Abstract
Background: Glucose-dependent insulinotropic polypeptide [also known as gastric inhibitory polypeptide (GIP)] and its receptor (GIPR) may link overnutrition to obesity, insulin resistance, and type 2 diabetes. A GIPR variant rs2287019 was recently associated with obesity and glucose metabolism.
Objective: We aimed to examine whether weight-loss diets that vary in fat content may modify the effect of this variant on changes in body weight, fasting glucose, and insulin resistance in the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial.
Design: We genotyped the GIPR rs2287019 in 737 overweight adults who were randomly assigned to 1 of 4 weight-loss diets that varied in macronutrient contents for 2 y. We assessed the percentage changes in body weight, fasting glucose, and insulin resistance (HOMA-IR) across genotypes by the low-fat and high-fat diets.
Results: At 6 mo of diet intervention, the T allele of rs2287019 was associated with greater weight loss (β ± SE: −1.05 ± 0.56%; P = 0.06) and greater decreases in fasting glucose (β ± SE: −2.33 ± 0.86%; P = 0.006), fasting insulin (β ± SE: −8.76 ± 4.13%; P = 0.03), and HOMA-IR (β ± SE: −10.52 ± 4.39%; P = 0.01) in participants who were assigned to low-fat diets, whereas there was no significant genotype effect on changes in these traits in the group assigned to the high-fat diet (all P > 0.44; P-interaction = 0.08, 0.04, 0.10, and 0.07, respectively). After correction for multiple tests (significant P = 0.008), the genotype effect on changes in fasting glucose remained significant. Sensitivity analysis in white participants showed that the interactions were more evident on changes in insulin and HOMA-IR (P-interaction < 0.008).
Conclusion: The T allele of GIPR rs2287019 is associated with greater improvement of glucose homeostasis in individuals who choose a low-fat, high-carbohydrate, and high-fiber diet. The POUNDS LOST trial was registered at clinicaltrials.gov as NCT00072995.
INTRODUCTION
GIP4 is an important incretin hormone principally secreted by intestinal K-cells after a meal, especially a meal that is high in fat (1–5). The ingestion of fat has stronger and more protracted effects than does the ingestion of carbohydrate or protein on GIP gene expression and GIP secretion, which leads to elevated circulating GIP (4). GIP exerts effects through GIPR, which is expressed in various tissues including pancreatic islets, adipose tissue, and the brain. Besides its classical physiologic action on insulin secretion in response to the ingestion of glucose, GIPR-mediated signaling also plays an important role in the deposition of excessive fat from diets into adipose tissues (1–5). Adipocyte GIPR activation may induce lipoprotein lipase activity, fatty acid synthesis, and fatty acid incorporation into adipose tissue (6, 7), which thereby promote fat storage. Animal studies showed that that inhibition of GIPR function might prevent high-fat diet– induced obesity and preserve glucose homeostasis (8–14). There is growing evidence that suggests that GIPR-mediated signaling directly links overnutrition to the development of obesity, insulin resistance, and type 2 diabetes (1–5).
Recent genome-wide association studies identified the common genetic variant rs2287019 in the GIPR locus that is associated with obesity risk (15), and major allele C, which increases BMI (in kg/m2), was also reported to be associated with higher fasting glucose but lower 2-h glucose concentrations in a glucose challenge test (16, 17). The direction of the genetic effect is concordant with the function of GIPR signaling (4, 5). Given the close relation between GIPR signaling and the ingestion of dietary fat, we hypothesized that diets that vary in fat content might differentially affect the genetic effect of GIPR on body weight and related metabolic traits.
We tested this hypothesis in participants in a 2-y randomized diet-intervention trial (POUNDS LOST) (18), in which 811 overweight participants were randomly assigned to 1 of 4 diets with different compositions of macronutrients. We investigated the effect of the newly identified GIPR variant rs2287019 on changes in body weight, fasting glucose, and insulin resistance in response to diets that varied in fat content in the intervention.
SUBJECTS AND METHODS
The POUNDS LOST trial
The POUNDS LOST trial was a 2-y randomized clinical trial that compared the effects of energy-reduced diets with different compositions of macronutrients on body weight. The study design, methods, and main results have been described in detail elsewhere (18). Briefly, a total of 811 overweight and obese subjects (25 ≤BMI ≤40) aged 30–70 y were randomly assigned to 1 of 4 diets; the target percentages of energy derived from fat, protein, and carbohydrate in the 4 diets were 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%. Thus, 2 diets were low-fat (20%), 2 diets were high-fat (40%), 2 diets were average-protein (15%), and 2 diets were high-protein (25%) diets, which constituted a 2-by-2 factorial design. Diets consisted of similar foods and met guidelines for cardiovascular health, and carbohydrate-rich foods were used that had a lower glycemic index. Major criteria for exclusion were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation. After 2 y, 80% of the participant (n = 645) completed the trial. The study was approved by the human subjects committee at the Harvard School of Public Health and Brigham and Women's Hospital and the Pennington Biomedical Research Center of the Louisiana State University System and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants gave written informed consent.
Participants
The current analyses were secondary analyses of a dataset obtained from the POUNDS LOST trial. A total of 737 participants with GIPR rs2287019 genotype data available were included in the current study (91% of participants in the POUNDS LOST trial). A total of 61% of participants were women, 80% of participants were white, 15% of participants were African American, 3% of participants were Hispanic, and 2% of participants were Asian or other ethnic groups by self-report. Consistent with the entire POUNDS LOST trial, the mean (±SD) age was 51 ± 9 y, and the mean BMI was 32.7 ± 3.9. There was no significant difference in the basic characteristics between participants with and without GIPR rs2287019 genotype data. At 6 mo, 662 participants with body-weight measurements and 632 participants with blood samples were included for the analyses. At 2 y, 601 participants with body-weight measurements and 537 participants with blood samples were included for analyses.
Measurements
Body weight and waist circumference were measured in the morning before breakfast on 2 d at baseline, 6 mo, and 2 y. Height was measured at baseline. Fasting blood samples, 24-h urine samples, and measurement of resting metabolic rate were obtained on 1 d, and blood pressure was measured on 2 d, at baseline, 6 mo, and 2 y. Concentrations of serum lipids, glucose, insulin, and urinary nitrogen were measured at the clinical laboratory at the Pennington Biomedical Research Center. Blood pressure was measured with the use of an automated device (HEM-907XL; Omron). BMI was calculated as weight in kilograms divided by height in square meters. Insulin resistance was estimated by the HOMA-IR calculated by the following equation:
To assess the dietary adherence across the intervention, dietary intake was assessed in a random sample of 50% of participants by a review of the 5-d diet record at baseline and by 24-h recall during a telephone interview on 3 nonconsecutive days at 6 mo and 2 y. Moore's Extended Nutrient Database was used to analyze diet recalls (20).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). SNP GIPR rs2287019 was genotyped by using the OpenArray SNP Genotyping System (BioTrove). The genotype success rate was 99%. Replicate quality-control samples (10%) were included and genotyped with > 99% concordance. The selected SNP rs2287019 was correlated with the genome-wide association study (17)-reported SNP rs10423928 [r2 = 0.79, 0.59, and 0.97, in the HapMap Centre d'Etude du Polymorphisme Humain from Utah, Yoruba from Ibadan, and Japanese population from Tokyo + Han Chinese from Beijing database, respectively] and another missense SNP rs1800437 (r2 = 0.83, 0.89, and 1.00, respectively) (http://hapmap.ncbi.nlm.nih.gov/). There were marginal differences in the genotype distributions of the GIPR rs2287019 in ethnic groups (P = 0.10). Because the numbers of Hispanic and Asian participants were relative small, we only compared the MAF of the GIPR rs2287019 between whites and African Americans. The MAF of the GIPR rs2287019 was higher in whites (MAF: 0.168) than in African Americans (MAF: 0.113), which was comparable with the HapMap CEU (MAF: 0.173) and YRI (MAF: 0.107) database. The allele frequency in all participants or in whites was in Hardy-Weinberg equilibrium (P > 0.53).
Statistical analysis
Primary endpoints for this study were percentage changes in body weight, fasting glucose, fasting insulin, and insulin resistance (estimated by HOMA-IR) from baseline to 6 mo of intervention. Changes in these traits at 2 y were secondary endpoints because most participants regained body weight, and the adherence to diets declined between 6 mo and 2 y in the POUNDS LOST trial (18, 21). Because GIP secretion is sensitive to dietary fat ingestion (1, 4, 5), we compared low-fat (20%) compared with high-fat (40%) diets in the primary analysis and compared average-protein (15%) with high-protein (25%) diets in the secondary analysis. General linear models for continuous variables and the chi-square test for categorical variables were applied for the comparison according to genotype groups at baseline. We compared changes in endpoints, biomarkers of adherence, and nutrient intakes across genotype groups according to diets groups at 6 mo and 2 y by using general linear models. To test gene–diet-intervention interactions, we examined genotype, diet intervention, and interaction of genotype by diet intervention as independent predictors of changes in endpoints adjusted for age, sex and ethnicity in the general linear models. We used additive inheritance models (GIPR CC, CT, and TT genotype groups were coded as 0, 1, and 2 in continuous form) in the analyses. Because HOMA-IR was calculated on the basis of glucose and insulin, and the endpoints at 6 mo and 2 y were correlated, we did not treat them as independent variables. Stratified analyses by 2 diet groups were followed by interaction tests. We used Bonferroni adjustment to adjust P values for 6 tests [3 dependently measured traits (body weight, glucose, and insulin) × 2 stratified analyses] at each time point. Because the majority of the participants were white (80%), similar analyses were repeated in white participants. All reported P values were nominal and 2-side, and P = 0.05 was considered statistical significant. We used Quanto 1.2.4 software (http://hydra.usc.edu/gxe; University of Southern California) to estimate the detectable interaction effects of genotype by diet intervention under an additive inheritance model. The study had 80% power to detect the gene–diet-intervention interaction by accounting for a 2.2% change for weight loss, 3.2% change in fasting glucose, 16.7% changes in fasting glucose, and 18.4% change in HOMA-IR at 6 mo at a significance level of 0.05. Statistical analyses were performed with SAS software (version 9.1; SAS Institute Inc).
RESULTS
Baseline characteristics of participants according to the GIPR rs2287019 genotype are shown in Table 1. Genotype frequencies were similar between men and women, in ethnic groups, and across diet groups, and no differences in baseline characteristics across the genotype were observed (all P ≥ 0.10).
TABLE 1.
Baseline characteristics of the study participants1
|
GIPR rs2287019 genotype |
||||
| CC (n = 504) | CT (n = 208) | TT (n = 25) | P | |
| Age (y) | 51 ± 92 | 51 ± 9 | 53 ± 10 | 0.71 |
| Sex [n (%)] | 0.13 | |||
| F | 316 (70.4) | 122 (27.2) | 11 (2.4) | |
| M | 188 (65.3) | 86 (41.4) | 14 (4.9) | |
| Race or ethnic group [n (%)] | 0.10 | |||
| White | 392 (66.4) | 178 (30.2) | 20 (3.4) | |
| Black | 89 (80.2) | 19 (17.1) | 3 (2.7) | |
| Hispanic | 16 (64.0) | 7 (28.0) | 2 (8.0) | |
| Asian or other | 7 (63.6) | 4 (36.4) | 0 (0) | |
| Diet groups [n (%)] | 0.62 | |||
| Low-fat diets (20% of calories) | 252 (67.9) | 104 (28.0) | 15 (4.1) | |
| High-fat diets (40% of calories) | 252 (68.9) | 104 (28.4) | 10 (2.7) | |
| Height (m) | 1.69 ± 0.09 | 1.69 ± 0.09 | 1.71 ± 0.09 | 0.63 |
| Weight (kg) | 93 ± 15 | 93 ± 16 | 95 ± 16 | 0.46 |
| BMI (kg/m2) | 32.7 ± 3.9 | 32.5 ± 3.8 | 32.3 ± 3.8 | 0.52 |
| Waist circumference (cm) | 104 ± 13 | 103 ± 13 | 106 ± 14 | 0.31 |
| Blood pressure (mm Hg) | ||||
| Systolic | 120 ± 13 | 118 ± 13 | 123 ± 17 | 0.24 |
| Diastolic | 76 ± 9 | 75 ± 9 | 78 ± 11 | 0.72 |
| Glucose (mg/dL) | 92 ± 12 | 93 ± 16 | 93 ± 12 | 0.75 |
| Insulin (μU/mL) | 12.2 ± 7.9 | 12.2 ± 7.3 | 12.2 ± 8.3 | 0.86 |
| HOMA-IR | 2.8 ± 2.0 | 2.8 ± 1.8 | 2.9 ± 2.1 | 0.81 |
| Total cholesterol (mg/dL) | 202 ± 37 | 201 ± 35 | 214 ± 47 | 0.30 |
| LDL cholesterol (mg/dL) | 126 ± 33 | 123 ± 30 | 138 ± 39 | 0.48 |
| HDL cholesterol (mg/dL) | 49 ± 14 | 49 ± 15 | 49 ± 14 | 0.62 |
| Triglycerides (mg/dL) | 140 ± 81 | 154 ± 97 | 136 ± 75 | 0.57 |
| Urinary nitrogen (g) | 12.1 ± 4.3 | 12.6 ± 4.8 | 12.4 ± 4.6 | 0.70 |
| Respiratory quotient | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.84 ± 0.03 | 0.79 |
| Dietary intake per day3 | ||||
| Energy (kcal) | 1923 ± 559 | 2021 ± 564 | 2294 ± 502 | 0.38 |
| Carbohydrate (%) | 45 ± 8 | 44 ± 8 | 43 ± 8 | 0.19 |
| Fat (%) | 37 ± 6 | 37 ± 7 | 39 ± 5 | 0.35 |
| Protein (%) | 18 ± 3 | 19 ± 4 | 17 ± 3 | 0.13 |
P values were calculated by using the chi-square test for categorical variables and general linear models for continuous variables.
Mean ± SD (all such values).
Information was collected from a 5-d diet record in a random sample of 50% of the participants.
Reported dietary intakes (total energy, fat, protein, and carbohydrate) and changes in biomarkers of adherence (urinary nitrogen and respiratory quotient) confirmed that participants modified their intakes of macronutrients in the direction of the intervention, but the targets were not fully achieved. These results were consistent with the entire POUNDS LOST trial (18). Reported fat intakes in low-fat and high-fat diet groups were both 37% at baseline; 26% and 34% at 6 mo; and 28% and 34% at 2 y. There were no significant differences in mean values or changes of nutrient intakes and biomarkers of adherence at 6 mo or 2 y across the GIPR rs2287019 genotype in each of the 2 diet groups (all P > 0.05) (Table 2).
TABLE 2.
Nutrient intake and biomarkers of adherence according to GIPR rs2287019 genotype and diets at 6 mo and 2 y1
| 6 mo |
2 y |
|||||||||||
| CC |
CT |
TT |
CC |
CT |
TT |
|||||||
| Value | Change2 | Value | Change2 | Value | Change2 | Value | Change2 | Value | Change2 | Value | Change2 | |
| Low-fat diets | ||||||||||||
| Dietary intake per day3 | ||||||||||||
| Energy (kcal) | 1578 ± 5424 | −393 | 1609 ± 530 | −467 | 1941 ± 468 | −524 | 1558 ± 474 | −442 | 1509 ± 414 | −535 | 2219 ± 513 | −547 |
| Carbohydrate (%) | 56 ± 11 | 9.3 | 54 ± 9 | 10.9 | 56 ± 7 | 14.4 | 52 ± 10 | 7.5 | 51 ± 10 | 9.6 | 59 ± 8 | 17 |
| Fat (%) | 26 ± 8 | −10.7 | 27 ± 7 | −10.7 | 28 ± 5 | −13.7 | 28 ± 8 | −9.8 | 28 ± 8 | −11 | 22 ± 4 | −18.1 |
| Protein (%) | 20 ± 4 | 2.2 | 20 ± 4 | 1.1 | 19 ± 4 | 3.4 | 20 ± 4 | 2.0 | 20 ± 4 | 2.0 | 20 ± 4 | 5.1 |
| Biomarkers of adherence | ||||||||||||
| Urinary nitrogen (g)5 | 11.6 ± 4.4 | 0.7 | 11.4 ± 3.9 | 1.6 | 11.2 ± 3.8 | −0.6 | 11.6 ± 4.1 | 1.7 | 12.5 ± 4.5 | −0.5 | 12.8 ± 5.2 | −14.7 |
| Respiratory quotient6 | 0.84 ± 0.04 | 2.68 | 0.84 ± 0.04 | −5.49 | 0.83 ± 0.03 | −10.37 | 0.83 ± 0.04 | −0.69 | 0.84 ± 0.04 | 1.67 | 0.81 ± 0.05 | −3.28 |
| High-fat diets | ||||||||||||
| Dietary intake per day7 | ||||||||||||
| Energy (kcal) | 1615 ± 498 | −401 | 1678 ± 530 | −335 | 1533 ± 275 | −291 | 1491 ± 497 | −350 | 1393 ± 498 | −549 | 1875 ± 133 | −329 |
| Carbohydrate (%) | 47 ± 9 | 3.2 | 45 ± 7 | 1.6 | 49 ± 11 | 3.6 | 47 ± 10 | 1.8 | 43 ± 9 | 1.6 | 48 ± 16 | −7.9 |
| Fat (%) | 34 ± 7 | −4.0 | 34 ± 7 | −4.0 | 34 ± 8 | −1.7 | 34 ± 8 | −4.0 | 34 ± 7 | −4.1 | 39 ± 11 | 8.1 |
| Protein (%) | 20 ± 5 | 2.0 | 21 ± 5 | 3.2 | 20 ± 5 | −1.3 | 20 ± 5 | 2.8 | 22 ± 5 | 2.6 | 16 ± 6 | 1.0 |
| Biomarkers of adherence | ||||||||||||
| Urinary nitrogen (g)8 | 11.3 ± 4.2 | −0.9 | 12.1 ± 5.7 | −0.7 | 12.3 ± 5.2 | 2.7 | 11.7 ± 4.7 | −0.2 | 12.6 ± 5.1 | 3.8 | 12.3 ± 4.7 | −3.2 |
| Respiratory quotient9 | 0.84 ± 0.04 | −2.14 | 0.84 ± 0.04 | −0.43 | 0.87 ± 0.06 | 12.02 | 0.83 ± 0.04 | −2.01 | 0.82 ± 0.04 | −2.74 | 0.83 ± 0.05 | −0.77 |
There were no significant differences in mean values or changes in nutrient intakes and biomarkers of adherence at 6 mo or 2 y across the GIPR rs2287019 genotype in each of the 2 diet groups (general linear models).
Change from baseline was the actual change in cases of nutrient intake per day and the percentage change in cases of biomarkers of adherence.
Data were included for 114 (CC), 45 (CT,) and 9 (TT) participants at 6 mo and 66 (CC), 19 (CT), and 3 (TT) participants at 2 y.
Mean ± SD (all such values).
Data were included for 187 (CC), 73 (CT), and 12 (TT) participants at 6 mo and 129 (CC), 48 (CT), and 7 (TT) participants at 2 y.
Data were included for 208 (CC), 80 (CT), and 14 (TT) participants at 6 mo and 162 (CC), 63 (CT), and 10 (TT) participants at 2 y.
Data were included for 107 (CC), 50 (CT), and 4 (TT) participants at 6 mo and 51 (CC), 26 (CT), and 3 (TT) participants at 2 y.
Data were included for 173 (CC), 88 (CT), and 7 (TT) participants at 6 mo and 122 (CC), 61 (CT), and 6 (TT) participants at 2 y.
Data were included for 194 (CC), 92 (CT), and 9 (TT) participants at 6 mo and 149 (CC), 72 (CT), and 6 (TT) participants at 2 y.
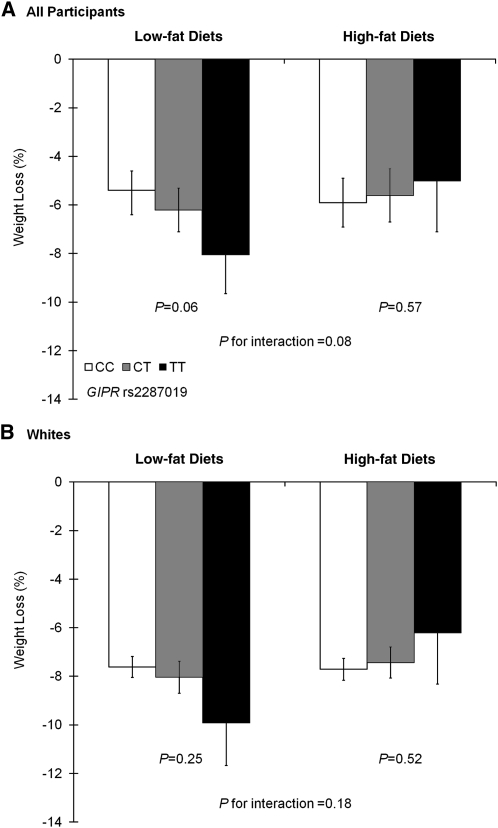
Means (±SDs) of body weight, fasting glucose, fasting insulin, and HOMA-IR across the GIPR rs2287019 genotype by the low-fat and high-fat diet groups at baseline, 6 mo, and 2 y are shown in Table 3. At 6 mo, the T allele of rs2287019 was marginally associated with greater weight loss [effect size (β ± SE): −1.05 ± 0.56%; P = 0.06] in participants who were assigned to low-fat diets (20% of calories), whereas no significant genotype effect on changes in weight loss was observed in subjects assigned to high-fat diets (40% of calories) (β ± SE: 0.34 ± 0.59%; P = 0.57) after adjustment for age, sex, and ethnicity (Figure 1A). There was a marginally significant interaction between GIPR rs2287019 variant and diet intervention on weight loss (P-interaction = 0.08). Sensitivity analysis in white participants showed similar trends for interaction, but results did not reach statistical significance (Figure 1B).
TABLE 3.
Body weight, fasting glucose, insulin, and HOMA-IR according to GIPR rs2287019 genotype and diets at baseline, 6 mo, and 2 y
| Baseline |
6 mo |
2 y |
|||||||
| CC | CT | TT | CC | CT | TT | CC | CT | TT | |
| Low-fat diets | |||||||||
| Participants (n) | 252 | 104 | 15 | 230 | 88 | 14 | 212 | 80 | 13 |
| Weight (kg) | 92.5 ± 14.01 | 93.7 ± 16.1 | 95.7 ± 15.3 | 86.0 ± 13.6 | 87.6 ± 16.3 | 88.2 ± 13.1 | 88.4 ± 14.6 | 89.8 ± 16.0 | 91.4 ± 13.7 |
| Participants (n) | 252 | 104 | 15 | 222 | 85 | 14 | 191 | 72 | 12 |
| Glucose (mg/dL) | 91.8 ± 11.5 | 92.1 ± 11.0 | 94.9 ± 14.6 | 89.7 ± 10.1 | 88.7 ± 8.1 | 88.7 ± 12.5 | 94.2 ± 11.5 | 94.3 ± 10.8 | 95.3 ± 11.7 |
| Insulin (μU/mL) | 12.1 ± 8.4 | 11.9 ± 6.6 | 12.1 ± 8.2 | 10.2 ± 7.1 | 9.0 ± 4.8 | 8.4 ± 4.4 | 11.5 ± 10.0 | 10.5 ± 5.4 | 10.8 ± 7.6 |
| HOMA-IR | 2.8 ± 2.2 | 2.8 ± 1.8 | 3.0 ± 2.2 | 2.3 ± 1.6 | 2.0 ± 1.2 | 1.9 ± 1.1 | 2.8 ± 2.5 | 2.5 ± 1.4 | 2.6 ± 1.9 |
| High-fat diets | |||||||||
| Participants (n) | 252 | 104 | 10 | 223 | 97 | 10 | 205 | 83 | 8 |
| Weight (kg) | 94.1 ± 16.8 | 92.6 ± 15.8 | 92.9 ± 17.2 | 87.1 ± 17.0 | 86.6 ± 16.3 | 86.6 ± 14.8 | 89.2 ± 17.9 | 89.4 ± 16.5 | 90.4 ± 17.2 |
| Participants (n) | 252 | 104 | 10 | 208 | 93 | 10 | 178 | 76 | 8 |
| Glucose (mg/dL) | 92.0 ± 11.8 | 91.7 ± 13.3 | 89.7 ± 4.2 | 90.7 ± 12.1 | 89.7 ± 13.0 | 91.2 ± 11.7 | 96.4 ± 15.4 | 94.1 ± 13.2 | 95.6 ± 10.2 |
| Insulin (μU/mL) | 12.3 ± 7.3 | 12.4 ± 7.9 | 12.4 ± 9.0 | 10.1 ± 6.1 | 9.8 ± 6.3 | 11.7 ± 8.0 | 11.0 ± 6.5 | 11.4 ± 8.7 | 14.0 ± 9.4 |
| HOMA-IR | 2.9 ± 1.8 | 2.9 ± 1.9 | 2.8 ± 2.1 | 2.3 ± 1.6 | 2.2 ± 1.5 | 2.8 ± 2.3 | 2.7 ± 1.9 | 2.7 ± 2.4 | 3.4 ± 2.5 |
Mean ± SD (all such values).
FIGURE 1.
Genotype effect of GIPR rs2287019 on mean (± SE) percent weight loss by low-fat and high-fat diet groups at 6 mo. A: Data were included for all participants [230 (CC), 88 (CT), and 14 (TT) participants in low-fat diet groups and 223 (CC), 97 (CT), and 10 (TT) participants in high-fat diet groups]. B: Data were included for white participants (182 (CC), 76 (CT), and 11 (TT) participants in low-fat diet groups and 179 (CC), 85 (CT), and 8 (TT) participants in high-fat diet groups]. Data were calculated by using general linear models after adjustment for age, sex, and ethnicity (if appropriate).
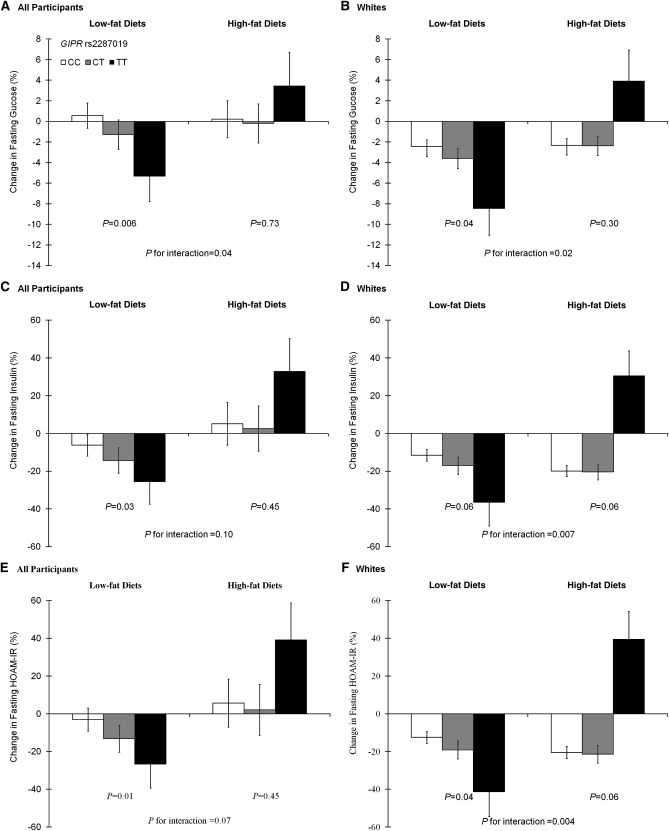
In the low-fat diet groups, the T allele of rs2287019 was associated with greater decreases in fasting glucose (β ± SE: −2.33 ± 0.86%; P = 0.006), fasting insulin (β ± SE: −8.76 ± 4.13%; P = 0.03), and HOMA-IR (β ± SE: −10.52 ± 4.39%; P = 0.01) (Figure 2, A, C, and E). In the high-fat diet groups, there was no significant genotype effect on changes in these traits (all P > 0.44). There was a significant interaction between GIPR rs2287019 and diet intervention on changes in fasting glucose (P-interaction = 0.04) and marginal interactions on changes in insulin and HOMA-IR (P-interaction = 0.10 and 0.07, respectively). After adjustment for multiple tests [significant P = 0.008 [(0.05 ÷ 6)], the genotype effect on changes in fasting glucose in low-fat diet groups remained significant. To test whether the observed effects were mediated by weight loss, we further adjusted for weight loss in the analysis models. Genotype effects on changes in fasting glucose, insulin, and HOMA-IR were attenuated (P = 0.02, 0.18, and 0.08, respectively) in the low-fat diet groups. The analysis in white participants showed more significant genotype–diet-intervention interactions on changes in fasting glucose, insulin, and HOMA-IR (P-interaction = 0.02, 0.007, and 0.004, respectively) (Figure 2, B, D, and F). Interactions on changes in insulin and HOMA-IR remained significant after adjustment for multiple tests [significant P = 0.008 [(0.05 ÷ 6)].
FIGURE 2.
Genotype effect of GIPR rs2287019 on mean (± SE) percent changes in fasting glucose (A and B), fasting insulin (C and D), and HOMA-IR (E and F) in the low-fat and high-fat diet groups at 6 mo. A, C, and E: Data were included for all participants [222 (CC), 85 (CT) and 14 (TT) participants in low-fat diet groups and 208 (CC), 93 (CT) and 10 (TT) participants in high-fat diet groups]. B, D, and F: Data were included for white participants [177 (CC), 75 (CT) and 11 (TT) participants in low-fat diet groups and 170 (CC), 82 (CT) and 8 (TT) participants in high-fat diet groups]. Data were calculated by using general linear models after adjustment for age, sex, and ethnicity (if appropriate).
At 2 y, most participants regained body weight (18), and there was no significant interaction or genotype effect on weight loss (see Figure S1 under “Supplemental data” in the online issue). For changes in fasting glucose, insulin and HOMA-IR, the results were also attenuated to nonsignificance, but with similar trends at 6 mo (see Figure S2 under “Supplemental data” in the online issue).
In secondary analyses, we did not find any genotype effects on changes in body weight, fasting glucose, and related traits in the average-protein diet or high-protein diet groups at 6 mo or 2 y (all P > 0.17). There was no significant interaction between GIPR genotype and diets (all P-interaction > 0.35).
DISCUSSION
In this 2 y weight-loss trial, we examined the genotype effects of the GIPR variant rs2287019 on weight loss and changes in fasting glucose and insulin resistance in response to diet interventions. We showed interactions between the GIPR variant and diets that varied in fat on weight loss and changes in fasting glucose, insulin, and HOMA-IR at 6 mo of follow-up. Our data indicated that the T allele of GIPR rs2287019 was associated with greater improvement of glucose and insulin resistance in individuals who consumed a low-fat diet.
The GIPR variant rs2287019 was not significantly related to baseline BMI and glucose concentrations in our study. This result may have been partly due to the overweight and obese nature of the study samples that was different from that of populations from which the variant was originally identified (15). We observed parallel genotype effects on weight loss and improvement of fasting glucose, fasting insulin, and HOMA-IR in the low-fat groups at 6 mo. The genotype-associated improvements of fasting glucose, fasting insulin, and HOMA-IR in low-fat diet groups were attenuated after adjustment for weight loss, which suggested that the genetic effects might have been partly mediated through changes in body weight. These findings support the pathophysiologic roles of GIPR-mediated signaling in obesity and insulin resistance (1–5).
There was no significant interaction between the GIPR variant rs2287019 and protein intakes in our study. This result was in agreement with the findings from previous studies that GIP secretion is largely regulated by the ingestion of fat and carbohydrate rather than by the ingestion of protein (4, 22, 23). In our study, the low-fat diets actually had high carbohydrate contents; therefore, it is difficult to distinguish the effects of dietary fat and carbohydrate. However, it has been suggested that fatty acids have stronger and more protracted actions than do sugars in the stimulation of GIP secretion (4, 24). In addition, carbohydrate-rich foods with low glycemic index were used in this intervention. The low-fat diets had higher fiber contents than did the high-fat diets. The consumption of low glycemic index foods and dietary fibers has been shown to lower GIP secretion (25). Taken together, we posit that dietary fat and/or fibers may be responsible for the observed interactions with the GIPR genotype. Because inhibition of GIPR-mediated signaling may prevent obesity, lower blood glucose and insulin, and improve insulin sensitivity (8–14), we speculate that the T allele (BMI-and fasting glucose–decreasing allele) of the GIPR variant rs2287019 is more likely to be associated with impaired GIPR signaling, although the functionality of this variant has not been clarified. In the current study, the T allele of GIPR rs2287019 was associated with greater decreases in body weight, fasting glucose and insulin, and HOMA-IR in participants in the low-fat diet groups. These observations may reflect the additive favorable effects of decreased GIP secretion by consumption of low-fat diets and the impaired GIPR function associated with GIPR genetic variation. Nevertheless, the underlying mechanisms responsible for the interactions between GIPR genetic variation and dietary interventions remain to be further clarified.
It was not surprising that the results were largely attenuated at 2 y because of the diminished adherence that occurred between 6 mo and 2 y in the POUNDS LOST study (18, 21). Substantially diminished adherence after the first few months is typical in weight-loss trails (26–29). Reduced statistical power might be another reason for the attenuated results because a relatively large number of participants did not have measurements of body weight (n = 136) or blood sample (n = 200) at 2 y for the current analysis. In sensitivity analyses, we showed similar results in white participants. The observed genotype effects and gene-diet interactions on changes in fasting glucose and related traits in white participants were even more evident than those observed in all participants. However, we did not perform a separate analysis in other ethnic groups because of the relative small numbers of minority participants.
To the best of our knowledge, this was the first study to investigate interactions between GIPR genetic variation and dietary fat on weight-loss and improvement of glucose homeostasis and insulin resistance in a large and long-term randomized trial. Previous evidence has suggested the potential therapeutic application of GIPR antagonism [(proline3) GIP] in the treatment of obesity and type 2 diabetes (1, 4, 5). Our findings suggest a new insight in the use of GIPR genetic variation in improving personalized dietary interventions of these disorders.
Several limitations of our study also need consideration. The euglycemic glucose clamp technique and 2-h glucose tolerance test were not performed because it was difficult to be applied in this large population-based trial. This study may have been underpowered in the detection of modest interactions and genotype effects, especially at 2 y. Although some of our results remained significant after adjustment for multiple tests, replication of these results is needed in future. In addition, most of the participants were white (80%) in our study, and it remains to be determined whether our findings can be generalized in other ethnic groups.
In conclusion, our data indicate weight-loss diets that vary in fat content may modify the genetic effect of the GIPR variant rs2287019 on changes in body weight, fasting glucose, fasting insulin, and HOMA-IR. The T allele of the GIPR rs2287019 was associated with greater improvement of glucose homeostasis in individuals in response to a low-fat, high-carbohydrate, and high-fiber diet. These findings may provide novel information to the development of an effective diet intervention in the prevention and treatment of obesity and related disorders.
Supplementary Material
Acknowledgments
We thank the participants in the trial for their dedication and contribution to the research.
The authors’ responsibilities were as follows—QQ, FMS, and LQ: designed the research; QQ, GAB, FMS, and LQ: conducted the research; QQ: analyzed data; QQ, FBH, and LQ: wrote the manuscript; GAB, FMS, and FBH: provided materials; LQ: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: GIP, glucose-dependent insulinotropic polypeptide (also known as gastric inhibitory polypeptide); GIPR, gastric inhibitory polypeptide receptor; MAF, minor allele frequency; POUNDS LOST, Preventing Overweight Using Novel Dietary Strategies; SNP, single nucleotide polymorphism.
REFERENCES
- 1.Gault VA, O'Harte FPM, Flatt PR. Glucose-dependent insulinotropic polypeptide (GIP): anti-diabetic and anti-obesity potential? Neuropeptides 2003;37:253–63 [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Miyawaki K, Tsukiyama K, Harada N, Yamada C, Seino Y. Pancreatic and extrapancreatic effects of gastric inhibitory polypeptide. Diabetes 2006;55(suppl 2):S86–91 [Google Scholar]
- 3.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–57 [DOI] [PubMed] [Google Scholar]
- 4.Flatt PR. Dorothy Hodgkin Lecture 2008. Gastric inhibitory polypeptide (GIP) revisited: a new therapeutic target for obesity–diabetes? Diabet Med 2008;25:759–64 [DOI] [PubMed] [Google Scholar]
- 5.Irwin N, Flatt P. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia 2009;52:1724–31 [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH, Fujimoto WY, Brunzell JD. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes 1979;28:1141–2 [DOI] [PubMed] [Google Scholar]
- 7.Beck B, Max J-P. Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regul Pept 1983;7:3–8 [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002;8:738–42 [DOI] [PubMed] [Google Scholar]
- 9.Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, Harriott P, O'Harte FPM, Flatt PR. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 2005;54:2436–46 [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Yamada Y, Tsukiyama K, Miyawaki K, Hosokawa M, Nagashima K, Toyoda K, Naitoh R, Mizunoya W, Fushiki T, et al. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun 2005;335:937–42 [DOI] [PubMed] [Google Scholar]
- 11.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 2007;117:143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin N, McClean P, O'Harte F, Gault V, Harriott P, Flatt P. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia 2007;50:1532–40 [DOI] [PubMed] [Google Scholar]
- 13.Gault VA, McClean P, Cassidy R, Irwin N, Flatt P. Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 2007;50:1752–62 [DOI] [PubMed] [Google Scholar]
- 14.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 2007;293:E1746–55 [DOI] [PubMed] [Google Scholar]
- 15.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan Ja, Magi R, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena R, Hivert M-F, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 20.Champagne CM, Bogle ML, McGee BB, Yadrick K, Allen HR, Kramer TR, Simpson P, Gossett J, Weber J. Dietary intake in the lower Mississippi delta region: results from the foods of our delta study. J Am Diet Assoc 2004;104:199–207 [DOI] [PubMed] [Google Scholar]
- 21.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoder SM, Yang Q, Kindel TL, Tso P. Differential responses of the incretin hormones GIP and GLP-1 to increasing doses of dietary carbohydrate but not dietary protein in lean rats. Am J Physiol Gastrointest Liver Physiol 2010;299:G476–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol 2009;297:G299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flatt PR, Bailey CJ, Kwasowski P, Page T, Marks V. Plasma immunoreactive gastric inhibitory polypeptide in obese hyperglycaemic (ob/ob) mice. J Endocrinol 1984;101:249–56 [DOI] [PubMed] [Google Scholar]
- 25.Gatenby SJ, Ellis PR, Morgan LM, Judd PA. Effect of partially depolymerized guar gum on acute metabolic variables in patients with non-insulin-dependent diabetes. Diabet Med 1996;13:358–64 [DOI] [PubMed] [Google Scholar]
- 26.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–90 [DOI] [PubMed] [Google Scholar]
- 27.Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, Ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia. Ann Intern Med 2004;140:769–77 [DOI] [PubMed] [Google Scholar]
- 28.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, weight watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53 [DOI] [PubMed] [Google Scholar]
- 29.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 2007;297:969–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.