to isolate rats from day 30 to 50 of age for the following reasons. Social isolation between day 25-45 of life in male rats, but not before day 25 or after day 45, delays emergence into an open field and slows the declines in novel object contact in adulthood at day 90 [23, 24]. Similarly, isolation during day 26-40 of age in males increases anxiety-related behavior in adulthood, whereas equivalent periods of isolation at later ages (day 65 or 130) have little effect in male rats [25, 26]. Thus, isolation form day 30-50 encompasses the period when the enduring effects of isolation have been observed. In addition, rats in the wild typically leave their burrows around 28 days of age and after this time, adolescent-typical neurobehavioral characteristics appear, vaginal opening occurs and increases in mature spermatids in seminiferous tubules are observed [27]. Based on such evidence, it is now increasingly accepted that day 28-50 of age encompasses the full extent of adolescence [17]. Finally, this study is based on our previous study in which rats were isolated from day 30-50 of age [15].
1.2.2. Forced Swim Test (FST)
The forced swim test (FST) test was adapted from the original procedures of Porsolt et al. [28] with modifications of Detke, Rickels and Lucki [20]. During adolescence, at P49, each of the 40 animals was subjected to 15 minutes of pre-exposure to the test environment – a plastic cylinder filled up to 37cm with 25 °C water. Animals could neither escape the water-filled cylinder nor support themselves by touching the bottom of the cylinder. Twenty-four hours later, on day 2, each animal was subjected to 5-minute swim in the same cylinder. Stressed rats remained isolated during the FST on day 49 and 50. Three behaviors were scored: immobility, swimming and climbing. Immobility was defined as no active movement besides minor efforts to keep the head afloat. Swimming was identified when the animal pedaled around the cylinder and moved more than ¼ of the circumference with all four paws immersed under water. Climbing was defined as the animal floating upright and actively attempting to climb out with their front paws extending above the water. These behaviors were coded every fifth second. The frequency of the occurrence of each behavior was converted to total time spent engaged in each behavior. The same 15-minute habituation and 5-minute tests were performed in adulthood, on P70 (day 1) and P71 (day 2), respectively, in all rats. Videorecordings were made on day 2 of the test in adolescence (day 50) and adulthood (day 71) and on day 1 of the test in adulthood (day 70). In forced swim test, at each age 10 control male rats, 10 male rats isolated during adolescence, 10 control female rats and 10 female rats isolated during adolescence were tested.
1.2.3. Sucrose Preference Test (SPT)
Based on Bechtholt et al. [19], a two-bottle choice paradigm was used to test for differences between the adult group-housed control rats and adult rats socially isolated during adolescence in their relative preference for sucrose over water. At P93, male rats were singly housed in new cages that allowed placement of two water bags. Animals were individually housed in order to assess fluid consumption for each individual rat. Animals were left to habituate to their new housing and drinking conditions for 3 days. Then, water consumption at baseline and body weights were taken for the next 48 hours, twice a day at 1100h and 1700h. For assessment of daily 24h consumption of water and of sucrose, the consumptions at 110h and 1700h were summed. Liquid consumption was conducted by weighing the water bags on a standard laboratory scale. The positions of the water bags were reversed after each measurement to control for any side preference throughout the study. Water baseline measurement was followed by the replacement of all water with sucrose solutions and 4 days of habituation to a 1% sucrose solution [19]. Again, consumptions were measured twice a day (1100h, 1700h) and side preference controlled. After the sucrose habituation period, all sucrose solutions were replaced by water. For the following 3 days, animals were subjected to an acute stress of 30-min restraint once a day at 1000h. Restraints were performed in rat decapitation cones. This was done to reinforce a stronger level of stress to induce a differential preference for sucrose between the control and isolated groups. Rats were permitted free access to water through the two water bags and chow throughout the restraint period. The restraint procedure was followed by an overnight, 20-hour deprivation of all fluids (chow was still available). The next morning (P107) at 1000h, one bag of water and one bag of 1% sucrose were made available randomizing to control side preference. Consumption measurements were taken after the first and second hour. All of the same procedures were performed on females with the habituation period starting at P117. Females were run separately due to limited availability of cages that could accommodate two water bags. Sucrose preference was calculated as a relative ratio (mass of sucrose solution intake/total fluid intake). Sucrose is highly palatable to rats, therefore, decreases in consumption of sucrose reflect a decreased sensitivity to normally rewarding stimuli, anhedonia, a major symptom of depression. This test has been extensively validated by anti-depressant drugs [29]. In this test, 10 rats/group were tested. However, due to leaks in water bags, some data were lost on some days. The group sizes varied from 8-10/group, depending on the measure. The specific group sizes for each measure are provided in Table 1 or in the legends for the figures.
1.2.4. Statistics
The day 2 FST data were analyzed by an Age (adolescent d50 or adult d71) × Stress (control or isolated during adolescence) × Sex repeated measures ANOVAs with Age being the repeated measure separately for immobility, swimming or climbing. The day 1 FST data at day 70 were analyzed by two-way ANOVAs for Sex × Treatment (control or isolated during adolescence) separately for time spent in immobility, swimming or climbing. For the sucrose preference test, the baseline water and sucrose habituation consumption data were analyzed separately by Day × Stress (control or isolated during adolescence) × Sex repeated measures ANOVAs with Day being the repeated measure separately for water consumption baseline and for sucrose consumption. For analysis of the preference test, an Hour (1st or 2nd hour) × Stress (control or isolated during adolescence) × Sex repeated measures ANOVA with Hour being the repeated measure was used. Data were analyzed using Prism 4 (GraphPad Software, LaJolla, CA). Significant Interaction effects were followed by Fisher’s post-hoc tests. A probability level of p<0.05 was used to indicate the statistical significance of the results.
1.3. Results
1.3.1. Forced Swim Test
Immobility on day 2
For immobility, there was a tendency for a significant effect of Sex (p<0.066) with female exhibiting greater immobility regardless of age or treatment (Figure 2a). There was no significant effect of Stress. There was a significant effect of Age ((F(1,36)=34.0; p<0.001) indicating that immobility was greater on day 71 than on day 50, regardless of sex or adolescent stress. There was a significant Age × Stress interaction (F(1,36)=4.01; p<0.05). Post hoc tests indicated that, regardless of sex, control rats on day 50 exhibited less immobility than control rats (p<0.001) and isolated rats at day 71 (p<0.018). Isolated rats on day 50 exhibited less immobility than control rats at day 70 (p<0.001) and tended to be lower than isolated rats at day 70 (p<0.06). This was consistent with the significant overall age effect.
Figure 2.
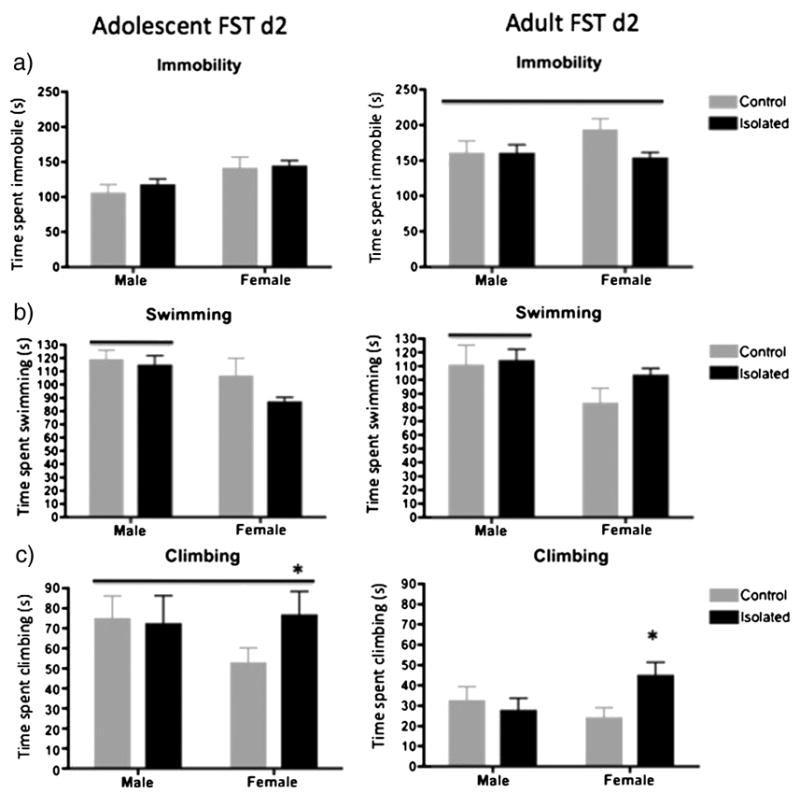
Male and female rats were exposed to the stress of isolation during adolescence or remained group housed (controls) from day 30-50 of age. On day 49 and 50 (adolescence), rats were tested in the two-day Porsolt forced swim test (FST). Rats were then (re-)group-housed and tested again in the 2d FST in adulthood (day 71,72). The behavioral results of from the 2nd day of the FST are shown. Times spent in immobility (a), swimming (b) or climbing (c) were assessed on day 2 and are shown here. Group sizes were 10/group. Adult rats exhibited significantly (p<0.05) increased time spent immobile compared to adolescent rats (shown by the bar in a) regardless of sex or adolescent stress experience. Male rats exhibited significantly more time spent swimming compared to females (p<0.05), regardless of age or adolescent stress experience (shown by the bars in b). Male rats exhibited significantly more time spent climbing compared to females (p<0.05), regardless of age or adolescent stress experience (shown by the bar in c). Female rats that had been isolated in adolescence exhibited significantly increased time spent climbing (p<0.05) compared to control females, regardless of age (indicated by the * in c).
Swimming on day 2
For swimming, there was a significant Sex effect (F(1,36)=6.21; p<0.017) with male rats exhibiting greater time spent swimming than female rats (p<0.007), regardless of age or stress (Figure 2b).
Climbing on day 2
For climbing, there was a significant Age effect (F(1,36)=43.0; p<0.001) with day 50 old animals exhibiting increased climbing compared to day 71 animals, regardless of sex or stress (Figure 2c). There was a significant Sex × Stress interaction (F(1,36)=4.71); p<0.037). Post hoc analyses indicated that females that were isolated during adolescence exhibited greater time spent climbing compared to control females regardless of age. These results indicate that on both day 50 and day 71 on the 2nd day of the FST, female rats that had been isolated during adolescence exhibited increased time spent climbing compared to female controls. In contrast, male rats that had been isolated during adolescence did not differ from their controls in their behavior in the FST at either age.
Day 1 of the forced swim test in adulthood
Because the finding of increased climbing in isolated females, behavior of on day 1 of the FST at this age was examined inorder to determine if the increased climbing occurred on the first exposure to swim (Figure 3). We observed a significant effect of Sex on immobility (F(1,32)=6.84; p<0.014) with males exhibiting greater time spent immobile compared to females, regardless of stress experience (Figure 3a). We observed a significant effect of Sex (F(1,32)=6.00; p<0.02) with females exhibiting greater time spent swimming compared to males, regardless of adolescent stress (Figure 3b). We observed a significant effect of Stress (F(1,32)=4.31; p<0.046) with animals exposed to adolescent isolation exhibiting greater time spent climbing compared to controls, regardless of sex (Figure 3c). Although these results suggest that both male and female rats exposed to adolescent isolation spent more time climbing in adulthood, the effect is largely due to the females, as apparent in Figure 3c.
Figure 3.
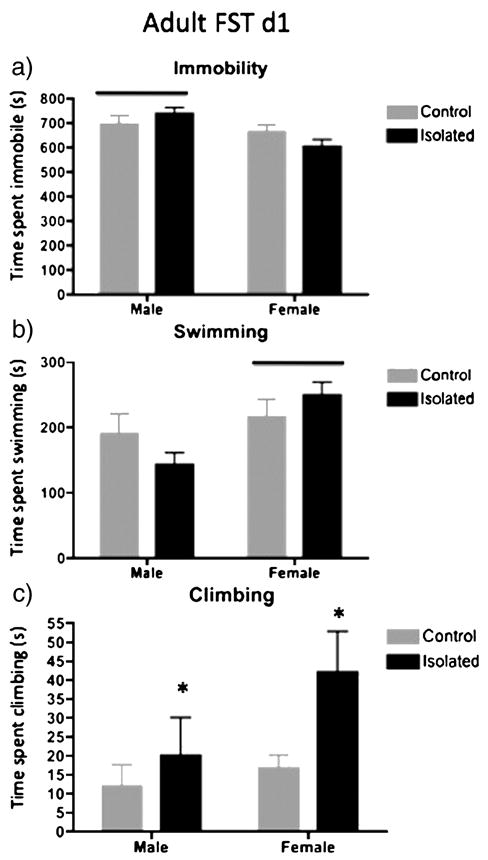
Time spent in immobility (a), swimming (b) or climbing (c) on the 1st day of the FST from the adult animals in Figure 2 is shown here. Adult male rats exhibited significantly more time spent immobile on the first day of the FST than females, regardless of adolescent stress (shown in a; p<0.05). Adult female rats spent significantly more time swimming on the first day of the FST than males, regardless of adolescent stress (shown in b; p<0.05). Isolated rats spent significantly more time climbing compared to control rats, regardless of stress (shown in c; p<0.05).
1.3.2. Sucrose Preference Test
Baseline
There was a significant Sex effect (F(1,34)=14.21; p<0.001) with females consuming more water at baseline compared to males (p<0.001; Table 1). There was a significant Sex × Stress interaction (F(1,34)=3.8; p<0.05). Post-hoc test indicated that females that had been isolated during adolescence exhibited significantly more water consumption compared to control females (p<.003), male controls (p<0.001) and males that had been isolated during adolescence (p<0.001). Control females also exhibited more water consumption compared to control males (p<0.04) and isolated males (p<0.037). There was no effect of Day indicating that water consumption was similar during both days of baseline water consumption (Table 1).
Table 1.
Consumption and body weight measures during baseline water consumption and sucrose habituation phases of the sucrose preference test.
| Dependent Measure | Male Control | Male Isolated | Female Control | Female Isolated |
|---|---|---|---|---|
| Baseline d1 water consumption (mg/kg) | 70.3 ±3.9 (n=10) | 66.7± 2.2 (n=10) | 88.9 ±8.4** (n=10) | 125.1±15.5*, ** (n=10) |
| Baseline d2 water consumption (mg/kg) | 69.9±4 (n=9) | 72.4±2.2 (n=10) | 100.7±21.9** (n=8) | 126.1±22*, ** (n=8) |
| Body weights on d2 of water consumption (g) | 499.8±10 (n=10) | 503.1±13.5 (n=10) | 308.2±6.9** (n=10) | 302.1±4.5** (n=10) |
| Sucrose d1 consumption (mg/kg) | 132.2±22 (n=10) | 118±9.6 (n=10) | 168.3±27.3** (n=10) | 203.3±25.4** (n=10) |
| Sucrose d4 consumption (mg/kg) | 263.1±13.7 (n=9) | 253.8±20.3 (n=10) | 443.6±63** (n=9) | 496±44** (n=10) |
| Body weights on d4 of sucrose habituation (g) | 519.6±12.9 (n=10) | 521.1±16.6 (n=10) | 311.3±7.0** (n=10) | 305.1±5.7** (n=10) |
| Change in body weight during repeated restraint (g) | -3.0±3.3 (n=8) | -3.1±2.4 (n=10) | -2.6±2.0 (n=10) | -4.3±1.6 (n=10) |
indicates that adult females that were isolated in adolescence exhibit increased water consumption compared to all other groups (p<0.05).
indicates significant Sex effects with female rats significantly different from male rats, regardless of stress treatment (p<0.05).
For sucrose consumption during habituation phase, there was a significant Sex effect (F(1,36)=24.03; p<0.001), a significant Day effect (F(1,36)=158.5; p<0.001), and a significant Day × Sex effect (F(1,36)=22.9; p<0.001). Post hoc analyses indicated that females consumed more sucrose than males (p<0.001) regardless of adolescent stress experience and, overall, consumption was greater on the fourth day compared to the first (p<0.001). Post-hoc analyses of the significant Day × Sex interaction effect indicated that females on the 4th day consumed more sucrose than they did on the 1st day and more than males on the first or fourth days (p<0.001 each day). There were no significant effect of adolescent isolation on body weight throughout the studies (Table 1).
Preference Test
On the day of the preference test, sucrose consumption as a percentage of total consumption was used to assess preference for sucrose, as in Bechtholt et al. [19]. An Hour (1st or 2nd hour) × Stress (control or isolated during adolescence) × Sex ANOVAs were conducted with Hour being the repeated measure for sucrose preference (Figure 4a). We observed a significant Hour effect (F(1,34)=5.11; p<0.03) and a significant Hour × Sex × Stress interaction (F(1,34)=4.15; p<0.049). Post hoc analyses indicated that intake was greater in the second hour compared to the first overall. Post hoc analyses of the significant 3-way interaction effect indicated that control females increased their consumption on the second hour compared to the first (indicated by bar in Figure 4a), but sucrose intake was similarly elevated in the first and second hour in isolated females. Isolated females also exhibited a tendency for greater consumption in the first hour compared to control females (p<.11) and compared to isolated males (p<0.07). Over the total 2hour preference test, there were no significant main effects and a tendency (p<0.06) for a significant Interaction effect (Figure 4b). Because of this trend, we conducted t-tests between female control and female isolated rats and between male control and male isolated rats. The results showed a significantly increased intake during the first hour in the isolated compared to control females (t=5.77; p<0.028). There was no difference in the second hour and no difference between male groups at either hour.
Figure 4.
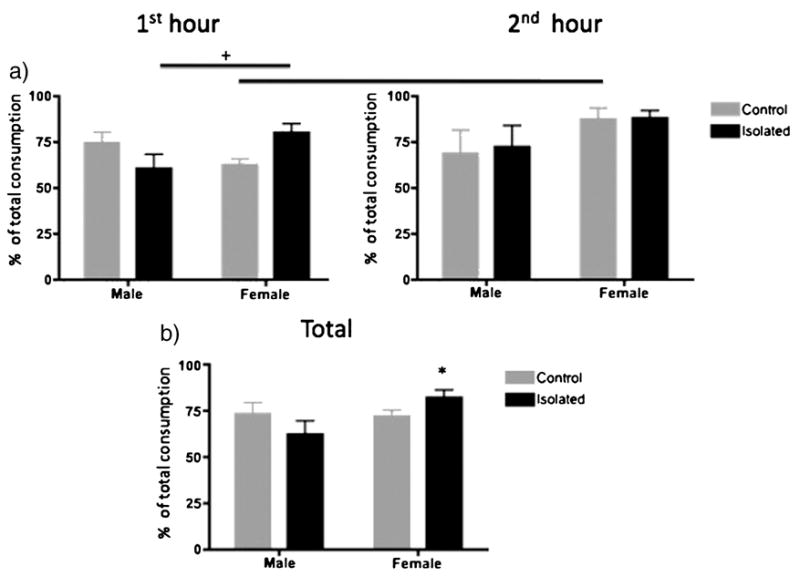
At least two weeks after the forced swim test in adulthood, rats were tested for sucrose preference over 2 hours. The percentage of sucrose consumed as a percentage of total fluid consumption (ie. Sucrose preference) during the first hour (a), second hour (b), and total 2hour consumption (c) are shown. Group sizes were Male control: n=9, male isolated: n=10, female control: n=9, female isolated: n=10. Overall, consumption was greater in the second hour compared to the first hour (not indicated), regardless of sex of adolescent stress experience. Furthermore, control females exhibited a significant (p<0.05) increase in consumption from the 1st to the 2nd hour, whereas isolated females exhibited similar consumption on the 1st to the 2nd hour (indicated by the bar in a). Adult females that had been isolated as adolescents exhibited a tendency (p<0.07) for increased sucrose consumption during the first hour compared to control adult male rats that had been isolated during adolescence (indicated by the + symbol). There was a tendency (p<0.06) for a significant interaction effect in total sucrose consumption and t-tests indicated that isolated females exhibited increased sucrose preference compared to control females (*; p<0.05).
1.4 Discussion
We examined the impact of social isolation throughout adolescence on behavior in adulthood in two tests of affective behaviors. We observed that female rats that had been isolated as adolescents exhibited increased climbing behavior during the second day of the forced swim test in both adolescence and adulthood and increased climbing on the first day of the test in adulthood. There were no differences between isolated and control females in time spent in immobility or swimming. Furthermore, adult females that had been isolated as adolescents exhibited similarly elevated sucrose consumption in the first and second hour of the preference test while control females exhibited an increase from the first to the second hour. Isolated females also exhibited a tendency to increased consumption of sucrose in the sucrose preference test compared to adult males that had been isolated as adolescents and a tendency for increased consumption compared to control females. In contrast to females, adolescent isolation did not impact behaviors in the forced swim test or the sucrose preference test in adult male rats. These results are the first to determine that adolescent social isolation produces long-term and sex-specific effects on behaviors relevant to affect.
Few studies have examined behavior in the forced swim test in adolescence, sex differences in this test in adolescence or the effects of stress during adolescence on behaviors in this test. Hefner and Holmes [30] demonstrated that behavior in this test develops with age since C57 mice exhibited decreased immobility at 4 weeks compared to adulthood. We observed a similar pattern in both males and female rats with immobility exhibited at lower levels on day 50 than on day 71. Leussis and Andersen [31] observed that in early adolescence (day 36 of age), group housed females exhibited increased time spent in immobility and decreased time spent swimming compared to males. Our results with the second day of the forced swim test in adolescence are consistent with these results as we observed that females exhibited increased immobility and decreased time spent swimming compared to males, independent of stress condition. Studies on the impact of stress during adolescence on behavior in the forced swim test have yielded inconsistent results. Leussis and Andersen [31] observed that isolation from day 30-35 increased immobility and decreased swim in males on day 36 without any effect on female rats. However, Jankford et al., [32] did not observe significant effects of chronic variable stress during adolescence (either day 35-48 or during day 50-64) in male rats on a one-day forced swim test paradigm. Our results did not indicate any significant effects of social isolation from day 30-50 of age on the forced swim test in adolescent male rats. With adolescent females, isolation increased time spent climbing on the second day as it also did in adult females. We also observed that on the fist day of the FST in adulthood, females already exhibited increased climbing. Variations in results across studies are likely due to the different types of stressors administered as well as methodological differences in the forced swim test. Another factor that contributes to the difficulty in comparing results across studies is the ages at which stressors are delivered. It seems increasingly likely that there are specific periods of sensitivity within adolescence during which stress can impact certain behaviors and physiological functions [32, 33]. As a result, the effects of stress during these specific periods may produce different effects than stress experienced throughout most of adolescence. The sum of the results with the forced swim test in the present study indicates that female rats isolated during adolescents display more climbing overall during adolescence and in adulthood, evidence of an active behavioral phenotype.
We then examined another test of relevance to affective behaviors, the sucrose preference test. Females isolated during adolescence exhibited increased water consumption during baseline habituation to the two water bags, increased preference for sucrose during the first hour of the 2hour test and a tendency for an overall increase in preference compared to control females. Our finding of increased sucrose consumption in isolated females is consistent with some previous studies that indicate that sucrose intake is increased after juvenile isolation in males [34]. Typically, changes in consumption in either direction are thought to reflect responsiveness to reward. Suppressed intake, as observed in the chronic mild stress protocol, is thought to indicate decreased responsiveness to a normally rewarding substance, sucrose (anhedonia; [35]). Anhedonia is a major symptom of depression and anti-depressant drugs increase consumption of sucrose [29]. The increased sucrose intake could reflect less pleasure derived from the normally pleasurable and palatable sucrose and could reflect increased reward-seeking [36]. This reward-seeking could be a way to reduce a dysphoric state as proposed by Panksepp and colleagues in Colonello et al., [34]. These interpretations suggest that the females isolated in adolescence preferred sucrose more than controls because they find it more rewarding or are seeking it to reduce a dysphoric state. Further studies, particularly of the neurobiological systems mediating reward, will be necessary to fully interpret the increased sucrose consumption in isolated females. It should be noted that all rats were exposed to repeated restraint to enhance responsivity in the sucrose preference test and the increased consumption in adult females isolated as adolescents is consistent with our previous findings that adult females isolated as adolescents exhibit increased reactivity to acute and to repeated restraint [15] All rats were also singly housed for the sucrose preference test. Single housing is necessary in order to measure the consumption of individual rats. Despite these similar conditions, social isolation produced a clear effect on sucrose consumption only in female rats. Thus, it is unlikely that the effects observed in this test were due to single housing or due to repeated restraint only although the possibility remains that the results were influenced by single housing interacting with the adolescent isolation to influence the isolated females only.
To our knowledge, the present study is the first to demonstrate that social isolation during adolescence produces an enduring and sex-specific impact on behavior in the forced swim and sucrose preference tests. Collectively, the results of the forced swim and the sucrose preference tests show that both adolescent and adult female rats exhibit increased climbing and adult females exhibited increased preference for sucrose after adolescent isolation. This result could be interpreted as a resistance to depressive behavior. An additional possibility is that isolated females are attentive to novelty or more exploratory. This is also supported by the finding that isolated females consumed more water when water was placed in two bags during the water baseline phase. In earlier work, we observed that adult females that had been isolated as adolescents exhibited increased reactivity to both acute and repeated stress in adulthood [15]. Other studies have indicated that isolation-rearing in adolescence tend to be more hyperactive and aggressive and to exhibit more perseverative behavior [25, 37, 38, 39]. The results from the present study extend these to demonstrate that the effects of isolation that is restricted to adolescence produces a sex specific increase in behaviors that reflect increased activity and exploration of novel environmental stimuli.
Previous studies indicate that social interactions in adolescence are important for appropriate affiliative and antagonistic behaviors in adulthood [8]. A recent study showed that social defeat in adolescent male rats promotes proactive behavior as assessed in the defensive burying test in adulthood [33]. The present results add to this growing literature by demonstrating that preventing social interactions throughout adolescence promotes a more behaviorally active phenotype in adulthood specifically in females. A more active, exploratory phenotype in adulthood may be adaptive if the adolescent isolation predicts an adult environment in which little social interactions take place. However, it may be maladaptive in an environment in which social engagement is necessary or in a changing social environment. It is possible that a similar phenotype is produced by adolescent isolation in adult males but that these phenotypes are expressed in other ways, such as aggression with conspecifics that were not assessed in the present study. Alternatively, it is possible that adolescent isolation does not have an impact on these behaviors in male rats. Indeed, our previous study indicated an anxiolytic phenotype in adult males after adolescent isolation accompanied by decreased orexin mRNA suggesting a less aroused animal [15]. Future studies are needed to address these questions. Additional work is also necessary to determine whether this sex-specific impact of social isolation in adolescence is also produced by other types of social stressors such as defeat which involves aggressive interactions. Finally, the increased climbing exhibited by adult females exposed to adolescent isolation observed in the forced swim test implicates the noradrenergic system [18] and suggest that this system may be specifically impacted by adolescent stress. Together with our previous work, the results suggest that adult behavioral phenotypes are moulded by the social environment during adolescence in a sex-specific way. These results have relevance for understanding how stressors experienced during adolescence contribute to an individual’s susceptibility to stress-related disorders throughout their life through changes in behavioral phenotypes.
Acknowledgments
This work was supported by MH090420 to SB. We thank Dr. Willem Heydendael for helpful discussions throughout these studies.
References
- 1.Hazen E, Schlozman S, Beresin E. Adolescent psychological development: a review. Pediatr Rev. 2008;29(5):161–7. doi: 10.1542/pir.29-5-161. [DOI] [PubMed] [Google Scholar]
- 2.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 3.Lenroot R, Geidd J. Brian Development in Children and Adolescents: Insights from Anatomical Magnetic Resonance Imaging. Neuroscience & Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Hankin BL, Mermelstein R, Roesch L. Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Development. 2007;78(1):279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 5.Garber J. Depression in Children and Adolescents Linking Risk Research and Prevention. American Journal of Preventive Medicine. 2006;31(6):104–125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Miller A. Social Neuroscience of Child and Adolescent Depression. Brain and Cognition. 2007;65:47–68. doi: 10.1016/j.bandc.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR. Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice. PLoS ONE. 2007;2(4):e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homberg JR, S OJ, Schoffelmeer AN, Cuppen E, Vanderschuren LJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195(2):175–82. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes, Brain and Behavior. 2007;6(7):661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Hol T, Van den Berg V, Van Ree JM, Sprijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behavioural Brain Research. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 12.Toth E, Avital A, Leshem M, Richter-Levin G, Braun K. Neonatal and juvenile stress induces changes in adult social behavior without affecting cognitive function. Behavior Brain Research. 2008;190:135–139. doi: 10.1016/j.bbr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Vidal J, de Bie J, Granneman A, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiology & Behavior. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 16.Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated Exposure to Stress Across the Childhood-Adolescent Period Alters Rats’ Anxiety- and Depression-Like Behaviors in Adulthood: The Importance of Stressor Type and Gender. Behavioral Neuroscience. 2007;121(3):462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- 17.McCormick C, Mathews I. HPA functions in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry and Behavior. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4-5):547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Bechtholt A, Smith K, Gaughan S, Lucki I. Sucrose intake and fasting glucose levels in 5-HT1A and 5-HT1B receptor mutant mice. Physiology & Behavior. 2007;93:659–65. doi: 10.1016/j.physbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 21.Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5(2):107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 22.Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10(2):123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- 24.Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Dev Psychobiol. 1978;11:213–225. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa H. The effects of isolation rearing on open-field behavior in male rats depends on developmental stages. Dev Psychobiol. 2003;43:11–19. doi: 10.1002/dev.10120. [DOI] [PubMed] [Google Scholar]
- 26.Arakawa H. Ontogenetic interaction between social relationships and defensive burying behavior in the rat. Physiol Behav. 2006;90:751–759. doi: 10.1016/j.physbeh.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 29.Muscat R, Papp M, Willner P. Reversal of stress induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 1992;109(4):433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- 30.Hefner K, Holmes AH. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behavioural Brain Research. 2007;176(2):210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 32.Jankford R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 2011;36(4):896–909. doi: 10.1038/npp.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colonnello V, Iacobucci P, Anderson MP, Panksepp J. Brief periods of positive peer interactions mitigate the effects of total social isolation in young Octodon degus. Dev Psychobiol. 2011 Apr;53(3):280–90. doi: 10.1002/dev.20520. Epub 2010 Dec 22. [DOI] [PubMed] [Google Scholar]
- 35.Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neuroscience and Biobehavioral Reviews. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- 36.Mourlon V, Baudin A, Blanc O, Lauber A, Giros B, Naudon L, Dauge V. Maternal deprivation induces depressive-like behaviours only in female rats. Behav Brain Research. 2010 Dec 1;213(2):278–87. doi: 10.1016/j.bbr.2010.05.017. Epub 2010 May 19. [DOI] [PubMed] [Google Scholar]
- 37.Einon DF, Humphreys AP, Chivers SM, Field S, Naylor V. Isolation has permanent effects upon the behavior of the rat, but not the mouse, gerbil, or guinea pig. Dev Psychobiol. 1981;14(4):343–355. doi: 10.1002/dev.420140407. [DOI] [PubMed] [Google Scholar]
- 38.Morgan MJ, Einon DF, Nicholas D. The effects of isolation rearing on behavioral inhibition in the rat. Quarterly Journal of Experimental Psychology. 1975;27:615–634. [Google Scholar]
- 39.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Research. 2008;188:298–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


