Abstract
Background: Caffeinated beverages are widely consumed among women of reproductive age, but their association with reproductive hormones, and whether race modifies any such associations, is not well understood.
Objective: We assessed the relation between caffeine and caffeinated beverage intake and reproductive hormones in healthy premenopausal women and evaluated the potential effect modification by race.
Design: Participants (n = 259) were followed for up to 2 menstrual cycles and provided fasting blood specimens for hormonal assessment at up to 8 visits per cycle and four 24-h dietary recalls per cycle. Weighted linear mixed models and nonlinear mixed models with harmonic terms were used to estimate associations between caffeine and hormone concentrations, adjusted for age, adiposity, physical activity, energy and alcohol intakes, and perceived stress. On the basis of a priori assumptions, an interaction between race and caffeine was tested, and stratified results are presented.
Results: Caffeine intake ≥200 mg/d was inversely associated with free estradiol concentrations among white women (β = −0.15; 95% CI: −0.26, −0.05) and positively associated among Asian women (β = 0.61; 95% CI: 0.31, 0.92). Caffeinated soda intake and green tea intake ≥1 cup/d (1 cup = 240 mL) were positively associated with free estradiol concentrations among all races: β = 0.14 (95% CI: 0.06, 0.22) and β = 0.26 (95% CI: 0.07, 0.45), respectively.
Conclusions: Moderate consumption of caffeine was associated with reduced estradiol concentrations among white women, whereas caffeinated soda and green tea intakes were associated with increased estradiol concentrations among all races. Further research is warranted on the association between caffeine and caffeinated beverages and reproductive hormones and whether these relations differ by race.
INTRODUCTION
Caffeine intake by women of childbearing age is common in the United States. Approximately 89% of women aged 18–34 y consume an average of 166 mg caffeine/d (equivalent to 1.5–2 cups caffeinated coffee) from a variety of sources but mostly from caffeinated beverages (1, 2). Despite the prevalence of intake, research relating caffeine and reproductive hormone concentrations among premenopausal women is limited and inconclusive (3–6). The inconsistent results may be due in part to interethnic variability in the endocrine dynamics of female reproductive hormones (7, 8) and caffeine metabolism (9, 10). The association between caffeine and hormones is of interest, because persistent elevation or insufficiency of reproductive hormones during the premenopausal years may not only contribute in the long term to the etiology of certain diseases, such as breast, endometrial, and ovarian cancers (11–14), but may also affect ovulatory function in the short term (15, 16). Further understanding of these associations can inform the development of appropriate guidelines regarding consumption levels for women of reproductive age (17).
A variety of pathophysiologic effects of caffeine and components of caffeinated beverages on sex hormones and ovulatory function exist. Animal models suggest that caffeine can inhibit oocyte maturation or enhance steroid production via inhibition of phosphodiesterase (4, 18) or, alternatively, may interfere with estrogen metabolism via inhibition of aromatase—the key enzyme responsible for converting androgens to estrogen (6, 19). Studies in women have suggested that caffeine may have a positive (4), inverse (6), or null association with E24 (5), but has no effect on ovulatory function (16, 20, 21), although no studies to date have prospectively measured caffeine intake at multiple time points and directly measured ovulation. Both caffeine and E2 are metabolized by the hepatic enzyme CYP1A2 (22, 23). Polymorphisms of CYP1A2 have been linked to variability in caffeine clearance (24) and serum E2 concentrations (25) and have been shown to modify relations between caffeine intake and adverse health outcomes (26, 27). Estrogen and caffeine metabolism and risk of breast and ovarian cancers have also been shown to differ between whites and Asians (28–30). It is unknown whether differences in caffeine consumption and metabolism could partially explain these differences.
The primary objective of this study was to determine whether caffeine and its associated beverages (coffee, tea, and soda) are related to serum concentrations of reproductive hormones in a cohort of 259 healthy premenopausal women and whether these associations differ by race. Our secondary objective was to determine whether caffeine and its associated beverages are associated with incident anovulation.
SUBJECTS AND METHODS
Study population
The BioCycle Study, conducted in 2005–2007, followed women from Western New York State for 1 (n = 9) or 2 (n = 250) complete menstrual cycles. The study population, materials, and methods were previously described in detail (31). In summary, healthy women aged 18–44 y had to be regularly menstruating (self-reported cycle length between 21 and 35 d for each menstrual cycle in the past 6 mo) and not currently using hormonal contraception (and for the 3 mo before study entry) to participate. Of 449 women who were screened, 318 met the eligibility criteria, of whom 276 enrolled. Seventeen women (6%) withdrew before completing the study (31). Women who withdrew were not markedly different (mean age: 25.9 ± 7.2 y) from women who completed the study (mean age: 27.3 ± 8.2 y), although a greater percentage of Asians (19%) than of blacks (6%) or whites (3%) withdrew. Reasons for withdrawal included scheduling conflicts (n = 8), loss to follow-up (n = 4), inability to tolerate blood draws (n = 3), inability to participate because of illness (n = 1), or loss of interest (n = 1). Women reporting conditions known to affect menstrual cycle function, such as polycystic ovary syndrome, uterine fibroids, or known ovulatory disorders, were excluded. The University at Buffalo Health Sciences Institutional Review Board approved the study and served as the Institutional Review Board designated by the NIH for this study under a reliance agreement. Written informed consent was obtained from all participants.
Hormone assessment
Women provided fasting blood specimens on up to 8 visits per cycle, with visit timing assisted by use of fertility monitors to correspond to menstruation; mid-follicular, late follicular, and LH/FSH surges; ovulation; and early luteal, mid-luteal, and late-luteal phases (32). Total E2 was measured via radioimmunoassay whereas progesterone, LH, FSH, SHBG, and insulin were measured by using solid-phase competitive chemiluminescent enzyme immunoassay (Immulite 2000). The albumin assay was tested with the Beckman LX20 auto analyzer by using bromocresol purple methods. Calculation of free E2 (ie, bioavailable E2) was performed via the equation proposed by Södergård et al (33) using total E2, SHBG, and albumin concentrations. All hormonal analyses were conducted by Kaleida Health Laboratories. Across the study period, the CVs for these tests were <10% for E2, SHBG, and insulin; <5% for LH, FSH, and albumin; and <14% for progesterone. Insulin resistance was calculated by using the homeostasis model assessment (34). Total cholesterol was measured in serum at each clinic visit by using an LX20 automated chemistry analyzer (Beckman), with a CV of <5%. We defined anovulation as any cycle with peak progesterone concentration ≤5 ng/mL and no observed serum LH peak at the mid– or late–luteal phase visits (n = 42 of 509 cycles; 8.3%). Study protocol compliance was high, and 94% of the participants completed 7 or 8 visits per cycle.
Dietary assessment
Participants completed a 24HDR at the clinic during the 4 visits corresponding to menstruation, mid-follicular phase, ovulation, and mid-luteal phase. Food and beverage intakes were collected and nutrient data were analyzed by using the NDSR (2005; Nutrition Coordinating Center). The NDSR program computed the nutrients (ie, total energy) and nonnutrients (ie, caffeine) along with beverage components (ie, coffee) consumed for each day of intake. Further abstraction was done from the raw 24HDR data to discriminate between caffeinated, decaffeinated, or noncaffeinated varieties of coffee, tea (black or green), and soda because this information is not included in standard NDSR output. Ninety-six percent of the participants completed four 24HDRs in at least one of their cycles under study; 99% completed at least three 24HDRs.
Covariate assessment
At study enrollment, waist-to-hip ratio was obtained by trained study staff using standardized protocols, whereas age, self-identified race, smoking, alcohol intake, reproductive history, and perceived stress were obtained by using validated questionnaires (31). For prospectively measured covariates, participants were provided a diary in which they were instructed to record daily vigorous exercise (min), cigarettes smoked (number), sexual intercourse (yes or no), sleep (h and min), and medication intake (type, dose, units, and number of times per day). Caffeine from medications (primarily from over-the-counter preparations with nonsteroidal antiinflammatory drugs) was averaged for each phase over the 2 cycles and added to the caffeine calculated from the eight 24HDRs. Eighty-nine percent of participants completed ≥75% of their daily diaries.
Statistical analyses
Descriptive characteristics of the study population were compared between tertiles of daily caffeine intake, averaged over the 2 cycles under study, and anovulatory status. We assessed differences using ANOVA per the Satterthwaite method for unequal variances and exact chi-square tests where appropriate (35). Reproductive hormone and serum cholesterol concentrations were log transformed for statistical analyses. The percentage of women consuming caffeinated and/or decaffeinated/noncaffeinated coffee, tea, and soda were also generated, based on average intakes across the 2 cycles. Caffeine source was also determined on the basis of total caffeinated food, beverage, or medication items reported.
Weighted linear mixed models were used to evaluate the association between visit-specific caffeine, coffee, tea, or soda intake and log serum concentrations of free and total E2, luteal progesterone, LH, and FSH. Generalized linear mixed models were used to estimate the odds of anovulation based on caffeine and caffeinated beverage consumption. These random-intercept models were chosen to account for between-women variation in baseline hormone concentrations and within-woman correlations of cycles. Recommended limits of caffeine for reproductive-aged women [≥200 compared with <200 mg/d (including no exposure)] (36) and relevant cutoffs for coffee, tea, or soda intake [≥1 compared with <1 cup/d (including no exposure)] (4) were assessed. Models were restricted to ovulatory cycles (n = 467), because the hormonal patterns for anovulatory cycles are distinctly different from ovulatory cycles.
On the basis of previous evidence for potential biologic effect modification (4, 37), we tested for interactions between caffeine or beverage exposure and both race and dietary cholesterol intake using the likelihood ratio test (α = 0.10). Lucero et al (4) reported that women whose cholesterol consumption was >217 mg/d had higher E2 concentrations than did women whose cholesterol intake was ≤217 mg/d. Thus, we tested whether there was an interaction between caffeine and cholesterol intakes at this cutoff (near our participants’ average intake of 214 mg/d). In addition to race and dietary cholesterol, we also separately evaluated effect modification by age and waist-to-hip ratio. Stratified analyses (with the use of models with relevant interaction terms) are presented in which significant effect modification was found. Potential confounders were determined a priori by using directed acyclic graphs (38). Age, race, waist-to-hip ratio, daily exercise, perceived stress, and total energy and alcohol intakes (all continuous) were included in our final models. Additional covariates, including other caffeinated beverage intake (ie, adjusted for caffeinated soda and tea when investigating coffee), cigarette use, reproductive history (ie, gravidity and past oral contraceptive use), sleep, sexual activity, dietary intake (ie, fiber, cholesterol, and percentage of energy from carbohydrate, fat, and protein), serum cholesterol, and insulin resistance were considered but did not appreciably alter the estimates (39). On the basis of our proposed acyclic graphs, the minimum set of confounders we adjusted for takes into account all sources of measured and known confounding. Because E2 concentrations change over the cycle in response to complex feedback mechanisms with other hormones, traditional regression adjustment for LH, FSH, and progesterone is inadequate. Therefore, we presented models that additionally adjusted for other reproductive hormones (eg, progesterone, FSH, and LH) through stabilized inverse probability of exposure weights (40, 41).
To assess how caffeine and caffeinated beverage intakes affect hormonal patterns, we applied nonlinear mixed models with harmonic terms. Whereas the linear mixed models allow for estimation of mean differences, the harmonic models used can additionally evaluate differences in amplitude (ie, difference between nadir and peak concentrations) and timing of phase shifts while taking into account between- and/or within-subject variation (42).
Sensitivity analyses were conducted to assess the effects of continuous and varying cutoffs of caffeine (tertiles and 100-mg increments) or caffeinated beverage (1-cup increments) intakes on reproductive hormones and anovulation. For reproductive hormones, we assessed the effects of including anovulatory cycles or excluding smokers. For anovulation, we assessed the effects of caffeine and caffeinated beverages on the less conservatively defined anovulation (ie, cycles with progesterone concentrations ≤5 ng/mL) or excluding smokers. The analyses were performed by using SAS version 9.2 (SAS Institute).
RESULTS
Caffeine and beverage consumption
Mean caffeine intake across both cycles was 90.9 mg/d (range: 0.0–475.4 mg/d). Caffeine intake was positively associated with age; white race; cigarette use; energy, alcohol, and fiber intakes; serum FSH; and serum cholesterol concentrations and inversely associated with nulligravidity, perceived stress, and insulin resistance. Anovulation was inversely associated with age, sleep, alcohol and caffeine intakes, total and free E2, and luteal progesterone and positively associated with nulligravidity (Table 1).
TABLE 1.
Characteristics of women participating in the BioCycle Study, by average caffeine intake and anovulatory status1
| Caffeine intake (mg/d) |
Anovulatory2 |
|||||||
| Total | <25 | 25–105 | >105 | P | Yes | No | P | |
| Number of participants [n (%)] | 259 | 86 (33.2) | 84 (32.4) | 89 (34.4) | 35 (13.5) | 224 (86.5) | ||
| Caffeine (mg/d) | 90.9 ± 94.03 | 10.9 ± 8.1 | 58.0 ± 23.7 | 199.4 ± 79.7 | <0.0001 | 60.2 ± 80.0 | 95.7 ± 95.8 | 0.04 |
| Demographic and lifestyle characteristics | ||||||||
| Age (y) | 27.3 ± 8.2 | 23.4 ± 5.8 | 26.5 ± 8.0 | 31.7 ± 8.3 | <0.0001 | 22.5 ± 5.6 | 28.0 ± 8.3 | 0.0002 |
| Race [n (%)] | <0.0001 | 0.27 | ||||||
| White | 154 (59.5) | 37 (43.0) | 49 (58.3) | 68 (76.4) | 20 (57.1) | 134(59.8) | ||
| Black | 51 (19.7) | 31 (36.1) | 14 (16.7) | 6 (6.7) | 8 (22.9) | 43 (19.2) | ||
| Asian | 37 (14.3) | 15 (17.4) | 13 (15.5) | 9 (10.1) | 7 (20.0) | 30 (13.4) | ||
| Other | 17 (6.6) | 3 (3.5) | 8 (9.5) | 6 (6.7) | 0 (0.0) | 17 (7.6) | ||
| BMI (kg/m2) | 24.1 ± 3.9 | 23.4 ± 3.6 | 24.6 ± 4.1 | 24.2 ± 3.8 | 0.11 | 23.4 ± 3.8 | 24.2 ± 3.9 | 0.25 |
| Waist-to-hip ratio | 0.75 ± 0.06 | 0.75 ± 0.06 | 0.75 ± 0.05 | 0.75 ± 0.05 | 0.90 | 0.75 ± 0.05 | 0.75 ± 0.06 | 0.51 |
| Nulligravid [n (%)] | 177 (69.4) | 71 (83.5) | 62 (74.7) | 44 (50.6) | <0.0001 | 28 (84.9) | 149 (67.1) | 0.04 |
| Perceived stress score | 20.2 ± 6.8 | 21.7 ± 74 | 20.3 ± 6.1 | 18.6 ± 6.5 | 0.01 | 20.2 ± 6.9 | 20.2 ± 6.8 | 0.99 |
| Cigarette use [n (%)] | 0.02 | 0.79 | ||||||
| No | 218 (84.2) | 80 (93.0) | 67 (79.8) | 71 (79.8) | 30 (85.7) | 188 (83.9) | ||
| Yes | 41 (15.8) | 6 (7.0) | 17 (20.2) | 18 (20.2) | 5 (14.3) | 36 (16.1) | ||
| Sleep (h) | 7.2 ± 0.8 | 7.1 ± 0.9 | 7.2 ± 0.7 | 7.2 ± 0.7 | 0.80 | 6.8 ± 0.7 | 7.2 ± 0.8 | 0.01 |
| Daily exercise (min/d) | 14.7 ± 21.9 | 10.9 ± 12.7 | 16.7 ± 27.0 | 16.5 ± 23.4 | 0.14 | 20.4 ± 25.2 | 13.9 ± 21.3 | 0.10 |
| Sex (intercourse/d) | 0.09 ± 0.13 | 0.07 ± 0.12 | 0.08 ± 0.12 | 0.11 ± 0.14 | 0.10 | 0.05 ± 0.11 | 0.09 ± 0.13 | 0.09 |
| Diet | ||||||||
| Total energy (kcal) | 1613.3 ± 367.3 | 1519.6 ± 342.2 | 1600.0 ± 351.5 | 1716.5 ± 382.6 | 0.002 | 1621.2 ± 424.7 | 1612.1 ± 358.6 | 0.89 |
| Alcohol (g) | 2.8 ± 5.5 | 1.0 ± 1.9 | 2.3 ± 4.7 | 4.9 ± 7.4 | <0.0001 | 1.0 ± 2.1 | 3.0 ± 5.8 | 0.0001 |
| Carbohydrates (% of energy) | 50.8 ± 7.3 | 51.7 ± 7.5 | 51.3 ± 7.3 | 49.4 ± 6.9 | 0.09 | 49.9 ± 8.4 | 50.9 ± 7.1 | 0.46 |
| Protein (% of energy) | 15.8 ± 3.1 | 16.0 ± 3.7 | 15.8 ± 3.0 | 15.7 ± 2.6 | 0.86 | 16.3 ± 3.8 | 15.7 ± 3.0 | 0.30 |
| Fat (% of energy) | 33.8 ± 5.6 | 33.3 ± 5.5 | 33.5 ± 5.7 | 34.6 ± 5.5 | 0.25 | 35.2 ± 5.9 | 33.6 ± 5.5 | 0.12 |
| Cholesterol (mg/d) | 213.3 ± 106.7 | 196.8 ± 112.0 | 220.9 ± 111.6 | 222.1 ± 95.4 | 0.21 | 231.3 ± 128.1 | 210.5 ± 103.3 | 0.28 |
| Fiber (g/d) | 13.6 ± 5.6 | 12.7 ± 5.5 | 13.2 ± 6.1 | 15.0 ± 4.8 | 0.02 | 15.7 ± 8.2 | 13.3 ± 5.0 | 0.11 |
| Total E2 (pg/mL) | 104.3 (83.3–134.4)4 | 111.1 (83.3–144.8) | 102.7 (83.9–131.6) | 101.6 (84.5–128.8) | 0.57 | 60.3 ± 1.4 | 90.0 ± 1.4 | <0.0001 |
| Free E2 (pg/mL) | 1.3 (1.1–1.6) | 1.3 (1.1–1.7) | 1.3 (1.1–1.5) | 1.3 (1.0–1.5) | 0.24 | 1.0 ± 0.3 | 1.4 ± 0.5 | <0.0001 |
| Luteal progesterone (ng/mL) | 3.4 (2.5–4.4) | 3.3 (2.5–4.5) | 3.0 (2.3–4.2) | 3.7 (2.8–4.4) | 0.19 | 0.69 ± 1.7 | 1.5 ± 1.5 | <0.0001 |
| LH (ng/mL) | 9.2 (7.5–11.4) | 9.2 (7.7–11.4) | 9.1 (7.5–11.4) | 9.2 (7.5–11.3) | 0.59 | 6.7 ± 15 | 6.0 ± 1.4 | 0.27 |
| FSH (mIU/mL) | 6.0 (5.1–7.0) | 5.7 (4.6–6.6) | 6.5 (5.2–7.1) | 7.0 (5.4–7.5) | 0.0004 | 5.3 ± 1.3 | 5.4 ± 1.3 | 0.78 |
| Other biomarkers | ||||||||
| Cholesterol (mg/dL) | 163.2 (147.4–177.9) | 159.8 (145.3–176.9) | 158.9 (146.1–176.4) | 169.1 (153.6–186.3) | 0.04 | 161.8 ± 25.3 | 164.8 ± 27.4 | 0.54 |
| Insulin resistance (mmol/L) | 1.5 (1.1–2.1) | 1.7 (1.2–2.6) | 1.5 (1.2–2.1) | 1.3 (1.0–1.9) | 0.02 | 2.0 ± 1.1 | 1.8 ± 1.3 | 0.42 |
ANOVA for continuous variables and exact chi-square tests for categorical variables were used to test associations between caffeine intake or ovulation status. Reproductive hormones and serum cholesterol were log transformed for normality for statistical analyses. E2, estradiol; FSH; follicle-stimulating hormone; LH, luteinizing hormone.
Defined as having at least one anovulatory cycle over the 2-cycle study period; 28 women had 1 anovulatory cycle, and 7 women had 2 anovulatory cycles.
Mean ± SD (all such values).
Median; interquartile range in parentheses (all such values).
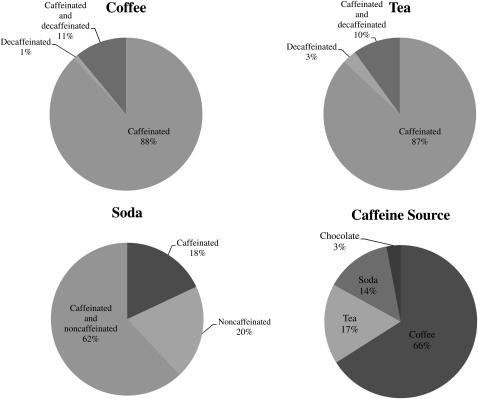
Over 2 cycles, 49% of the subjects consumed coffee (88% exclusively caffeinated, 1% exclusively decaffeinated, and 11% caffeinated and decaffeinated), 60% consumed tea (87% exclusively caffeinated, 3% exclusively decaffeinated, and 10% caffeinated and decaffeinated), and 70% consumed soda (18% exclusively caffeinated, 20% exclusively noncaffeinated, and 62% caffeinated and noncaffeinated) (Figure 1). Few women consumed energy drinks (2%); therefore, caffeinated energy drink consumption was combined with caffeinated soda consumption. Overall, 66% of caffeine intake came from coffee, 17% from tea, 14% from soda, 3% from chocolate, and 0.003% from caffeinated medications (Figure 1).
FIGURE 1.
Percentage of women consuming caffeinated, decaffeinated, and/or decaffeinated/noncaffeinated beverages based on average intakes captured by eight 24-h dietary recalls across 2 menstrual cycles (n = 259). Caffeine sources—based on total caffeinated food, beverage, or medication items reported—are also shown (n = 3079).
Fifty-eight percent of whites reported consuming coffee, followed by Asians (46%) and blacks (25%). Whereas roughly half of all races reported consuming black tea, 27% of Asians reported consuming green tea, followed by whites (18%) and blacks (12%). Blacks reported the highest frequency of soda consumption (75%), followed by whites (71%) and Asians (59%). Among whites, 70% of caffeine came from coffee, 14% from tea, 13% from soda, 3% from chocolate, and 0.004% from caffeinated medications. Among blacks, 33% of caffeine came from coffee, 40% from tea, 23% from soda, 4% from chocolate, and 0.001% from caffeinated medications. Among Asians, 60% of caffeine came from coffee, 25% from tea, 12% from soda, and 4% from chocolate (no caffeinated medication was reported among Asians).
Reproductive hormones
Interactions between race and caffeine intake (≥200 compared with <200 mg/d) on total and free E2 and LH concentrations were significant (likelihood ratio test: P = 0.01, P = 0.02, and P = 0.01, respectively). Similar effect modification was seen between race and coffee intake (≥1 compared with <1 cup/d) on total and free E2, LH, and FSH concentrations (P = 0.06, P = 0.14, P = 0.01, and P = 0.03, respectively). No significant interactions were found between caffeine and cholesterol intakes, age, or waist-to-hip ratio and reproductive hormone concentrations.
We observed that white women who consumed an average of ≥200 mg caffeine/d had lower free (and total) E2 concentrations (free E2: β = −0.15; 95% CI: −0.26, −0.05) than did those who consumed <200 mg/d after adjustment for age, waist-to-hip ratio, perceived stress, daily exercise, energy and alcohol intakes, and FSH, LH, and progesterone concentrations (Table 2). In contrast, black and Asian women who consumed ≥200 mg caffeine/d had elevated free (and total) E2: β = 0.27 (95% CI: −0.01, 0.56) and β = 0.44 (95% CI: 0.13, 0.74), respectively. Additionally, black women who consumed ≥200 mg caffeine/d had reduced FSH (β = −0.36; 95% CI: −0.57, −0.14), whereas Asian women had elevated LH (β = 0.52; 95% CI: 0.19, 0.85). Sensitivity analyses showed some evidence of a dose response: white women who consumed ≥400 mg caffeine/d (n = 17) had lower free (and total) E2 concentrations (free E2; β = −0.39; 95% CI: −0.82, 0.04) compared with white women who consumed less (data not shown). Too few blacks and Asians reported higher caffeine intakes to adequately assess dose response.
TABLE 2.
Mean difference in log serum concentrations of reproductive hormones, stratified by race of participants according to caffeine and coffee intakes (n = 433 ovulatory cycles)1
| Caffeine≥200 compared with <200 mg/d |
Coffee≥1 compared with <1 cup/d |
|||
| Log hormone | Model 12 | Model 23 | Model 12 | Model 23 |
| White (n = 277 cycles) | ||||
| E2 (pg/mL) | −0.09 (−0.19, 0.005) | −0.14 (−0.25, −0.03) | −0.04 (−0.14, 0.05) | −0.06 (−0.16, 0.03) |
| Free E2 (pg/mL) | −0.11 (−0.21, −0.01) | −0.15 (−0.26, −0.05) | −0.05 (−0.14, 0.04) | −0.03 (−0.07, 0.01) |
| Luteal progesterone (ng/mL) | −0.01 (−0.24, 0.23) | 0.00 (−0.27, 0.27) | −0.06 (−0.26, 0.15) | 0.04 (−0.17, 0.26) |
| FSH (mIU/mL) | −0.02 (−0.10, 0.05) | −0.04 (−0.12, 0.04) | 0.03 (−0.05, 0.10) | 0.02 (−0.05, 0.10) |
| LH (ng/mL) | −0.04 (−0.15, 0.06) | −0.12 (−0.23, −0.002) | −0.05 (−0.15, 0.05) | −0.06 (−0.16, 0.03) |
| Black (n = 92 cycles) | ||||
| E2 (pg/mL) | 0.14 (−0.16, 0.45) | 0.24 (−0.05, 0.53) | −0.03 (−0.28, 0.22) | −0.03 (−0.25, 0.20) |
| Free E2 (pg/mL) | 0.17 (−0.14, 0.47) | 0.27 (−0.01, 0.56) | 0.003 (−0.24, 0.25) | −0.01 (−0.23, 0.21) |
| Luteal progesterone (ng/mL) | 0.04 (−0.56, 0.65) | −0.10 (−0.74, 0.55) | −0.05 (−0.56, 0.47) | −0.05 (−0.56, 0.46) |
| FSH (mIU/mL) | −0.36 (−0.59, −0.14) | −0.36 (−0.57, −0.14) | −0.06 (−0.24, 0.13) | −0.08 (−0.25, 0.09) |
| LH (ng/mL) | −0.06 (−0.39, 0.28) | −0.08 (−0.39, 0.24) | 0.08 (−0.19, 0.35) | −0.003 (−0.25, 0.24) |
| Asian (n = 64 cycles) | ||||
| E2 (pg/mL) | 0.44 (0.15, 0.73) | 0.61 (0.31, 0.92) | 0.35 (0.16, 0.54) | 0.32 (0.13, 0.51) |
| Free E2 (pg/mL) | 0.27 (−0.02, 0.56) | 0.44 (0.13, 0.74) | 0.28 (0.09, 0.47) | 0.26 (0.07, 0.44) |
| Luteal progesterone (ng/mL) | 0.18 (−0.60, 0.96) | 0.28 (−0.60, 1.17) | −0.21 (−0.66, 0.25) | −0.11 (−0.56, 0.34) |
| FSH (mIU/mL) | 0.20 (−0.02, 0.42) | 0.17 (−0.06, 0.40) | 0.19 (0.04, 0.33) | 0.11 (−0.04, 0.25) |
| LH (ng/mL) | 0.43 (0.11, 0.74) | 0.52 (0.19, 0.85) | 0.27 (0.06, 0.47) | 0.14 (−0.06, 0.34) |
All values are mean differences (95% CIs). Coffee includes caffeinated (98%) and decaffeinated (2%) forms. Anovulation was defined as any cycle with a peak progesterone concentration ≤5 ng/mL and no observed serum LH peak at the mid– or late–luteal phase visits. There were 433 ovulatory cycles among whites, blacks, and Asians. The analyses were performed by using linear mixed-effects models on the log scale of hormones. Interactions between race and caffeine intake (≥200 compared with <200 mg/d) on total and free E2 and LH concentrations were significant (likelihood ratio test: P = 0.01, P = 0.02, and P = 0.01, respectively) and between race and coffee intake (≥1 compared with <1 cup/d) on total and free E2, LH, and FSH were significant (P = 0.06, P = 0.14, P = 0.01, and P = 0.03, respectively). E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Adjusted for age, waist-to-hip ratio, perceived stress, daily exercise, and energy and alcohol intakes (all continuous).
Adjusted for age, waist-to-hip ratio, perceived stress, daily exercise, energy and alcohol intakes, and relevant phase-specific hormone concentrations by using inverse probability of exposure weights.
Whereas no statistically significant associations between coffee intake ≥1 cup/d and reproductive hormones for whites and blacks were found, Asian women who consumed ≥1 cup coffee/d had elevated free (and total) E2 concentrations (free E2: β = 0.26; 95% CI: 0.07, 0.44) (Table 2). Assessment of continuous coffee intake (cups/d) showed similar results. As shown in Table 3, green (but not black) tea intake ≥1 cup/d was associated with elevated free (and total) E2 (free E2: β = 0.26; 95% CI: 0.07, 0.45) after adjustment for age, waist-to-hip ratio, perceived stress, daily exercise, energy and alcohol intakes, and FSH, LH, and progesterone concentrations. Additionally, caffeinated soda intake ≥1 cup/d was associated with elevated free (and total) E2 (free E2: β = 0.14; 95% CI: 0.06, 0.22) and LH (β = 0.13; 95% CI: 0.04, 0.21) concentrations (Table 3). For each 1-cup increase in green tea consumption, free (and total) E2 concentrations increased (free E2: β = 0.09; 95% CI: 0.02, 0.16) (data not shown). Sensitivity analyses showed that LH concentrations were not associated with each 1-cup increase in caffeinated soda intake, but that free (and total) E2 concentrations increased (free E2: β = 0.04; 95% CI: 0.001, 0.08) (data not shown). The results were similar for all models when the anovulatory cycles were included (n = 42 cycles) or when the analyses were restricted to nonsmokers (n = 218 women).
TABLE 3.
Mean difference in log serum concentrations of reproductive hormones according to beverage intake (n = 467 ovulatory cycles)1
| Beverage ≥1 compared with <1 cup/d |
||
| Log hormone | Model 12 | Model 23 |
| Black tea | ||
| E2 (pg/mL) | −0.02 (−0.09, 0.06) | 0.002 (−0.08, 0.08) |
| Free E2 (pg/mL) | −0.01 (−0.08, 0.07) | 0.01 (−0.08, 0.09) |
| Luteal progesterone (ng/mL) | −0.08 (−0.26, 0.11) | −0.05 (−0.26, 0.16) |
| FSH (mIU/mL) | 0.04 (−0.02, 0.10) | 0.04 (−0.02, 0.10) |
| LH (ng/mL) | 0.05 (−0.04, 0.14) | 0.03 (−0.06, 0.12) |
| Green tea | ||
| E2 (pg/mL) | 0.15 (−0.004, 0.31) | 0.28 (0.09, 0.47) |
| Free E2 (pg/mL) | 0.14 (−0.02, 0.30) | 0.26 (0.07, 0.45) |
| Luteal progesterone (ng/mL) | 0.02 (−0.32, 0.36) | −0.13 (−0.59, 0.33) |
| FSH (mIU/mL) | −0.10 (−0.22, 0.02) | −0.04 (−0.18, 0.10) |
| LH (ng/mL) | 0.01 (−0.16. 0.18) | 0.09 (−0.11. 0.30) |
| Caffeinated soda | ||
| E2 (pg/mL) | 0.11 (0.03, 0.19) | 0.15 (0.07, 0.23) |
| Free E2 (pg/mL) | 0.10 (0.03, 0.18) | 0.14 (0.06, 0.22) |
| Luteal progesterone (ng/mL) | −0.04 (−0.22, 0.15) | −0.10 (−0.30, 0.10) |
| FSH (mIU/mL) | 0.01 (−0.05, 0.07) | 0.03 (−0.03, 0.09) |
| LH (ng/mL) | 0.08 (−0.003, 0.16) | 0.13 (0.04, 0.21) |
All values are mean differences (95% CIs). Anovulation was defined as any cycle with a peak progesterone concentration ≤5 ng/mL and no observed serum LH peak at the mid– or late–luteal phase visits. There were 467 ovulatory cycles among all BioCycle Study participants. The analyses were performed by using linear mixed-effects models on the log scale of hormones. E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Adjusted for age (continuous), race (white, black, Asian, other), waist-to-hip ratio (continuous), perceived stress (continuous), daily exercise (continuous), and energy and alcohol intakes (continuous).
Adjusted for age, race, waist-to-hip ratio, perceived stress, daily exercise, energy and alcohol intakes, and relevant phase-specific hormone concentrations by using inverse probability of exposure weights.
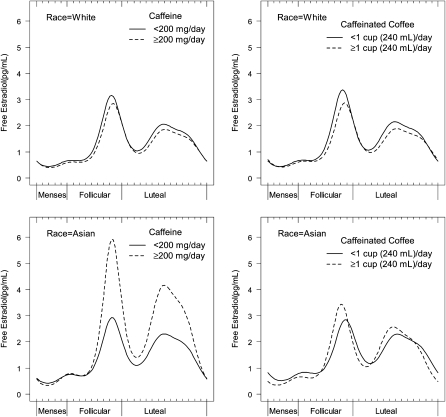
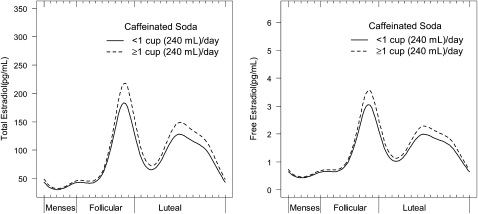
Similar relations, with some differences in patterns, were observed when the nonlinear harmonic models were used. Whereas intakes of ≥200 mg/d and ≥1 cup coffee/d were associated with decreased mean concentrations of free E2 in white women [β = −0.09 (95% CI: −0.18, −0.01) and β = −0.10 (95% CI: −0.17, −0.02), respectively], increased amplitude in free E2 was observed for Asian women who consumed ≥200 mg/d (β = 0.39; 95% CI: 0.01, 0.77) and ≥1 cup coffee/d (β = 0.29; 95% CI: 0.12, 0.47) (Figure 2), compared with women who consumed less. White and Asian women also had a significant free E2 phase shift with coffee intake ≥1 cup/d, although in different directions. Whites peaked later (β = 0.05; 95% CI: 0.03, 0.08), whereas Asians peaked earlier (β = −0.10; 95% CI: −0.16, −0.04) (Figure 2). The relation between mean caffeinated soda consumption (≥1 compared with <1 cup) and free E2 concentrations among all races mirrored the mixed-model results (β = 0.11; 95% CI: 0.03, 0.19) (Figure 3). Similar trends were seen for free and total E2, and no further statistically significant differences in hormonal patterns for other caffeinated beverages were found in the harmonic models.
FIGURE 2.
Adjusted mean serum concentrations of free estradiol across the menstrual cycle for white and Asian women according to caffeine and caffeinated coffee intakes (n = 277 cycles for whites and n = 64 cycles for Asians). The analyses were conducted by using nonlinear mixed models with harmonic terms centered on the day of ovulation. Display set: women with a mean age of 27 y, waist-to-hip ratio of 0.75, perceived stress of 20.2, daily exercise of 14.7 min, and energy intake of 1614 kcal who were consumers of alcohol.
FIGURE 3.
Adjusted mean serum concentrations of total and free estradiol across the menstrual cycle according to intake of caffeinated soda (n = 467 cycles). The analyses were conducted by using nonlinear mixed models with harmonic terms centered on the day of ovulation. Display set: women with a mean age of 27 y, waist-to-hip ratio of 0.75, perceived stress of 20.2, daily exercise of 14.7 min, and energy intake of 1614 kcal who were consumers of alcohol.
Anovulation
Green tea intake (≥1 compared with <1 cup/d) was associated with increased odds of anovulation (adjusted OR: 4.14; 95% CI: 1.26, 13.60); however, results lacked precision because only 16% (n = 42 women) reported consuming ≥1 cup green tea/d on at least one of their 24HDRs. No further significant associations between caffeine (≥200 compared with <200 mg/d) and caffeinated beverages (≥1 compared with <1 cup/d) and ovulatory function were found (Table 4). Sensitivity analyses for caffeine (≥100, ≥300, and ≥400 mg/d) and all caffeinated beverages (1-cup increments) indicated that ≥2 cups coffee/d was marginally associated with decreased odds of anovulation (adjusted OR: 0.23; 95% CI: 0.05, 1.02) (data not shown). Assessment of anovulation based on the less-conservative definition (progesterone ≤5 ng/mL) did not alter the findings from our initial analyses nor did the restriction of the analyses to nonsmokers.
TABLE 4.
Odds of anovulation according to recommended caffeine [≥ 200 (high) compared with <200 (low) mg/d] and beverage [≥1 (high) compared with <1 (low) cup/d] intakes1
| Recommended intake | Multivariate adjustedOR (95% CI)2 |
| Caffeine, ≥200 compared with <200 mg/d | 0.82 (0.31, 2.21) |
| Coffee, ≥1 compared with <1 cup/d | 0.56 (0.22, 1.40) |
| Black tea, ≥1 compared with <1 cup/d | 0.74 (0.32, 1.70) |
| Green tea, ≥1 compared with <1 cup/d | 4.14 (1.26, 13.60) |
| Caffeinated soda, ≥1 compared with <1 cup/d | 0.76 (0.30, 1.94) |
Anovulation was defined as any cycle with a peak progesterone concentration ≤5 ng/mL and no observed serum luteinizing hormone peak at the mid– or late–luteal phase visits (n = 42 of 509 cycles; 8.3%); 28 women had 1 anovulatory cycle, and 7 women had 2 anovulatory cycles. Intake was assessed 4 times during each cycle (menses, midfollicular, ovulation, and midluteal) at clinic visits via 24-h dietary recall. The analyses were performed by using generalized linear mixed models.
Adjusted for age (continuous), race (white, black, Asian, other), waist-to-hip ratio (continuous), perceived stress (continuous), daily exercise (continuous), and energy and alcohol intakes (continuous).
DISCUSSION
We observed that caffeine intake was significantly associated with premenopausal reproductive hormone concentrations and varied across race-ethnicity groups. Higher caffeine intake was associated with decreased free E2 concentrations among whites and with increased free E2 concentrations among Asians. Although we observed differences by race, these results are based on a relatively small sample size and should be interpreted with caution. In addition, caffeinated soda and green tea intake were positively associated with increases in total and free E2 concentrations among all races. Caffeine intake above the recommended levels was not associated with anovulation; however, green tea intake ≥1 cup/d was associated with increased odds for anovulation.
Our finding of an inverse association between caffeine and free E2 concentrations in premenopausal white women concurs with the findings of 2 studies. Kotsopoulos et al (6) examined free E2 plasma concentrations among 524 predominately white (13) women and found an inverse association between caffeine and luteal free E2 concentrations. Similarly, Choi et al (43) found a significant inverse association between increased caffeine intake and decreased peak E2 in 2474 women (race not specified) undergoing infertility treatment. In contrast, Lucero et al (4) found that caffeine intake was associated with increasing early follicular E2 in 498 predominately white (97%) premenopausal women. Kinney et al (5) found no association between caffeine and total E2 in the early follicular phase in a study of 188 predominately white (95%) premenopausal women, whereas Nagata et al (3) found no significant association between caffeine and E2 in the follicular or luteal phase among college-aged Asian women (n = 50). Comparison of these latter 3 studies with ours is limited, because they measured caffeine via a single food-frequency questionnaire and obtained at most 2 serum samples per cycle without the use of a validated method to time menstrual cycle phase. Because menstrual cycle phase has been shown to affect caffeine metabolism (44), women's caffeine intake may vary, which demonstrates the need to capture acute compared with habitual patterns when assessing the effect of caffeine on reproductive hormone concentrations.
Similar associations of coffee and caffeine on E2, both in our study and others (6), suggest that caffeine is the component that influences estrogen metabolism. Whereas we did not measure testosterone, evidence has shown higher testosterone concentrations with higher caffeine and coffee intakes (6), which supports the hypothesis that caffeine's effect on E2 is via aromatase inhibition (6, 19). Estrogen metabolism has also been shown to differ between premenopausal Asians and whites (28–30). It has been hypothesized that gene-environment interactions may partially explain these differences (28–30). CYP1A2 genotypes have been shown to have interethnic variability, eg, Asians and Africans appear to metabolize caffeine more slowly than do whites (10, 37). Our finding of higher E2 concentrations among Asians and blacks with higher caffeine intakes might be due to CYP polymorphisms, but corroboration by other studies is lacking, and we did not directly measure genotype. Additionally, race as a construct represents a complex interplay between many social, lifestyle, environmental, and genetic factors. Further studies are needed to explore differences in the effects of caffeine on reproductive hormones by race.
Despite evidence that elevated or insufficient E2 concentrations can inhibit ovulation (15), we found no association between caffeine, coffee, and anovulation. Our finding corroborates the findings of previous studies (16, 20, 21) and suggests that even though moderate caffeine and coffee intakes may alter E2, these alterations are not within a range that affects ovulatory function. Our results are in line with those of Choi et al's (43) finding that, despite lower E2 concentrations in women with moderate to high caffeine intake, the number of oocytes retrieved did not differ by caffeine category. Recent systematic reviews do not support a positive relation between caffeine consumption and adverse reproductive outcomes (45, 46).
The effects of tea on reproductive hormones have not been well studied. One study, restricted to Asian women, differentiated exposure by tea type and found green tea to be inversely correlated with follicular E2 (3). In contrast, women of all races in the BioCycle Study (including Asian women) had statistically elevated free E2 concentrations at green tea intakes ≥1 cup/d compared with those who consumed lesser amounts and with an increased odds of anovulation—a finding supported by animal research (47). Increases in E2 in response to green tea intake may also lead to an increase in oxidative stress (48); thus, more antioxidants are required to compensate for the increase in oxidative stress. Given that green tea is high in antioxidants and recent evidence suggests that antioxidants adversely affect ovulatory function (49), further research is justified. Caution is warranted regarding the marginal association we found between anovulation and green tea because of the limited intake among study participants and lack of comparative studies.
Our finding that soda intake significantly increases E2 concentrations is novel, but mirrors results from animal studies. Celec and Behuliak (50) found that the intake of 3 different sweetened cola drinks was associated with elevated E2 concentrations in adult male Wistar rats (n = 40). The only other human study that we are aware of that investigated soda consumption and premenopausal E2 concentrations found no effect after adjustment for age, BMI, and cycle length; however, their investigation was restricted to cola, had a small sample size (n = 50), and had a limited exposure/outcome assessment (3).
The BioCycle Study had several strengths, including multiple measures of hormone assessment over 2 menstrual cycles (with the use of standardized methods to time cycle phase) and multiple measures of not only caffeine and caffeinated beverage intake but also of important dietary and lifestyle factors. Whereas self-report of diet is subject to measurement error (51–53), our study used multiple validated 24HDRs to reduce the potential for exposure misclassification.
Nevertheless, the study had several limitations, including the low percentage of women who consumed ≥200 mg caffeine/d and ≥1 cup caffeinated beverages/d. US premenopausal women consume daily an average of 166 mg total caffeine, 19 oz soda, 6 oz coffee, and 5 oz tea (1, 54), whereas the BioCycle Study participants consumed daily an average of 91 mg caffeine, 3 oz soda, 4 oz coffee and 3 oz tea (1 oz = 28 g). Additionally, whereas the BioCycle Study had greater racial diversity than comparable studies (3–6), our study was limited by different sample sizes among the racial groups, which may have limited our power to detect significant differences in some of our subgroup analyses.
In conclusion, within moderate ranges of consumption, caffeine was associated with reduced E2 concentrations among white women and elevated E2 concentrations among Asian women. Caution regarding effect modification by race in this study is warranted because of limited numbers of Asians with high exposure. Understanding the relation between caffeine and E2 has substantial implications for women's health, both in regard to reproductive health and hormonally dependent cancers. Higher concentrations of E2 are found in women with endometriosis—a well-known risk factor for infertility (55). Additionally, evidence indicates an increased risk of breast cancer with elevated E2 concentrations among premenopausal women (56) and possibly of endometrial and ovarian cancers as well (12). Furthermore, bone mineral density is known to vary between races and ethnicities and may be influenced by sex hormones (57–59). Given these public health implications, further research of whether caffeine or other components of caffeinated beverages play a role in reproductive hormone synthesis, and whether these relations differ by race, is needed.
Acknowledgments
We thank Don McMahon (Division of Endocrinology, Columbia University Medical Center) for his assistance with calculating the free E2 concentrations, James VanDerslice (Division of Public Health, University of Utah) for his invaluable comments, and the women who participated in the BioCycle Study for their extraordinary commitment to the study.
The authors’ responsibilities were as follows—KCS and EFS: had full access to all of the data in the study and responsible for the integrity of the data, accuracy of the data analysis, and final content; EFS and JW-W: responsible for the study concept and design and supervision and acquisition of the data; KCS, SLM, AY, and EFS: analyzed the data; KCS and SLM: drafted the manuscript; and KCS, EFS, SLM, AZP, CZ, AY, JBS, AOH, CAP, and JW-W: interpreted the data and assisted with the manuscript revisions. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: CYP1A2, cytochrome P450 1A2; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NDSR, Nutrition Data System for Research; SHBG, sex hormone–binding globulin; 24HDR, 24-h dietary recall.
REFERENCES
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 2005;105:110–3 [DOI] [PubMed] [Google Scholar]
- 2.McCusker RR, Goldberger BA, Cone EJ. Caffeine content of specialty coffees. J Anal Toxicol 2003;27:520–2 [DOI] [PubMed] [Google Scholar]
- 3.Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer 1998;30:21–4 [DOI] [PubMed] [Google Scholar]
- 4.Lucero J, Harlow BL, Barbieri RL, Sluss P, Cramer DW. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil Steril 2001;76:723–9 [DOI] [PubMed] [Google Scholar]
- 5.Kinney A, Kline J, Kelly A, Reuss ML, Levin B. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod 2007;22:1175–85 [DOI] [PubMed] [Google Scholar]
- 6.Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 2009;115:2765–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, Hall JE. Estrogen levels are higher across the menstrual cycle in African-American women compared with caucasian women. J Clin Endocrinol Metab 2011;96:3199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Med 2006;119:S23–30 [DOI] [PubMed] [Google Scholar]
- 9.Lin K-M, Poland RE. Ethnicity, culture, and psychopharmacology : Bloom FE, Kupfer DJ. Psychopharmacology: the fourth generation of progress Brentwood, TN: American College of Neuropsychopharmacology, 2000 [Google Scholar]
- 10.Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev 2003;35:99–106 [DOI] [PubMed] [Google Scholar]
- 11.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst 2006;98:1406–15 [DOI] [PubMed] [Google Scholar]
- 12.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol 2008;630:148–65 [PubMed] [Google Scholar]
- 13.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer 2008;112:1169–77 [DOI] [PubMed] [Google Scholar]
- 14.Yong M, Atkinson C, Newton KM, Aiello Bowles EJ, Stanczyk FZ, Westerlind KC, Holt VL, Schwartz SM, Leisenring WM, Lampe JW, et al. Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control 2009;20:1039–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speroff L, Glass RH, Kase NG. Clinical gynecologic endocrinology and infertility. 6th ed Philadelphia, PA: Lippincott Williams & Wilkins, 1999 [Google Scholar]
- 16.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology 2009;20:374–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson BL, Juliano LM, Schulkin J. Caffeine's implications for women's health and survey of obstetrician-gynecologists’ caffeine knowledge and assessment practices. J Womens Health (Larchmt) 2009;18:1457–66 [DOI] [PubMed] [Google Scholar]
- 18.Miao YL, Shi LH, Lei ZL, Huang JC, Yang JW, Ouyang YC, Sun QY, Chen DY. Effects of caffeine on in vivo and in vitro oocyte maturation in mice. Theriogenology 2007;68:640–5 [DOI] [PubMed] [Google Scholar]
- 19.Yen SSC, Strauss JF, Barbieri RL. Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology, and clinical management. 5th ed Philadelphia, PA: Elsevier/Saunders, 2004 [Google Scholar]
- 20.Grodstein F, Goldman MB, Ryan L, Cramer DW. Relation of female infertility to consumption of caffeinated beverages. Am J Epidemiol 1993;137:1353–60 [DOI] [PubMed] [Google Scholar]
- 21.Fenster L, Quale C, Waller K, Windham GC, Elkin EP, Benowitz N, Swan SH. Caffeine consumption and menstrual function. Am J Epidemiol 1999;149:550–7 [DOI] [PubMed] [Google Scholar]
- 22.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA 1989;86:7696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 1998;11:659–65 [DOI] [PubMed] [Google Scholar]
- 24.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 1999;47:445–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurie G, Maskarinec G, Kaaks R, Stanczyk FZ, Le Marchand L. Association of genetic polymorphisms with serum estrogens measured multiple times during a 2-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev 2005;14:1521–7 [DOI] [PubMed] [Google Scholar]
- 26.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006;295:1135–41 [DOI] [PubMed] [Google Scholar]
- 27.Palacios N, Weisskopf M, Simon K, Gao X, Schwarzschild M, Ascherio A. Polymorphisms of caffeine metabolism and estrogen receptor genes and risk of Parkinson's disease in men and women. Parkinsonism Relat Disord 2010;16:370–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldin BR, Adlercreutz H, Gorbach SL, Woods MN, Dwyer JT, Conlon T, Bohn E, Gershoff SN. The relationship between estrogen levels and diets of Caucasian American and Oriental immigrant women. Am J Clin Nutr 1986;44:945–53 [DOI] [PubMed] [Google Scholar]
- 29.Kotsopoulos J, Vitonis AF, Terry KL, De Vivo I, Cramer DW, Hankinson SE, Tworoger SS. Coffee intake, variants in genes involved in caffeine metabolism, and the risk of epithelial ovarian cancer. Cancer Causes Control 2009;20:335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman MT, Tung KH, McDuffie K, Wilkens LR, Donlon TA. Association of caffeine intake and CYP1A2 genotype with ovarian cancer. Nutr Cancer 2003;46:23–9 [DOI] [PubMed] [Google Scholar]
- 31.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol 2009;23:171–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 2009;169:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Södergård R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–10 [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 35.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics 1946;2:110–4 [PubMed] [Google Scholar]
- 36.Carl J, Hill DA. Preconception counseling: make it part of the annual exam. J Fam Pract 2009;58:307–14 [PubMed] [Google Scholar]
- 37.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 2008;9:625–37 [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 39.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 2009;169:1182–90 [DOI] [PubMed] [Google Scholar]
- 40.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60 [DOI] [PubMed] [Google Scholar]
- 42.Albert PS, Hunsberger S. On analyzing circadian rhythms data using nonlinear mixed models with harmonic terms. Biometrics 2005;61:1115–20 [DOI] [PubMed] [Google Scholar]
- 43.Choi JH, Ryan LM, Cramer DW, Hornstein MD, Missmer SA. Effects of caffeine consumption by women and men on the outcome of in vitro fertilization. J Caffeine Res 2011;1:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balogh A, Klinger G, Henschel L, Borner A, Vollanth R, Kuhnz W. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur J Clin Pharmacol 1995;48:161–6 [DOI] [PubMed] [Google Scholar]
- 45.Peck JD, Leviton A, Cowan LD. A review of the epidemiologic evidence concerning the reproductive health effects of caffeine consumption: a 2000-2009 update. Food Chem Toxicol 2010;48:2549–76 [DOI] [PubMed] [Google Scholar]
- 46.Brent RL, Christian MS, Diener RM. Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol 2011;92:152–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteiro R, Assuncao M, Andrade JP, Neves D, Calhau C, Azevedo I. Chronic green tea consumption decreases body mass, induces aromatase expression, and changes proliferation and apoptosis in adult male rat adipose tissue. J Nutr 2008;138:2156–63 [DOI] [PubMed] [Google Scholar]
- 48.Schisterman EF, Gaskins AJ, Mumford SL, Browne RW, Yeung E, Trevisan M, Hediger M, Zhang C, Perkins NJ, Hovey K, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol 2010;172:430–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci USA 2011;108:1462–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celec P, Behuliak M. Behavioural and endocrine effects of chronic cola intake. J Psychopharmacol 2010;24:1569–72 [DOI] [PubMed] [Google Scholar]
- 51.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 52.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13 [DOI] [PubMed] [Google Scholar]
- 53.Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, Schoeller DA, Troiano RP, Freedman LS. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol 2003;32:1054–62 [DOI] [PubMed] [Google Scholar]
- 54.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc 2006;106:1992–2000 [DOI] [PubMed] [Google Scholar]
- 55.Bulun SE, Zeitoun K, Takayama K, Noble L, Michael D, Simpson E, Johns A, Putman M, Sasano H. Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis. Endocr Relat Cancer 1999;6:293–301 [DOI] [PubMed] [Google Scholar]
- 56.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer: does the association vary by a woman's predicted breast cancer risk? J Clin Oncol 2006;24:1823–30 [DOI] [PubMed] [Google Scholar]
- 57.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 2002;87:3057–67 [DOI] [PubMed] [Google Scholar]
- 58.Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int 2003;14:44–52 [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura N, Kasamatsu T, Sakata K, Hashimoto T, Cooper C. The relationship between endogenous estrogen, sex hormone-binding globulin, and bone loss in female residents of a rural Japanese community: the Taiji Study. J Bone Miner Metab 2002;20:303–10 [DOI] [PubMed] [Google Scholar]