Abstract
Background: Flavonoids are plant-based phytochemicals with cardiovascular protective properties. Few studies have comprehensively examined flavonoid classes in relation to cardiovascular disease mortality.
Objective: We examined the association between flavonoid intake and cardiovascular disease (CVD) mortality among participants in a large, prospective US cohort.
Design: In 1999, a total of 38,180 men and 60,289 women in the Cancer Prevention Study II Nutrition Cohort with a mean age of 70 and 69 y, respectively, completed questionnaires on medical history and lifestyle behaviors, including a 152-item food-frequency questionnaire. Cox proportional hazards modeling was used to calculate multivariate-adjusted hazard RRs and 95% CIs for associations between total flavonoids, 7 flavonoid classes, and CVD mortality.
Results: During 7 y of follow-up, 1589 CVD deaths in men and 1182 CVD deaths in women occurred. Men and women with total flavonoid intakes in the top (compared with the bottom) quintile had a lower risk of fatal CVD (RR: 0.82; 95% CI: 0.73, 0.92; P-trend = 0.01). Five flavonoid classes—anthocyanidins, flavan-3-ols, flavones, flavonols, and proanthocyanidins—were individually associated with lower risk of fatal CVD (all P-trend < 0.05). In men, total flavonoid intakes were more strongly associated with stroke mortality (RR: 0.63; 95% CI: 0.44, 0.89; P-trend = 0.04) than with ischemic heart disease (RR: 0.90; 95% CI: 0.72, 1.13). Many associations appeared to be nonlinear, with lower risk at intakes above the referent category.
Conclusions: Flavonoid consumption was associated with lower risk of death from CVD. Most inverse associations appeared with intermediate intakes, suggesting that even relatively small amounts of flavonoid-rich foods may be beneficial.
See corresponding editorial on page 271.
INTRODUCTION
CVD5 remains the leading cause of death for adults over the age of 65 y, despite declining rates over the past 2 decades (1). Medical and pharmacologic interventions can reduce cardiovascular mortality among individuals at risk, but they are costly and sometimes have adverse side effects (2, 3). Consumption of plant foods is also associated with lower risk of CVD (4) and hypertension (5, 6). These associations are ascribed, at least in part, to their fiber, potassium, and magnesium content. However, other constituents, such as flavonoids, may be responsible for some of the protective associations that link plant foods to CVD prevention. Flavonoids, found primarily in fruit, vegetables, nuts, cocoa, and beverages such as tea and wine, are bioactive polyphenolic, noncaloric, nonnutrient secondary metabolites in plants that cannot be synthesized by humans. Many have potent antioxidant or antiinflammatory activity, reduce LDL cholesterol oxidation, and regulate endothelial nitric oxide synthesis; some also have weak estrogenic activity (7).
Asian populations consume large amounts of soy foods that contain relatively high amounts of isoflavonoids, a class of flavonoids associated with lower CVD risk in ecologic (8) and prospective cohort (9) studies, particularly in women. In Western populations, however, isoflavonoid consumption is relatively low, and other classes of flavonoids are eaten in much larger quantities. Most prospective epidemiologic studies of flavonoids and CVD risk have examined only 1 or 2 classes of flavonoid compounds (10, 11). They provide some support for flavonol, flavone, and flavan-3-ol (catechin) consumption in lowering CVD risk, although the results have been mixed (10, 12). Many studies included <200 cases (10, 12). Comprehensive epidemiologic investigation of other classes of flavonoids, including anthocyanidins, flavanones, isoflavones, and proanthocyanidins, in relation to CVD risk in Western populations has been limited (13–15). In the past decade, new flavonoid composition databases (16–18) have enabled more in-depth evaluation of the role of these dietary constituents in chronic disease prevention.
Given the limited evidence relating flavonoid intake to CVD risk in Western populations, we examined the relation between total flavonoids and 7 flavonoid classes and fatal CVD, IHD, and stroke in the American Cancer Society's CPS-II Nutrition Cohort, a large US prospective study in adult men and women.
SUBJECTS AND METHODS
Study population
Participants in this analysis were drawn from the 86,404 men and 97,786 women enrolled in the CPS II Nutrition Cohort, a subset of the 1.2 million US adults in the larger CPS-II study established in 1982 who reside in 21 states (19). In 1992–1993, CPS-II Nutrition Cohort participants completed a mailed 10-page confidential, self-administered questionnaire that included information on demographic, medical, lifestyle, anthropometric, and dietary factors, with follow-up questionnaires sent out to cohort participants in 1997 and biennially thereafter to update outcome and exposure information. The response rate for each of the follow-up questionnaires was ≥89%. The Emory University School of Medicine Institutional Review Board (Atlanta, GA) approved all aspects of the CPS-II Nutrition Cohort study.
Dietary assessment
At baseline (1992–1993) participants in the cohort completed a semiquantitative 68-item modified Block FFQ (19). Dietary information was updated in 1999 by using a modified, semiquantitative 152-item Willett FFQ, which was shown to be valid and reliable in similar US populations (20, 21). For this analysis, we set the baseline at 1999 because flavonoid-containing foods were more broadly represented on the 152-item FFQ. For each item on the FFQ, a common food or beverage serving size was specified (eg, 0.5 cup blueberries or 1 cup soy milk), and participants were asked how often, on average, they had consumed this amount over the previous year. The 9 possible frequency responses ranged from “never or less than once per month” to “6 or more times per day,” depending on the item.
Flavonoid values were derived from 3 USDA databases on flavonoids (16), proanthocyanidins (17), and isoflavones (18) and other research publications (22–24), as described previously (25). For mixed dishes (eg, pizza, coleslaw), we assigned weighted content values based on recipes from standard cookbooks. When data for cooked foods were unavailable, raw data were used. Data for some items on the FFQ were not available for all flavonoid classes (eg, anthocyanidins for strawberry jam). No data were available for cereals or bread and starch items because flavonoids have been removed in processing. Analytic data were also unavailable for brown rice, oat bran, other bran, wheat germ, liquor, popcorn, and olive oil, but most of these items were infrequently consumed in this cohort. To obtain the daily estimated flavonoid nutrient intake values, we multiplied the reported frequency of standard portion sizes by the flavonoid compound content of each food (per 100 g) and summed flavonoid intakes across all foods and beverages. All flavonoid subgroups that were estimated, their respective compounds, and some major food sources on our questionnaire are listed in Table 1.
TABLE 1.
Flavonoid classes, compounds, and sources
| Respective compounds | Common plant sources | |
| Flavonoid class | ||
| Anthocyanidins | Cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin | Blueberries, red wine, strawberries |
| Flavan-3-ols | Epicatechin, epicatechin 3-gallate, epigallocatechin, epigallocatechin gallate, catechin, gallocatechin | Apples, black tea, blueberries, chocolate, red wine |
| Flavanones | Eriodictyol, hesperetin, naringenin | Citrus fruit and juices, herbal tea |
| Flavones | Apigenin, luteolin | Celery, garlic, green peppers, herbal tea |
| Flavonols | Isorhamnetin, kaempferol, myricetin, quercetin | Blueberries, garlic, kale, onions, spinach, tea, broccoli, red wine, cherry tomatoes |
| Proanthocyanidins | Monomers, dimers, trimers, 4–6mers, 7–10mers, polymers | Apples, black tea, blueberries, chocolate, mixed nuts, peanuts, red wine, strawberries, walnuts |
| Isoflavones | Daidzein, genistein, glycitein, coumestrol, formononetin, biochanin A | Soy products, peanuts |
| Total flavonoids | ||
| >50 mg/100 g | Apples, black tea, blueberries, chocolate, garlic powder, grapefruits, grapes, herbal tea, mixed nuts, oranges, pears, red wine, strawberries, walnuts | |
| >50 mg/serving1 | Apples, black tea, blueberries, chocolate, grapefruit juice, grapefruit, grapes, herbal tea, oranges, pears, red wine, strawberries | |
Serving sizes that are predefined on the food-frequency questionnaire are available from www.cancer.org and are generally similar to those of the USDA Nutrient Database for Common Reference (http://www.ars.usda.gov).
Analytic cohort
We excluded from this analysis participants who reported a history of myocardial infarction (n = 9379), angina pectoris (n = 4491), coronary bypass or angioplasty (n = 3858), stroke (n = 2040), transient ischemic attack (n = 2259), carotid surgery (n = 427), or diabetes (n = 7653) at baseline in 1999 because these individuals are at greater risk of CVD, IHD, or stroke mortality and might have changed their diets in response to diagnosis. We also excluded an individual who died before the date of baseline survey return (eg, sent in by proxy; n = 1). Because few women were premenopausal (and thus could not be evaluated separately), we excluded women who were pre- or perimenopausal at baseline or with unknown menopausal status (n = 192). We also excluded men and women who in 1999 reported extreme energy intakes (<800 or >4200 kcal/d for men and <600 or >3500 kcal/d for women) or excessive missing values (>70 blanks) (n = 22,033). We also excluded the top 0.1% of reported individual flavonoid class intakes to remove possible erroneous values (n = 547). A total of 98,469 participants (38,180 men and 60,289 women) remained for analysis. A total of 2771 deaths due to CVD occurred during follow-up (1589 men and 1182 women), with 1286 deaths due to IHD (803 men and 483 women) and 573 deaths due to stroke (281 men and 292 women).
Outcome ascertainment
The vital status of study participants was determined through 31 December 2006, and deaths were ascertained through automated linkage with the National Death Index (26). The underlying causes of death were coded according to the ICD-9 and ICD-10 (27, 28). Total CVD deaths were defined as ICD-9 codes 390–459 and ICD-10 codes I20–I99, CHD deaths as ICD-9 codes 410–414 (ischemic heart disease) and 492.2 (atherosclerotic heart disease) and ICD-10 codes I20–I25, and stroke as ICD-9 codes 430–438 (ischemic and hemorrhagic cerebrovascular disease) and ICD-10 codes I60–-I69 (cerebrovascular diseases). The follow-up time was calculated from the date of return of the baseline questionnaire in 1999 to the date of death, loss to follow-up, or 31 December 2006, whichever came first.
Statistical analysis
We used Cox proportional hazards modeling to calculate the hazard rate ratios and 95% CIs for CVD mortality associated with quintile categories of total flavonoids, anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, isoflavones, and proanthocyanidins, each adjusted for energy by using the residual method (29). IHD and stroke mortality were also examined as separate endpoints. We grouped flavonoids into quartile categories when examining stroke endpoints because of smaller numbers.
Age adjustment was accomplished by stratifying on single year of age within each Cox model. Covariates included history of hypertension (yes or no), history of high cholesterol (yes or no), family history of myocardial infarction (yes or no), BMI [weight (in kg)/height (in m2); <22.5, 22.5 to <25.0, 25.0 to <30.0, 30 to <35.0, ≥35.0, or missing data], recreational physical activity in quartiles (metabolic equivalent-hours/wk), smoking status (never, current, former, unknown, or invalid smoking status), quintiles of total energy intake (kcal/d), and aspirin use (pills/mo: none, 1–14, 15–29, 30–59, or ≥60) for both men and women. The multivariate models for women (all were postmenopausal) also included HRT, which included both estrogen-only and combined replacement therapies (never, current, past, or other HRT/unknown/missing type and unknown use). To control for alcohol, we included terms for beer intake (drinks/d: never, >0–<0.14, ≥0.14) and liquor intake (drinks/d: never, >0 to <1.0, ≥1.0), but not wine to avoid overcontrolling for sources of certain flavonoids. However, when we controlled for total ethanol (g) instead, results were not materially different. History of hypertension, history of high cholesterol, aspirin use, and HRT were modeled as time-varying covariates by using information obtained in 1999, 2001, and 2003. Other variables considered for the analysis but not included in the final model because they had negligible effects on our results included education, race, multivitamin use, and vitamin C and E use.
To test for linear trend, we used the median value for each quintile category and modeled this as a continuous variable. We also examined the possibly nonlinear relation between intake of individual flavonoid classes and total flavonoids (modeled as continuous variables) and death from CVD, IHD, or stroke nonparametrically with restricted cubic splines (30). Tests for nonlinearity used the likelihood ratio test to compare the model with only the linear term to the model that includes both the linear and the cubic spline terms.
We tested for effect modification by smoking status (ever or never smoker), multivitamin use [never use, occasional use (<6 pills/wk), regular use (≥6 pills/wk)], physical activity (bottom 2 compared with top 2 quartiles of metabolic equivalent-hours/wk), and age (>70 and ≤70 y) and sex. Interaction terms were created between follow-up time and quintiles of total and individual flavonoid subclasses to test for violations of the Cox proportional hazards assumption. Statistical interaction and the Cox proportional hazards assumption were assessed by using the likelihood ratio test (31). Results from 2-sided chi-square tests were considered significant at P ≤ 0.05. All analyses were conducted by using SAS version 9.2 (SAS Institute).
RESULTS
Energy-adjusted mean and median total flavonoid intakes for men were 268 and 203 mg/d, respectively (10th–90th percentile distribution: 99–498 mg/d), and for women were 268 and 201 mg/d, respectively (10th–90th percentile distribution: 92–522 mg/d); quintile cutoffs were therefore defined from intake distributions in men and women combined. Pearson correlations between flavonoids ranged from weak (r = 0.03 for isoflavones and flavanones) to high (r = 0.99 for proanthocyanidins and total flavonoids). Baseline characteristics according to quintile categories of total flavonoid consumption in 1999 are presented in Table 2. Individuals of both sexes with higher total flavonoid intakes were more educated, less likely to have a history of hypertension but more likely to have a history of high cholesterol, exercised more, had a slightly lower BMI, and were less likely to smoke. As expected, participants with higher total flavonoid intakes consumed more fruit and vegetables as well as less trans and saturated fats. They were also more likely to be regular users of vitamin supplements.
TABLE 2.
Baseline characteristics by quintile of total flavonoid intake of men and women in the CPS II Nutrition Cohort, 1999–20061
| Quintiles of total flavonoid intake2 |
|||||||||
| Men (n = 38,180) |
Women (n = 60,289) |
Men and women (n = 98,469) |
|||||||
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| Sociodemographic characteristics | |||||||||
| n (per quintile) | 6926 | 8074 | 7434 | 12,767 | 11,620 | 12,260 | 19,693 | 19,694 | 19,694 |
| Mean age (y) | 69.6 ± 5.83 | 70.2 ± 5.8 | 70.5 ± 5.9 | 68.1 ± 6.2 | 68.9 ± 6.2 | 68.5 ± 6.3 | 68.6 ± 6.1 | 69.4 ± 6.0 | 69.3 ± 6.2 |
| BMI in 1999 (%) | |||||||||
| <22.5 kg/m2 | 11.1 | 10.9 | 13.7 | 23.5 | 26.2 | 29.7 | 19.1 | 19.9 | 23.7 |
| 22.5 to <25.0 kg/m2 | 20.4 | 23.6 | 24.8 | 21.6 | 24.4 | 24.6 | 21.2 | 24.0 | 24.7 |
| 25.0 to <30.0 kg/m2 | 44.9 | 44.9 | 43.2 | 32.6 | 30.9 | 29.6 | 37.0 | 36.7 | 34.7 |
| 30 to <35.0 kg/m2 | 13.4 | 11.6 | 10.1 | 12.6 | 10.7 | 9.0 | 12.9 | 11.1 | 9.4 |
| ≥35.0 kg/m2 | 2.9 | 2.2 | 2.0 | 5.0 | 3.7 | 3.0 | 4.3 | 3.1 | 2.6 |
| Race (%) | |||||||||
| White | 98.3 | 98.0 | 97.5 | 98.1 | 97.6 | 97.5 | 98.1 | 97.8 | 97.5 |
| African American | 0.7 | 0.9 | 0.9 | 0.9 | 1.4 | 1.0 | 0.8 | 1.2 | 0.9 |
| Other | 0.7 | 0.8 | 1.3 | 0.8 | 0.8 | 1.3 | 0.8 | 0.8 | 1.3 |
| College graduate (%) | 43.7 | 53.3 | 57.8 | 28.2 | 36.2 | 36.2 | 33.7 | 43.2 | 44.3 |
| Family history of MI (%) | 39.5 | 40.4 | 41.2 | 43.5 | 43.5 | 44.0 | 42.1 | 42.2 | 42.9 |
| History of hypertension in 1999 (%) | 32.1 | 32.0 | 30.0 | 33.9 | 30.9 | 30.2 | 33.3 | 31.3 | 30.1 |
| History of high cholesterol in 1999 (%) | 25.6 | 29.0 | 29.2 | 34.4 | 35.5 | 36.1 | 31.3 | 32.9 | 33.4 |
| Smoking history (%) | |||||||||
| Never smoker | 28.0 | 35.4 | 37.7 | 49.8 | 56.0 | 56.9 | 42.1 | 47.6 | 49.6 |
| Former smoker | 61.8 | 59.8 | 58.4 | 40.7 | 39.6 | 39.0 | 48.2 | 47.8 | 46.3 |
| Current smoker | 8.6 | 3.2 | 2.5 | 8.4 | 3.4 | 3.1 | 8.5 | 3.3 | 2.9 |
| Exercise quartiles (%)4 | |||||||||
| <5.5 MET-h/wk | 29.6 | 20.2 | 19.4 | 33.7 | 23.9 | 23.7 | 32.3 | 22.4 | 22.1 |
| 5.5 to <13.4 MET-h/wk | 21.3 | 22.6 | 22.1 | 25.2 | 25.9 | 25.9 | 23.8 | 24.6 | 24.4 |
| 13.4 to <24.5 MET-h/wk | 21.7 | 24.1 | 25.8 | 21.7 | 25.4 | 25.1 | 21.7 | 24.9 | 25.4 |
| ≥24.5 MET-h/wk | 26.0 | 31.9 | 31.5 | 16.8 | 22.3 | 23.1 | 20.0 | 26.2 | 26.3 |
| HRT in 1999 (%) | |||||||||
| Never | — | — | — | 43.6 | 41.3 | 41.2 | 28.2 | 24.4 | 25.7 |
| Current | — | — | — | 29.6 | 32.2 | 32.0 | 19.1 | 19.0 | 20.0 |
| Past | — | — | — | 19.0 | 18.7 | 18.8 | 12.3 | 11.1 | 11.6 |
| Aspirin use in 1999 (%) | |||||||||
| None | 52.6 | 48.7 | 49.7 | 57.4 | 57.0 | 58.2 | 55.7 | 53.6 | 55.0 |
| 1–14 pills/mo | 10.8 | 11.3 | 10.5 | 12.2 | 12.7 | 12.1 | 11.7 | 12.1 | 11.5 |
| 15–29 pills/mo | 7.0 | 8.6 | 7.8 | 6.4 | 6.3 | 6.0 | 6.6 | 7.3 | 6.7 |
| 30–59 pills/mo | 26.1 | 28.5 | 29.1 | 20.3 | 21.3 | 20.8 | 22.3 | 24.2 | 23.9 |
| ≥60 pills/mo | 3.5 | 2.9 | 2.9 | 3.6 | 2.7 | 2.8 | 3.6 | 2.8 | 2.8 |
| Dietary variables | |||||||||
| Alcohol intake (%) | |||||||||
| Nondrinker | 38.2 | 30.2 | 33.1 | 50.8 | 46.0 | 49.3 | 46.3 | 39.6 | 43.2 |
| <1 drink/d | 29.9 | 35.5 | 34.4 | 31.2 | 37.9 | 36.0 | 30.7 | 36.9 | 35.5 |
| 1 drink/d | 12.6 | 18.7 | 17.3 | 8.9 | 11.0 | 9.2 | 10.2 | 14.1 | 12.2 |
| >1 drink/d | 18.2 | 14.9 | 15.0 | 8.4 | 4.8 | 5.3 | 11.8 | 9.0 | 8.9 |
| Multivitamin use (%) | |||||||||
| Never | 51.7 | 44.2 | 41.0 | 40.5 | 34.7 | 32.6 | 44.5 | 38.6 | 35.8 |
| Occasional | 6.2 | 6.6 | 6.6 | 8.0 | 8.0 | 7.9 | 7.4 | 7.4 | 7.4 |
| Regular | 34.5 | 42.1 | 45.2 | 41.6 | 47.3 | 49.8 | 39.1 | 45.1 | 48.1 |
| Vitamin C use (%) | |||||||||
| Never | 66.7 | 59.1 | 53.3 | 58.6 | 50.7 | 46.9 | 61.5 | 54.2 | 49.4 |
| Occasional | 15.3 | 19.9 | 22.6 | 17.3 | 21.7 | 22.2 | 16.6 | 20.9 | 22.4 |
| Regular | 8.1 | 10.5 | 13.4 | 9.6 | 12.1 | 14.8 | 9.1 | 11.4 | 14.3 |
| Vitamin E use (%) | |||||||||
| Never | 59.2 | 50.3 | 44.7 | 48.3 | 39.8 | 36.3 | 52.1 | 44.1 | 39.5 |
| Occasional | 4.9 | 6.0 | 6.4 | 5.3 | 6.6 | 6.6 | 5.2 | 6.4 | 6.6 |
| Regular | 26.9 | 35.0 | 39.9 | 34.2 | 40.6 | 44.0 | 31.6 | 38.3 | 42.5 |
| Energy intake in 1999 (kcal) | 1883 ± 7.0 | 1970 ± 6.4 | 1868 ± 6.7 | 1586 ± 4.4 | 1658 ± 4.6 | 1560 ± 4.4 | 1691 ± 3.9 | 1786 ± 3.9 | 1676 ± 3.9 |
| Fruit intake (servings/wk) | 10.2 ± 0.1 | 18.5 ± 0.1 | 20.9 ± 0.1 | 11.8 ± 0.08 | 19.5 ± 0.08 | 19.9 ± 0.08 | 11.2 ± 0.06 | 19.1 ± 0.06 | 20.3 ± 0.06 |
| Vegetable intake (servings/wk) | 16.0 ± 0.1 | 21.3 ± 0.1 | 23.0 ± 0.1 | 18.9 ± 0.1 | 24.1 ± 0.1 | 24.5 ± 0.1 | 17.8 ± 0.09 | 23.0 ± 0.09 | 23.9 ± 0.09 |
| Saturated fat intake (g/d)5 | 22.8 ± 0.06 | 19.9 ± 0.06 | 18.5 ± 0.06 | 19.4 ± 0.04 | 17.0 ± 0.04 | 16.2 ± 0.04 | 20.6 ± 0.04 | 18.2 ± 0.04 | 17.1 ± 0.04 |
| Total trans fat intake (g/d)5 | 4.3 ± 0.02 | 3.6 ± 0.01 | 3.3 ± 0.01 | 3.3 ± 0.009 | 2.8 ± 0.009 | 2.7 ± 0.009 | 3.7 ± 0.008 | 3.2 ± 0.008 | 2.9 ±0.008 |
| n–3 Fatty acid intake (g/d)5 | 0.1 ± 0.002 | 0.2 ± 0.001 | 0.2 ± 0.002 | 0.1 ± 0.001 | 0.2 ± 0.001 | 0.2 ± 0.001 | 0.1 ± 0.001 | 0.2 ± 0.001 | 0.2 ± 0.001 |
All variables except for age were standardized to the age distribution of the entire cohort. CPS II, Cancer Prevention Study II; HRT, hormone replacement therapy; MET-h, metabolic equivalent hours; MI, myocardial infarction.
Quintiles of total flavonoid intake (same for men, women, men and women combined): 1, <121.5 mg/d; 3, 172.4 to <238.1 mg/d; 5, ≥359.7 mg/d.
Mean ± SD (all such values).
Metabolic equivalents were defined for each type of exercise-related physical activity as a multiple of the metabolic equivalent of sitting quietly for 1 h.
Energy-adjusted.
The associations between total flavonoids, flavonoid classes, and CVD mortality are shown in men, women, and both combined in Table 3. Men and women with total flavonoid intakes in the highest (compared with lowest) quintile had an 18% lower risk of fatal CVD (RR: 0.82; 95% CI: 0.73, 0.92; P-trend = 0.01). Inverse associations were observed for anthocyanidins, flavan-3-ols, flavones, flavonols, and proanthocyanidins (P-trend < 0.05 for all). We observed no significant heterogeneity in results by sex. In results examined separately in men and women, age-adjusted associations for most flavonoid classes were significantly inversely related to CVD risk. Relations were attenuated after control for confounders, more so in men, although men in the top (compared with bottom) quintile of total flavonoids remained at lower risk of fatal CVD (RR: 0.83; 95% CI: 0.71, 0.98; P-trend = NS). Cigarette smoking and physical activity were the strongest confounders in both men and women. In women, flavone consumption was associated with the greatest reduction in CVD risk (RR: 0.74; 95% CI: 0.61, 0.90 for quintile 5 compared with quintile 1; P-trend = 0.001). Because isoflavone intake in the cohort was low, we also examined the association with isoflavones comparing quintile 5 to a reference of quintiles 1–4 combined, and results were similarly null (data not shown).
TABLE 3.
RRs and 95% CIs for cardiovascular disease mortality by quintile of energy-adjusted flavonoid intake in men and women in the CPS II Nutrition Cohort, 1999–20061
| Men (n = 38,180) |
Women (n = 60,289) |
Men and women (n = 98,469)2 |
||||||||
| Quintile | Median intake (range) | Deaths | Age-adjusted RR3 | Multivariate-adjusted RR (95% CI)4 | Deaths | Age-adjusted RR3 | Multivariate-adjusted RR (95% CI)4 | Deaths | Age-adjusted RR3 | Multivariate-adjusted RR (95% CI)4 |
| mg/d | no. | no. | no. | |||||||
| Total flavonoids | ||||||||||
| 1 | 94.5 (<121.5) | 322 | 1.00 (—) | 1.00 (—) | 293 | 1.00 (—) | 1.00 (—) | 615 | 1.00 (—) | 1.00 (—) |
| 2 | 146.5 (121.5–172.3) | 305 | 0.76 (0.65, 0.89) | 0.84 (0.72, 0.98) | 233 | 0.79 (0.67, 0.94) | 0.86 (0.72, 1.02) | 538 | 0.77 (0.69, 0.87) | 0.84 (0.75, 0.95) |
| 3 | 201.9 (172.4–238.0) | 330 | 0.79 (0.68, 0.92) | 0.91 (0.78, 1.06) | 222 | 0.73 (0.62, 0.88) | 0.82 (0.69, 0.98) | 552 | 0.77 (0.68, 0.86) | 0.87 (0.77, 0.98) |
| 4 | 286.0 (238.1–359.6) | 343 | 0.80 (0.69, 0.93) | 0.95 (0.81, 1.10) | 208 | 0.67 (0.56, 0.80) | 0.76 (0.63, 0.91) | 551 | 0.74 (0.66, 0.83) | 0.86 (0.76, 0.96) |
| 5 | 512.5 (≥359.7) | 289 | 0.72 (0.61, 0.84) | 0.83 (0.71, 0.98) | 226 | 0.72 (0.61, 0.86) | 0.81 (0.68, 0.97) | 515 | 0.72 (0.64, 0.80) | 0.82 (0.73, 0.92) |
| P-trend5 | 0.003 | 0.2 | 0.002 | 0.05 | <0.0001 | 0.01 | ||||
| Anthocyanidin | ||||||||||
| 1 | 3.8 (<5.5) | 323 | 1.00 (—) | 1.00 (—) | 313 | 1.00 (—) | 1.00 (—) | 636 | 1.00 (—) | 1.00 (—) |
| 2 | 6.8 (5.5–8.1) | 284 | 0.83 (0.70, 0.97) | 0.92 (0.79, 1.09) | 244 | 0.74 (0.62, 0.87) | 0.81 (0.68, 0.95) | 528 | 0.78 (0.70, 0.88) | 0.87 (0.77, 0.98) |
| 3 | 9.8 (8.2–11.4) | 318 | 0.82 (0.71, 0.96) | 0.94 (0.81, 1.11) | 230 | 0.71 (0.60, 0.84) | 0.80 (0.68, 0.96) | 548 | 0.77 (0.68, 0.86) | 0.87 (0.78, 0.98) |
| 4 | 13.7 (11.5–16.6) | 339 | 0.78 (0.67, 0.91) | 0.95 (0.82, 1.11) | 199 | 0.63 (0.53, 0.76) | 0.74 (0.62, 0.88) | 538 | 0.72 (0.64, 0.80) | 0.86 (0.76, 0.96) |
| 5 | 22.2 (≥16.7) | 325 | 0.72 (0.62, 0.84) | 0.91 (0.77, 1.06) | 196 | 0.70 (0.58, 0.84) | 0.82 (0.69, 0.99) | 521 | 0.70 (0.62, 0.79) | 0.86 (0.76, 0.97) |
| P-trend5 | 0.0002 | 0.3 | 0.0002 | 0.06 | <0.0001 | 0.04 | ||||
| Flavan-3-ols | ||||||||||
| 1 | 7.0 (<9.5) | 305 | 1.00 (—) | 1.00 (—) | 316 | 1.00 (—) | 1.00 (—) | 621 | 1.00 (—) | 1.00 (—) |
| 2 | 11.8 (9.5–14.0) | 295 | 0.84 (0.71, 0.98) | 0.92 (0.78, 1.08) | 239 | 0.76 (0.64, 0.90) | 0.83 (0.70, 0.99) | 534 | 0.79 (0.71, 0.89) | 0.88 (0.78, 0.98) |
| 3 | 16.8 (14.1–20.3) | 333 | 0.81 (0.69, 0.94) | 0.95 (0.81, 1.11) | 215 | 0.73 (0.61, 0.87) | 0.82 (0.69, 0.98) | 548 | 0.77 (0.69, 0.86) | 0.88 (0.79, 0.99) |
| 4 | 26.3 (20.4–37.1) | 354 | 0.81 (0.69, 0.94) | 0.98 (0.84, 1.15) | 194 | 0.70 (0.58, 0.84) | 0.79 (0.66, 0.95) | 548 | 0.76 (0.68, 0.85) | 0.89 (0.79, 1.01) |
| 5 | 63.7 (≥37.2) | 302 | 0.74 (0.63, 0.87) | 0.87 (0.74, 1.02) | 218 | 0.70 (0.59, 0.84) | 0.79 (0.66, 0.94) | 520 | 0.72 (0.64, 0.81) | 0.83 (0.74, 0.93) |
| P-trend5 | 0.004 | 0.1 | 0.005 | 0.05 | <0.0001 | 0.02 | ||||
| Flavanones | ||||||||||
| 1 | 3.5 (<7.0) | 317 | 1.00 (—) | 1.00 (—) | 289 | 1.00 (—) | 1.00 (—) | 606 | 1.00 (—) | 1.00 (—) |
| 2 | 10.4 (7.0–13.7) | 258 | 0.81 (0.68, 0.95) | 0.90 (0.76, 1.06) | 201 | 0.67 (0.56, 0.81) | 0.74 (0.62, 0.89) | 459 | 0.74 (0.66, 0.84) | 0.82 (0.73, 0.93) |
| 3 | 17.4 (13.8–21.6) | 301 | 0.81 (0.69, 0.95) | 0.95 (0.81, 1.12) | 231 | 0.75 (0.63, 0.90) | 0.86 (0.72, 1.03) | 532 | 0.78 (0.70, 0.88) | 0.91 (0.81, 1.02) |
| 4 | 27.3 (21.7–35.3) | 331 | 0.84 (0.72, 0.98) | 0.98 (0.84, 1.15) | 228 | 0.72 (0.61, 0.86) | 0.85 (0.71, 1.01) | 559 | 0.78 (0.70, 0.88) | 0.92 (0.82, 1.03) |
| 5 | 49.9 (≥35.4) | 382 | 0.82 (0.71, 0.96) | 0.99 (0.85, 1.15) | 233 | 0.70 (0.59, 0.83) | 0.80 (0.67, 0.96) | 615 | 0.77 (0.68, 0.86) | 0.90 (0.80, 1.01) |
| P-trend5 | 0.1 | 0.7 | 0.005 | 0.1 | 0.002 | 0.5 | ||||
| Flavones | ||||||||||
| 1 | 0.4 (<0.5) | 401 | 1.00 (—) | 1.00 (—) | 292 | 1.00 (—) | 1.00 (—) | 693 | 1.00 (—) | 1.00 (—) |
| 2 | 0.7 (0.5–0.8) | 306 | 0.84 (0.72, 0.97) | 0.92 (0.79, 1.07) | 264 | 0.90 (0.76, 1.06) | 0.98 (0.83, 1.16) | 570 | 0.86 (0.77, 0.96) | 0.94 (0.84, 1.05) |
| 3 | 1.1 (0.9–1.2) | 301 | 0.79 (0.68, 0.92) | 0.92 (0.79, 1.07) | 238 | 0.82 (0.69, 0.97) | 0.93 (0.78, 1.11) | 539 | 0.79 (0.71, 0.89) | 0.91 (0.81, 1.02) |
| 4 | 1.6 (1.3–2.0) | 290 | 0.76 (0.65, 0.88) | 0.90 (0.78, 1.05) | 227 | 0.79 (0.66, 0.94) | 0.92 (0.77, 1.10) | 517 | 0.77 (0.68, 0.86) | 0.91 (0.81, 1.02) |
| 5 | 3.0 (≥2.1) | 291 | 0.73 (0.63, 0.85) | 0.89 (0.76, 1.04) | 161 | 0.62 (0.51, 0.75) | 0.74 (0.61, 0.90) | 452 | 0.68 (0.60, 0.77) | 0.82 (0.72, 0.92) |
| P-trend5 | 0.0002 | 0.2 | <0.0001 | 0.001 | <0.0001 | 0.001 | ||||
| Flavonols | ||||||||||
| 1 | 6.9 (<8.5) | 333 | 1.00 (—) | 1.00 (—) | 324 | 1.00 (—) | 1.00 (-) | 657 | 1.00 (—) | 1.00 (—) |
| 2 | 9.9 (8.5–11.3) | 316 | 0.80 (0.68, 0.93) | 0.88 (0.75, 1.03) | 226 | 0.73 (0.62, 0.87) | 0.79 (0.67, 0.94) | 542 | 0.76 (0.68, 0.86) | 0.83 (0.74, 0.93) |
| 3 | 13.0 (11.4–14.7) | 341 | 0.82 (0.71, 0.96) | 0.95 (0.81, 1.11) | 214 | 0.72 (0.61, 0.86) | 0.79 (0.67, 0.94) | 555 | 0.77 (0.69, 0.87) | 0.87 (0.78, 0.98) |
| 4 | 17.2 (14.8–20.5) | 317 | 0.78 (0.67, 0.91) | 0.92 (0.79, 1.08) | 199 | 0.66 (0.55, 0.79) | 0.74 (0.62, 0.89) | 516 | 0.72 (0.64, 0.81) | 0.84 (0.74, 0.94) |
| 5 | 27.2 (≥20.6) | 282 | 0.76 (0.65, 0.89) | 0.88 (0.74, 1.03) | 219 | 0.72 (0.61, 0.86) | 0.82 (0.69, 0.97) | 501 | 0.74 (0.66, 0.83) | 0.84 (0.75, 0.94) |
| P-trend5 | 0.006 | 0.2 | 0.002 | 0.07 | <0.0001 | 0.03 | ||||
| Proanthocyanidins | ||||||||||
| 1 | 53.1 (<71.6) | 307 | 1.00 (-) | 1.00 (—) | 283 | 1.00 (—) | 1.00 (—) | 590 | 1.00 (—) | 1.00 (—) |
| 2 | 90.0 (71.6–109.2) | 327 | 0.93 (0.80, 1.09) | 1.02 (0.87, 1.20) | 246 | 0.86 (0.72, 1.02) | 0.92 (0.77, 1.09) | 573 | 0.89 (0.80, 1.00) | 0.97 (0.86, 1.09) |
| 3 | 132.0 (109.3–160.2) | 358 | 0.96 (0.83, 1.12) | 1.07 (0.92, 1.26) | 199 | 0.68 (0.57, 0.82) | 0.75 (0.63, 0.91) | 557 | 0.83 (0.74, 0.94) | 0.92 (0.82, 1.04) |
| 4 | 196.8 (160.3–253.5) | 317 | 0.86 (0.74, 1.01) | 0.99 (0.84, 1.16) | 223 | 0.74 (0.62, 0.88) | 0.81 (0.68, 0.97) | 540 | 0.80 (0.71, 0.90) | 0.90 (0.80, 1.01) |
| 5 | 379.4 (≥253.6) | 280 | 0.81 (0.69, 0.95) | 0.91 (0.77, 1.08) | 231 | 0.75 (0.63, 0.89) | 0.83 (0.70, 0.99) | 511 | 0.78 (0.69, 0.88) | 0.87 (0.77, 0.98) |
| P-trend5 | 0.006 | 0.1 | 0.01 | 0.09 | 0.0001 | 0.02 | ||||
| Isoflavones | ||||||||||
| 1 | 0.024 (<0.027) | 249 | 1.00 (—) | 1.00 (—) | 290 | 1.00 (-) | 1.00 (-) | 539 | 1.00 (-) | 1.00 (-) |
| 2 | 0.029 (0.027–0.031) | 291 | 0.90 (0.76, 1.07) | 0.94 (0.79, 1.11) | 254 | 0.99 (0.84, 1.18) | 1.02 (0.86, 1.21) | 545 | 0.96 (0.85, 1.08) | 0.99 (0.88, 1.12) |
| 3 | 0.036 (0.032–0.041) | 353 | 0.86 (0.73, 1.01) | 0.91 (0.77, 1.08) | 277 | 1.18 (1.00, 1.40) | 1.24 (1.05, 1.46) | 630 | 1.01 (0.90, 1.13) | 1.06 (0.94, 1.19) |
| 4 | 0.055 (0.042–0.141) | 433 | 0.80 (0.68, 0.93) | 0.83 (0.71, 0.98) | 186 | 1.02 (0.85, 1.23) | 1.04 (0.86, 1.25) | 619 | 0.90 (0.80, 1.01) | 0.93 (0.82, 1.05) |
| 5 | 0.713 (≥0.142) | 263 | 0.72 (0.60, 0.86) | 0.84 (0.71, 1.01) | 175 | 0.85 (0.71, 1.03) | 0.99 (0.82, 1.20) | 438 | 0.79 (0.70, 0.90) | 0.92 (0.81, 1.05) |
| P-trend5 | 0.004 | 0.3 | 0.02 | 0.4 | 0.0001 | 0.1 | ||||
(—), referent; CPS II, Cancer Prevention Study II.
There was no heterogeneity in RRs by sex, tested by using likelihood ratio tests of models with and without an interaction term of flavonoid (quintiles) and sex.
Age-adjusted models for men and women combined were additionally adjusted for sex.
Multivariate model was adjusted for age, smoking, beer and liquor intake, history of hypertension, history of cholesterol, family history of myocardial infarction, BMI, physical activity, energy intake, aspirin use, hormone replacement therapy (in women only), and sex (in combined model only) by using Cox proportional hazards regression.
Test for trend by using median values for each quintile and modeling as a continuous variable.
The associations between flavonoid intakes and death due to IHD and stroke are provided in Supplemental Tables 1 and 2 under “Supplemental data” in the online issue. In men and women combined, flavone consumption was associated with lower risk of fatal IHD (quintile 5 compared with quintile 1—RR: 0.75; 95% CI: 0.62, 0.90; P-trend = 0.009), especially among women (quintile 5 compared with quintile 1—RR: 0.60; 95% CI: 0.44, 0.82; P-trend = 0.002). Total flavonoid intake was inversely associated with fatal stroke in men (quartile 4 compared with quartile 1—RR: 0.63; 95% CI: 0.44, 0.89; P-trend = 0.04). Including total flavonoids in the models of individual flavonoid class weakened most associations, except for flavones (not shown). The correlation between flavones and total flavonoids was lower (r = 0.18) than most, especially proanthocyanidins and total flavonoids (r = 0.99).
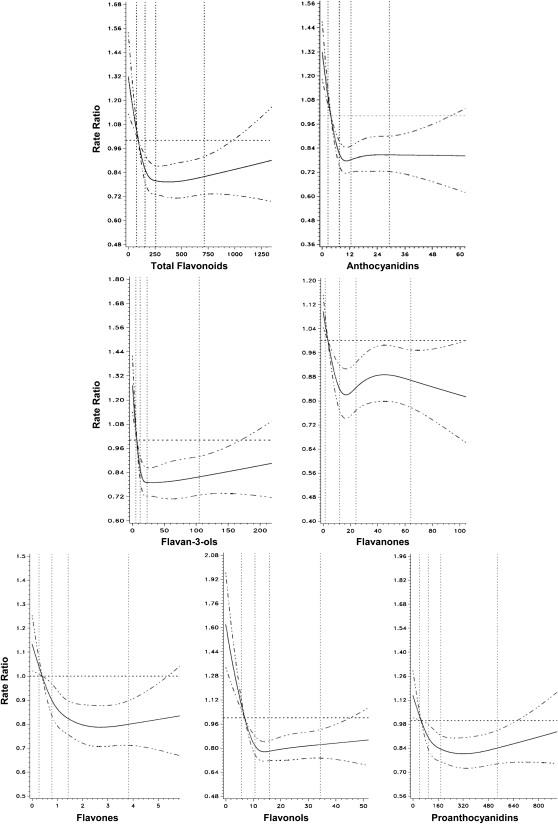
Many relations appeared to be nonlinear, with risk reduction emerging at the second quintile. Indeed, several associations were statistically nonlinear by the spline test (30), and the test of significance for the spline curve (compared with a model with no main exposure variable) indicated significant P values for most flavonoids (P < 0.01). Spline curves for the multivariate model of CVD risk associated with flavonoid consumption among men and women combined are shown in Figure 1. These findings reinforce the interpretation of Table 3 and Supplemental Tables 1 and 2, which suggest possible threshold associations.
FIGURE 1.
Cubic spline curves for the association between flavonoid intake (mg) and cardiovascular disease mortality among men and women. All associations (except isoflavones) were nonlinear (P ≤ 0.01) and significant at P < 0.01 using the likelihood ratio test. Isoflavone graph not included due to low intake estimates.
No effect modification by smoking or multivitamin use was observed for total flavonoids or flavonoid class in either sex or both sexes combined. For total flavonoids and proanthocyanidin intakes in the third compared with bottom tertile, older men (age >70 y) had a lower risk of fatal CVD compared with men aged ≤70 y, but all CIs included 1.0 (P-interaction = 0.03 and 0.05, respectively; data not shown).
DISCUSSION
In this large prospective cohort of US men and women, a greater intake of total flavonoids, and of most flavonoid classes, was associated with a lower risk of fatal CVD in men and women after several important CVD risk factors were controlled for. In women, the strongest inverse association was observed with flavones, particularly for fatal IHD. In men, total flavonoid intake was associated with lower risk of fatal stroke. Associations were somewhat attenuated with adjustment for smoking and physical activity, suggesting possible confounding by associated lifestyle factors. Many of the associations we observed were nonlinear, with low risks seen at even modest intakes. This suggests that consumption of even relatively small amounts of flavonoid-rich foods may be beneficial for reducing risk of fatal CVD.
Most of the prior epidemiologic literature on flavonoids and CVD risk has focused on flavones, flavonols, and flavan-3-ols (catechins) (10, 12). Earlier studies combined flavones with flavonols in analyses (11, 32–40), and some (32–36, 40) reported significant inverse associations with CVD risk. The current study contributes to the body of evidence for a role of flavonols and especially flavones in lowering the risk of fatal CVD. Flavan-3-ols were associated with lower CVD risk in the current study, in agreement with some (41, 42), but not all (13, 14, 43), prospective cohorts. In addition, we found that the less-studied anthocyanidins and proanthocyanidins were associated with lower CVD risk. Isoflavones were not related to fatal CVD in the present study or in other studies in US (14) and European women (44). Consumption of soy products, however, are ~10-fold lower in Western populations than in Asian populations, in whom some protection against cerebral and myocardial infarctions was observed (9).
Over the past decade, accumulation of analytic data on flavonoids and the expansion of flavonoid composition databases has facilitated the comprehensive study of several flavonoid classes in relation to health and disease (16, 45). This evolution complicates comparison of intakes and risk estimates among studies, however, because “total flavonoids” will vary depending on the number of compounds quantified, and data sources used. Use of different dietary assessment tools will also contribute to variation across studies. We used an FFQ designed to estimate usual intake of major nutrient sources in the United States with a defined food list, and derived flavonoid values primarily from USDA sources, as did 2 other US studies (14, 15). Pérez-Jimenez (46) analyzed multiple 24-h dietary records from 4952 French adults by using an online polyphenol database and reported higher daily estimates of flavonols, flavones, flavan-3-ols, and anthocyanidins than the US cohorts (14, 15). The polyphenol database includes some additional compounds not included in USDA databases. Flavonoid estimates will also differ because of true differences in dietary habits across populations. Despite these sources of variation, rank ordering of exposure allows the comparison of risk by high compared with low intake amounts within a study population.
Our methods are most comparable to those of Mink et al (14), who examined associations between 7 flavonoid classes and CVD, IHD, and stroke mortality in a cohort of white postmenopausal women by using a similar FFQ. Total flavonoids were not significantly related to lower CVD mortality after multivariate adjustment, but anthocyanidin and flavanone intakes were associated with both fatal CVD and IHD (14). In the current study, both classes were inversely associated with fatal CVD and IHD in women (associations were inverse, but weaker in men), but CIs for IHD included 1.0. Flavones were not associated with risk in the Iowa study, in contrast to the strong risk reduction we observed among women in the CPS-II Nutrition Cohort. Our flavone and anthocyanidin estimates were higher, and flavanone estimates lower, than in the Mink study (14) which used the earlier 2003 USDA flavonoid database.
The reproducibility and validity of the questionnaire used in the current study was tested in similar populations of mostly educated, white US adults (20, 21, 47). In particular, the FFQ was validated by using serum measures of carotenoids (47), which are found in similar foods as flavonoids; therefore, we expect that the validity and reproducibility are reasonable for flavonoids. Flavonoid content of plants varies depending on the growing conditions, storage, and cooking methods. Interindividual differences in absorption and microbial transformation in the gut, as well as general nutritional composition of the meal, contribute to variability in metabolism and internal exposure to relevant flavonoid metabolites (48). Given these sources of variability in intake and absorption, validation using biomarkers would be ideal (49). Few large epidemiologic studies, however, collected repeated urine samples, which may be needed to estimate usual intake.
The mechanism for a cardioprotective role of flavonoids likely involves more than one pathway, including antioxidant and antiinflammatory functions (7) and vascular effects (50). Using flavan-3-ol compounds as an example, observational data from indigenous populations (51) and other mechanistic studies in humans (50, 52) suggest a protective role for flavan-3-ols in blood pressure regulation. Cocoa induces nitric oxide synthase, which is important in the vasodilator response (50). Meta-analyses of randomized controlled trial data showed that consumption of flavonoid-rich foods (eg, green tea, soy protein isolates, and cocoa or chocolate) was associated with increased flow-mediated dilatation, reduced LDL cholesterol, and reduced blood pressure (53).
Several limitations of our study deserve mention. Flavonoid intake may be misclassified, and risk estimates attenuated. By their very nature, FFQs cannot capture all potential sources of flavonoids. For example, dark chocolate and herbs such as peppermint and parsley were not included on the current questionnaire, but consumption is assumed to have been relatively low in the United States at the time of this survey. We believe that the majority of important flavonoid sources in the US diet were captured because total estimates (mean: 268 mg/d; median: 202 mg/d) were intermediate between estimates derived from the 2003 USDA database [for 6 (54, 55) or 7 (14) flavonoid classes] and from the 2007 USDA database (in health professionals) (15). Due to multiple associations examined, some of our significant findings would be expected by chance. We regard these findings as suggestions for hypothesis generation and further testing, and not as evidence of cause-and-effect. We were unable to investigate the relation between diet and incident, nonfatal CVD. Fatal CVD contains a higher proportion of sudden death, for which the etiologic factors are not completely clear, but dietary factors have been implicated (56–58).
Strengths of this study include the prospective design and large sample size, the inclusion of both men and women, and the use of a well-validated dietary assessment instrument that contained important common sources of several major flavonoids in the United States. We also had detailed information on important risk factors and confounders for CVD that were absent from some of the earlier studies.
In conclusion, our findings indicate that total flavonoids and several classes, especially flavones, are associated with lower risks of fatal CVD. Relatively little is known about the long-term biological effects of these compounds in humans. The finding that benefits of flavonoid consumption were realized at relatively low intake thresholds deserves further examination. If these findings are replicated, recommendations for food sources rich in specific flavonoids should be considered for CVD risk reduction.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MLM, JTD and PFJ: designed the research; MLM, JJP, and JTD: conducted the research; RP and RS: conducted statistical analyses; MLM, JTD and JJP: wrote the manuscript; and MLM: had primary responsibility for final content. All authors read and approved the final manuscript. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: CPS II, Cancer Prevention Study II; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; HRT, hormone replacement therapy; ICD-9, International Classification of Diseases, 9th revision; ICD-10, International Classification of Diseases, 10th revision; IHD, ischemic heart disease.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–146 Erratum in: Circulation 2010;122(1):e10 [DOI] [PubMed] [Google Scholar]
- 2.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–83 [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe JH, Carter MD, Lavie CJ. Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc 2009;84:741–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dauchet L, Amouyel P, Dallongeville J. Fruits, vegetables and coronary heart disease. Nature Reviews. Cardiology 2009;6:599–608 [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore T, Obarzanek E, Vollmer W, Svetkey L, Sacks F, Bray G, Vogt T, Cutler J, Windhauser M, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 6.Djoussé L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr 2009;28:10–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000;52(4):673–751 [PubMed] [Google Scholar]
- 8.Nagata C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. Int J Epidemiol 2000;29:832–6 [DOI] [PubMed] [Google Scholar]
- 9.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation 2007;116:2553–62 [DOI] [PubMed] [Google Scholar]
- 10.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 2005;81:317S–25S [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Rexrode KM, Hu FB, Albert CM, Chae CU, Rimm EB, Stampfer MJ, Manson JE. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am J Epidemiol 2007;165:1305–13 [DOI] [PubMed] [Google Scholar]
- 12.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2003;57(8):904–8 [DOI] [PubMed] [Google Scholar]
- 13.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 2008;100:890–5 [DOI] [PubMed] [Google Scholar]
- 14.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DRJ. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909 [DOI] [PubMed] [Google Scholar]
- 15.Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman J, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2010;93:338–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USDA USDA database for the flavonoid content of selected foods, release 2.1. Beltsville, MD: Agricultural Research Service, Nutrient Data Laboratory, 2007 [Google Scholar]
- 17.USDA USDA database for the proanthocyanidin content of selected foods. Beltsville, MD: Agricultural Research Service, Nutrient Data Laboratory, 2004 [Google Scholar]
- 18.USDA Iowa State University database on the isoflavone content of foods, release 1.3. Beltsville, MD: Agricultural Research Service, Nutrient Data Laboratory, 2002 [Google Scholar]
- 19.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, Feigelson HS, Thun MJ. The American Cancer Society Cancer Prevention Study II Nutrition Cohort—rationale, study design, and baseline characteristics. Cancer 2002;94:2490–501 [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of a expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 22.Peterson JJ, Beecher GR, Bhagwat SA, Dwyer JT, Gebhardt SE, Haytowitz DB. Flavanones in grapefruit, lemons, and limes: a compilation and review of the data from the analytical literature. J Food Compost Anal 2006;19(suppl):S74–80 [Google Scholar]
- 23.Peterson JJ, Dwyer JT, Beecher GR, Bhagwat SA, Gebhardt SE, Haytowitz DB. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compost Anal 2006;19(suppl):S66–73 [Google Scholar]
- 24.Peterson J, Dwyer J, Bhagwat S, Haytowitz D, Holden J, Eldridge AL, Beecher G, Aladesanmi J. Major flavonoids in dry tea. J Food Compost Anal 2005;18:487–501 [Google Scholar]
- 25.Bobe G, Peterson JJ, Gridley G, Hyer M, Dwyer JT, Brown LM. Flavonoid consumption and esophageal cancer among black and white men in the United States. Int J Cancer 2009;125(5):1147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among Cancer Prevention Study II participants. Am J Epidemiol 1993;137:235–41 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization International classification of diseases, injuries, and causes of death. Based on recommendations of the 9th revision conference, 1975, and adopted by the 29th World Health Assembly. Geneva, Switzerland: WHO, 1977–1978 [Google Scholar]
- 28.World Health Organization International statistical classification of diseases and related health problems, 10th revision. 10th ed. Geneva, Switzerland: WHO, 1992 [Google Scholar]
- 29.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61 [DOI] [PubMed] [Google Scholar]
- 31.Hosmer D, Lemeshow S. Applied logistic regression. New York, NY: John Wiley and Sons, 1989 [Google Scholar]
- 32.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ 1996;312:478–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol 1999;149:943–9 [DOI] [PubMed] [Google Scholar]
- 34.Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology 2001;12:62–7 [DOI] [PubMed] [Google Scholar]
- 35.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993;342:1007–11 [DOI] [PubMed] [Google Scholar]
- 36.Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet 1997;349:699. [DOI] [PubMed] [Google Scholar]
- 37.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med 1996;125:384–9 [DOI] [PubMed] [Google Scholar]
- 38.Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr 2003;77:1400–8 [DOI] [PubMed] [Google Scholar]
- 39.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 2000;31(10):2301–6 [DOI] [PubMed] [Google Scholar]
- 40.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med 1996;156:637–42 [PubMed] [Google Scholar]
- 41.Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr 2001;74:227–32 [DOI] [PubMed] [Google Scholar]
- 42.Mennen LI, Sapinho D, de Bree A, Arnault N, Bertrais S, Galan P, Hercberg S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr 2004;134:923–6 [DOI] [PubMed] [Google Scholar]
- 43.Arts IC, Jacobs DR, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology 2001;12:668–75 [DOI] [PubMed] [Google Scholar]
- 44.van der Schouw YT, Kreijkamp-Kaspers S, Peeters PH, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation 2005;111:465–71 [DOI] [PubMed] [Google Scholar]
- 45.Neveu V, Perez-Jiminez J, Vox F, Crespy V, duChaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010. doi: 10.1093/database/bap024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pérez-Jiminez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Galan P, Scalbert A. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr 2011;93:1220–8 [DOI] [PubMed] [Google Scholar]
- 47.Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, Willett WC. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev 1998;7(4):283–90 [PubMed] [Google Scholar]
- 48.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 2001;90:157–77 [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Jimenez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 2010;92:801–9 [DOI] [PubMed] [Google Scholar]
- 50.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 2003;21(12):2281–6 [DOI] [PubMed] [Google Scholar]
- 51.McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, Coletti C, Campos H, Hollenberg NK. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol 2006;47(suppl 2):S103–9 [DOI] [PubMed] [Google Scholar]
- 52.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103(4):1024–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88(1):38–50 [DOI] [PubMed] [Google Scholar]
- 54.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 2007;137:1244–52 [DOI] [PubMed] [Google Scholar]
- 55.Dwyer J, Peterson J, Winters B, Liu W, Mitchell DC, Atkinson K. Do flavonoid intakes of postmenopausal women with breast cancer vary on very low fat diets? Nutr Cancer 2008;60:450–60 [DOI] [PubMed] [Google Scholar]
- 56.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008;138:1746S–51S [DOI] [PubMed] [Google Scholar]
- 57.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 58.Scrutinio D. The potential of lifestyle changes for improving the clinical outcome of patients with coronary heart disease: mechanisms of benefit and clinical results. Rev Recent Clin Trials 2010;5:1–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.